Abstract
BACKGROUND AND PURPOSE:
There is gathering evidence to suggest that agenesis of the corpus callosum is associated with delayed fetal sulcation; it is possible that the corpus callosum facilitates normal gyral development. In this paper we sought to confirm whether delayed sulcation is found in fetuses with isolated agenesis of the corpus callosum as judged by in utero MR imaging.
MATERIALS AND METHODS:
Retrospective analysis of 20 fetuses with isolated corpus callosum agenesis investigated by in utero MR imaging and 20 aged-matched normal fetuses was performed in the second or third trimester. All fetuses were singleton pregnancies with known gestational age, imaged on a 1.5T superconducting MR system. Estimation of sulcation maturity was made with reference to a standard atlas and subgroup analysis of earlier gestation (group 1, 21–26 weeks) and later gestation (group 2, 30–34 weeks) fetuses was performed.
RESULTS:
Group 1 (n = 12) did not show a statistically significant difference between the 2 subgroups (P = .44) in terms of sulcation. A significant difference was demonstrated in the later gestation, group 2 (n = 8) fetal analyses; mean difference between consensus and actual gestation for normal fetuses was 0.9 weeks (SD of 1.5 weeks) versus −0.5 weeks (SD of 1.1 weeks) for the agenesis of corpus callosum cases (P = .046), suggestive of delayed sulcation in callosal agenesis.
CONCLUSIONS:
Delayed sulcation encountered in third trimester fetuses with agenesis of the corpus callosum may be seen and does not in itself imply an additional brain abnormality.
The development of the cortex of the cerebral hemispheres follows a predictable course in the normal fetus in terms of sulcation and has been used as a method to estimate gestational age, both on neuropathologic and fetal neuroimaging studies.1–6 Assessing gyral and sulcal patterns of the fetal brain is difficult by using ultrasonography, but in utero MR imaging affords excellent anatomic detail and improved diagnostic accuracy over sonography in a wide range of fetal brain pathology.3,7–9
Gyral formation can be first recognized macroscopically by the appearance of sulci around 14 weeks of gestational age.10 There are numerous hypotheses regarding the process of normal gyral and sulcal development, and it is likely that multiple factors determine the normal process.10–17 One mechanism that has received attention recently relates to the possible role of white matter tracts “pinning” some regions of the cortex and that mechanical axonal tension holds the cortex at the base of a future cortical sulcus.12,13 The corpus callosum develops between 8 and 20 weeks of gestation and is the principal commissural structure (fiber pathways extending between the hemispheres); as such, the corpus callosum would be expected to be highly involved in cortical pinning.7,18 It would be predicted if that theory is correct that a fetus without a corpus callosum would have delayed sulcation when compared with a normal fetus. The purpose of this study was to see if delayed sulcation is present in second and third trimester fetuses with agenesis of the corpus callosum but with no other detectable brain abnormality.
Materials and Methods
All of the cases in this report were recruited as part of a larger research program of fetal MR imaging based at our institution, directed at investigating the diagnostic value of iuMR imaging as an adjunct to sonography in evaluation of fetal brain abnormalities.19 That program was performed with the approval of the local, regional ethics committee, and fully informed, written consent was obtained from all of the women taking part.
We identified 20 consecutive fetuses with complete isolated ACC from our research data base, and those cases were reviewed by an experienced neuroradiologist (P.D.G.) to confirm the absence of any other brain abnormality within the limits of the examination. None of the cases had associated lipomas based on the in utero imaging. Any fetus with a malformation of cortical development, as defined by Barkovich et al, was excluded.20 Ventriculomegaly was not deemed an additional specific finding, as ACC is frequently associated with dilation of the posterior portions of the lateral ventricles (colpocephaly).7 All of the cases had iuMR performed between July 2002 and October 2008 during which time an equal number of age-matched fetuses with normal iuMR brain studies were identified. All were singleton pregnancies with an accurately estimated gestational age based on last menstrual period and refined by the second trimester dating sonography examination. We quote gestational ages rounded to the nearest week for the purpose of subsequent analyses.
The iuMR imaging was performed on a 1.5T superconducting system (Eclipse or Infinion; Philips Medical Systems, Cleveland, Ohio) with a flexible phased array abdominal coil. Single-shot fast spin-echo sequences were used with 5- and 3 mm-sections in the 3 natural orthogonal planes. The sequence parameters for the 5-mm SSFSE were: TR, 3000 ms; TE, 100 ms; echo-train length, 8; number of averages, 2; FOV, 240 mm; matrix size, 352 × 512; and acquisition time, 210 s. The sequence parameters for the 3-mm SSFSE acquisitions were: TR, 33,804 ms; TE (effective), 156 ms; echo-train length, 139; number of averages, 1; FOV, 255 mm; matrix size, 256 × 256. Additionally, T1 radio-frequency–spoiled Fourier-acquired steady-state images were acquired in the axial plane: TR, 132 ms; TE, 4.5 ms. No maternal sedation or fetal muscular blockade was used in any of the cases.
The iuMR images were then reviewed by 2 neuroradiologists (D.J.A.C., D.J.W.) blinded to clinical history, previous imaging results, and the actual gestational age. The neuroradiologists formed a consensus opinion of gestational age, made by reference to a standard atlas of fetal brain development.1 That atlas demonstrates a 2-week lag between initial sulcal detection (present in 25–75% of cases) and sulcal presence (>75% of cases); our gestational age assessment was based upon Garel's documentation of a sulcus being present.1 The atlas presents data on sulcal presence from an earliest gestation of 22–23 weeks to 34 weeks inclusive; any fetus that demonstrated a sulcal pattern of either <22 weeks or >34 weeks was documented as such, and for purposes of analysis any result within this gestational period was considered as only a single time period (week). It is well documented that different hemispheres can show different degrees of cortical maturity in the same case. In this situation the most mature hemisphere was recorded.
A matched pairs t test was performed to establish whether there was significance between the 2 groups sampled. The optimal time period for most precise correlation between MR sulcal pattern and actual gestational age is considered to occur between 28 and 34 weeks' gestation; we elected, therefore, to make subgroup analyses dependent on gestational age, with group 1 consisting of fetuses whose actual gestational age was 21–26 weeks and group 2 consisting of 30- to 34-week-old fetuses. Note that there were no fetuses studied between 27 and 29 weeks of gestation age, which reflects our referral patterns into the wider study.
Results
The 20 fetuses with ACC had a median gestational age of 25 weeks at the time of iuMR (range, 21–34 weeks), and the 20 normal fetuses had a median gestational age of 24 weeks (range, 21–34 weeks). The actual and estimated gestational ages based on cortical development are shown in Table 1. The prediction of gestational age from sulcation patterns in the normal fetuses was considered to be good with only 1 of 20 (5%) being estimated as >2 weeks different from the actual gestation.
Table 1:
GA predicted from iuMR imaging compared with the actual gestational age in 20 cases of fetal ACC and age-matched normal cases
| Pair Number | ACC |
Normal |
||||
|---|---|---|---|---|---|---|
| Predicted GA | Actual GA | Difference (Weeks) | Predicted GA | Actual GA | Difference (weeks) | |
| 1 | 21 | 21 | 0 | 22 | 21 | +1 |
| 2 | 21 | 22 | −1 | 22 | 21 | +1 |
| 3 | 22 | 22 | 0 | 22 | 21 | +1 |
| 4 | 22 | 22 | 0 | 22 | 22 | 0 |
| 5 | 22 | 22 | 0 | 23 | 23 | 0 |
| 6 | 22 | 22 | 0 | 24 | 23 | +1 |
| 7 | 23 | 24 | −1 | 23 | 24 | −1 |
| 8 | 23 | 24 | −1 | 23 | 24 | −1 |
| 9 | 24 | 24 | 0 | 24 | 24 | 0 |
| 10 | 23 | 25 | −2 | 23 | 24 | −1 |
| 11 | 25 | 25 | 0 | 24 | 24 | 0 |
| 12 | 28 | 25 | +3 | 24 | 26 | −2 |
| 13 | 29 | 30 | −1 | 30 | 30 | 0 |
| 14 | 30 | 30 | 0 | 32 | 30 | +2 |
| 15 | 31 | 31 | 0 | 33 | 31 | +2 |
| 16 | 29 | 31 | −2 | 34 | 31 | +3 |
| 17 | 32 | 32 | 0 | 30 | 32 | −2 |
| 18 | 31 | 33 | −2 | 34 | 33 | +1 |
| 19 | 34 | 33 | +1 | 34 | 33 | +1 |
| 20 | 34 | 34 | 0 | 34 | 34 | 0 |
Note:—Cases 1–12 were included as group 1 for subgroup analysis, while cases 13–20 were group 2.
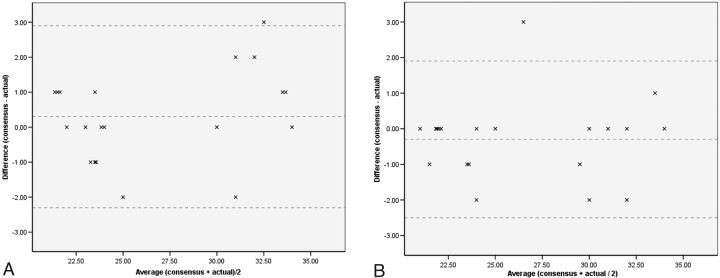
The gestational age estimated from sulcal morphology in all 20 of the normal group had a mean difference from the actual gestation of 0.3 weeks with a standard deviation of 1.3 weeks. The 20 fetuses with ACC had a mean difference of −0.3 weeks with a standard deviation of 1.1 weeks. The base data are presented in Table 1 and the results are also displayed graphically by using a Bland-Altman plot (Fig 1).21 There was no statistically significant difference between the 2 groups by using a 1-tailed, paired t test (P = .096).
Fig 1.
Results displayed by using a Bland-Altman plot (A) normal subgroup. B, ACC subgroup, demonstrating average gestational age (weeks) versus mean difference (weeks). The mean and mean ± 2 SD reference lines are demonstrated.
There were statistically significant differences when groups of different gestational ages were considered. In group 1 (21–26 weeks of gestation, n = 12) there remained no statistically significant difference between the normal and ACC cases. The normal fetuses had a mean difference of 0.1 weeks with a standard deviation of 1.0 weeks, and the ACC group had a mean difference of −0.2 weeks and standard deviation of 1.2 weeks (P = .44). It can be seen from both Fig 1B and Table 1 that this subgroup contains a solitary outlier within pair 12 (defined by Moore and McCabe as a point that falls >1.5 times the interquartile range above the third quartile22). If this data point and its partner data (Table 1, pair 12) are removed from further analysis, then there is a trend toward significance with a mean difference for the ACC subgroup of −0.5 weeks, SD of 0.7 weeks (P = .06). In contrast, group 2 (30–34 weeks, n = 8) did show statistically significant differences; the mean difference for the normal group was 0.9 weeks with a SD of 1.5 weeks, while the ACC group had a mean difference of −0.5 weeks and a SD of 1.1 weeks (P = .046). Examples are shown in Figs 2–4.
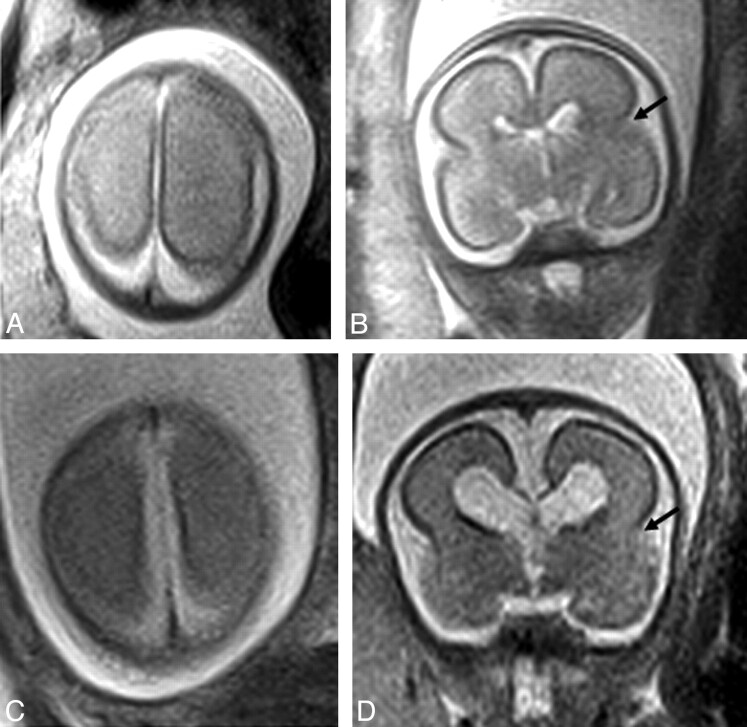
Fig 2.
Axial (A) and (B) coronal plane iuMR of a normal 24 week fetus (group 1); C (axial) and D (coronal) represent an aged-matched ACC case. No discernable difference of the surface sulcal pattern is present. The lateral sulcus is demonstrated on B and D (black arrow).
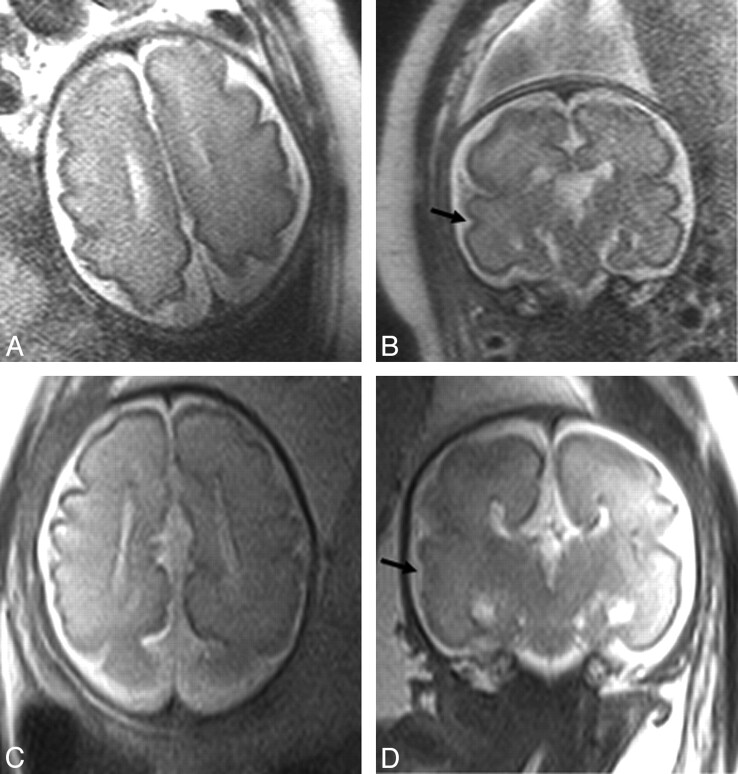
Fig 3.
In utero MR of 31 week gestation fetuses; A (axial) and B (coronal) views of a normal fetus are shown; C and D represent an aged-matched ACC case. This case demonstrates relative sulcation delay with ACC, most obvious within the temporal lobes on coronal imaging where delay in formation of the superior temporal sulcus (black arrow) can be appreciated between the normal case (B) and ACC case (D).
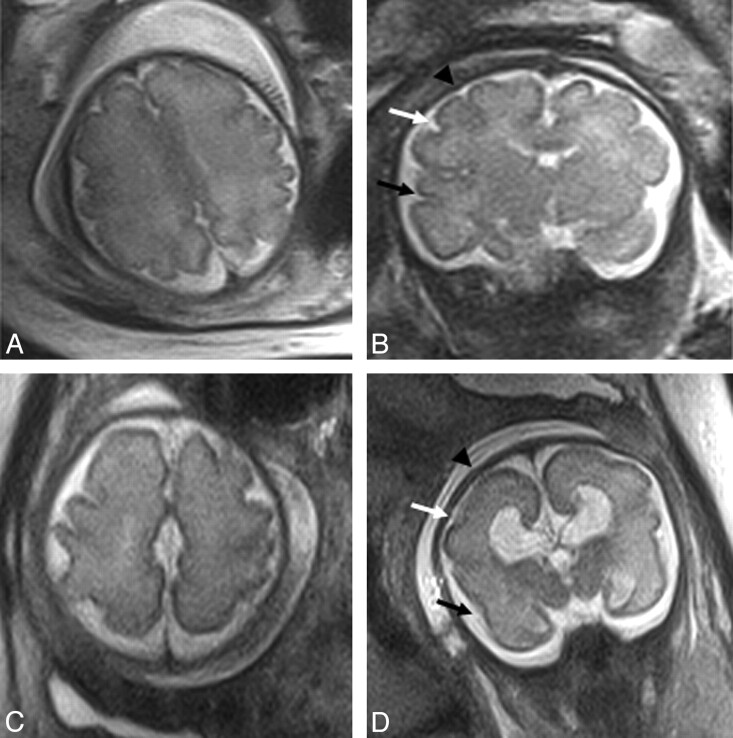
Fig 4.
In utero MR of a 30 week (A and B) normal fetus and (C and D) aged-matched ACC case from group 2. Sulcation delay is apparent between the normal and ACC case; the normal case demonstrates clear formation of the superior frontal sulcus (black arrowhead), inferior frontal sulcus (white arrow), and the superior temporal sulcus (black arrow), while these are not formed on the ACC case (expected location indicated).
Discussion
In this paper we have studied sulcation in fetuses with structurally normal brains including a normal corpus callosum and compared that feature with fetuses with ACC and no other obvious brain abnormality. Failed commissuration is not always complete but we have not included any fetuses with hypogenesis of the corpus callosum. We recognize that most authorities have pointed out that ACC is an embryologically incorrect term as the fibers of the corpus callosum have not failed to form, rather they have not crossed the midline, remaining ipsilateral as the bundles of Probst. While acknowledging this embryologic truism we have elected to use the term ACC in this paper for reasons of common usage and brevity.
It is becoming recognized that the diagnosis of isolated ACC is relatively unusual as it is frequently associated with a range of other brain anomalies and is implicated in >50 congenital syndromes.23 Hetts et al reviewed the postnatal MR imaging of 142 patients with corpus callosum anomalies and showed that 51.4% had associated cortical developmental malformations, with gray matter heterotopia being the most common (present in 29% of their patients with ACC).23 In the review by Coakley et al, half of the fetuses with ACC studied had additional CNS anomalies including Dandy-Walker syndrome, Chiari II malformation, gray matter heterotopia, and holoprosencephaly.8 We attempted to define 20 cases of isolated ACC diagnosed on iuMR for this study, but we must stress that our prenatal diagnosis may be incorrect. This is particularly true for the earlier gestational age cases, as the commonly associated neocortical formation abnormalities may be difficult to diagnose at that stage. We have not been able to follow up all of these cases (either by postmortem studies or by postnatal imaging) to confirm the absence of other brain abnormalities. We do not think, however, that the fundamental diagnosis of ACC would be changed in any of our cases, as the literature points to a high sensitivity and specificity for iuMR to show ACC.
It is well known that there is disruption of the normal sulcal pattern in children and adults with ACC involving the paramedian sulci of the cerebral hemispheres, which show a radial pattern as a direct effect of the absent corpus callosum.5,17,23 That feature, however, was not the subject of our study, though it can be seen in fetuses with ACC after 32 weeks' gestational age. As described previously, the etiology of cortical sulcation delay could be due to the presence of other brain abnormalities and unrelated to the commissural abnormality per se. Our attempt to include only isolated ACC cases was to try to circumvent that confounding factor.
We must consider why sulcation might be delayed in a fetus with ACC. Numerous hypotheses have been proposed regarding the processes intrinsic to normal gyral/sulcal development, including tailored growth during gyrogenesis,11,12 competitive mechanical tension along axons in the white matter,12,13 genetic factors,14,15 and environmental factors.10,16 Toro and Burnod17 described a morphogenetic model for development of cortical convolutions combining a mechanical theory of gyrification and genetic factors. It is likely that a number of factors determine normal gyral development; mechanical factors do however appear central to many theories, specifically the organization of long-range projections and resultant gyral morphology relating specifically to underlying cortical pinning.12,13,17 The work presented in this paper supports the theory that an integral corpus callosum facilitates optimal gyral development and that ACC may lead to delayed gyral formation.
Apparent delayed sulcation has been described recently by Tang et al in a review of 29 cases of fetal ACC.18 Those authors found evidence of sulcal delay and/or abnormal sulcal morphology in 23 of 29 cases with a statistically significant delay in sulcation in cases of ACC when compared with a control group (P < .0001); not all of those cases, however, were isolated ACC cases. They found that sulcal delay occurred exclusively in fetuses before 30 weeks' gestational age with no detectable delay in those ≥30 weeks' gestational age or on postnatal assessment. Indeed, they presented a case of delayed sulcation identified at 26.4 weeks, in which sulcation became “normal for age” on repeat iuMR imaging at 32.4 weeks. Our own results are somewhat at variance with that; in our assessment of earlier gestation fetuses (group 1, 21–26 weeks gestation), though there was a trend toward significance, no actual statistically significant difference between the normal and ACC cases was identified. Comparative review of the assessments performed during the most accurate dating period according to Garel and colleagues (group 2, 30–34 weeks gestation) did however reveal a statistically significant difference, suggesting that sulcation is delayed in cases of fetal ACC at this stage (30–34 weeks) of neurodevelopment.1,4 We demonstrated excellent correlation between consensus assessments of gestation versus actual gestational age with 95% of all cases being accurately dated to within 2 weeks.
It is important for us to review some methodologic considerations at this point. While numerous pathologic studies have documented progressive gyral development with increasing gestation, there is a relative paucity of MR studies depicting normal fetal cortical development patterns in utero. One of the largest series published to date was by Garel et al who reported the findings of 225 normal iuMR fetal studies, between 22 to 38 weeks' gestation; they presented the gestational age at which particular sulci were present in 75% of cases (Table 2).1 Neuropathologic studies24,25 assessing sulcal pattern typically demonstrate sulcal formation before its visualization on iuMR.4,5 Levine and Barnes, for example, reported a mean lag of 1.9 ± 2.2 weeks in MR appearance of sulci when compared with neuropathologic studies, with the greatest lag occurring between 20 and 32 weeks' gestation.5 The sections used in neuropathologic studies are significantly thinner than those in MR imaging (15–30 μm vs 3–5 mm), thus allowing detection of subtle surface indentations that may initially be overlooked on MR imaging.1,3,6 Autopsy specimens also allow true orthogonal sections to be obtained, which can be difficult to achieve with fetal movement during imaging acquisition, with oblique sections limiting accurate sulcal detection. Chi et al25 report left-right hemispheric asymmetry in formation of a number of the secondary sulci and an approximately 2 week sulcal delay in twin fetuses; these various factors confound the difficulty of establishing an accurate gestational age and also make formation of an accurate, reproducible, dating atlas extremely difficult.3–6,25 It is also possible that the fixation process involved in making histologic sections may make sulcation more prominent.
Table 2:
Sulcal presence according to GA, adapted from Garel1
| GA (Weeks) | Sulci Present | Additional Sulci Present |
|---|---|---|
| 22–23 | Interhemispheric fissure | |
| Lateral sulcus | ||
| Internal parieto-occipital fissure | ||
| Hippocampal fissure | ||
| Callosal sulcus | ||
| 24–25 | Calcarine fissure | |
| Cingular sulcus | ||
| 26 | Central sulcus | |
| Collateral sulcus | ||
| 27 | Marginal sulcus | |
| Precentral sulcus | ||
| Superior (posterior) temporal sulcus | ||
| 28 | Postcentral sulcus | |
| Intraparietal sulcus | ||
| 29 | Superior frontal sulcus | |
| Inferior frontal sulcus | ||
| 30 | None | |
| 31 | None | |
| 32 | Superior (anterior) temporal sulcus | |
| Inferior temporal sulcus | ||
| 33 | Occipitotemporal sulcus | |
| Secondary cingular sulcus | ||
| 34 | Insular sulci | |
| Secondary occipital sulci |
Note:—The presence of a given sulcus is defined by Garel as present in at least 75% of the population for a particular gestation.
The reference atlas utilized for this work was that of Garel1; in assessment of delayed sulcation, the author recommends comparative analysis be made with respect to sulcal presence rather than detectability alone.1,4 A temporal delay of ∼2 weeks exists between when a sulcus is detectable (seen in 25%–75% cases) and present (seen in >75% cases); definitive, accurate prediction of gestational age is accordingly necessarily limited.4–6 Our assessment of gestational age was based on sulcal presence. The sulcal depth and shape were not separately examined; however, with progressive cortical maturation the sulci deepen and demonstrate increased complexity. Dating correlation between neuropathology studies and MR imaging improves with increased actual gestation; specifically, the later the normal development of a sulcus the closer is the dating correlation. Optimal time for most precise correlation between MR sulcal pattern and actual gestational age is considered to occur between 28 and 34 weeks' gestation.1,4 At 34 weeks' gestation, most secondary and all primary sulci are present, and estimation of gestation subsequent to this gestation becomes increasingly difficult to delineate.1,4
When our data are displayed graphically (Fig 1) it is apparent that there is a relative paucity of fetuses between 27 and 29 weeks actual gestational age. This reflects the referral pathway whereby many of the earlier gestation fetuses are referred via screening in contrast to the later gestations, which are imaged for indications such as reduced fetal movement; there is resultantly a natural dearth of cases in the interim.
In our experience, a paucity of primary sulci in early gestation makes clear determination of delayed gestation difficult if not impossible (Fig 2). With increased gestation (ie, between 28 and 34 weeks) discrimination between normal and delayed fetal gestation was more apparent (Figs 3 and 4). Limited study numbers prohibit us to conclude whether a true delay in sulcation in association with fetal ACC is identified more frequently with increased gestation or whether this reflects that accurate gestational assessment is difficult and the most accurate assessment time period is in later gestation; primary and secondary sulci do eventually form, and thus any delay identified in utero does appear to correct during the postnatal period.18
Conclusions
Delayed sulcation is seen with high frequency in fetuses with ACC in the third trimester when compared with normal fetuses. The delay is not present on imaging in fetuses with ACC in the late second trimester. We think that the delayed sulcation is most likely to be secondary to the failed commisuration and does not necessarily imply the presence of additional brain malformations.
Abbreviations
- ACC
agenesis of the corpus callosum
- CNS
central nervous system
- GA
gestational age
- iuMR
in utero MR
- SSFSE
single shot fast spin-echo
References
- 1. Garel C. MRI of the Fetal Brain: Normal Development and Cerebral Pathologies. Berlin: Springer; 2004 [Google Scholar]
- 2. Lan LM, Yamashita Y, Tang Y, et al. Normal fetal brain development: MR imaging with a half-Fourier rapid acquisition with relaxation enhancement sequence. Radiology. 2000;215:205–10 [DOI] [PubMed] [Google Scholar]
- 3. Rich P, Jones R, Britton J, et al. MRI of the foetal brain. Clin Radiol 2007;62:303–13 [DOI] [PubMed] [Google Scholar]
- 4. Garel C, Chantrel E, Brisse H, et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol 2001;22:184–89 [PMC free article] [PubMed] [Google Scholar]
- 5. Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology 1999;210:751–58 [DOI] [PubMed] [Google Scholar]
- 6. Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol 2006;27:1604–11 [PMC free article] [PubMed] [Google Scholar]
- 7. Glenn OA, Goldstein RB, Li KC, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med 2005;24:791–804 [DOI] [PubMed] [Google Scholar]
- 8. Coakley FV, Glenn OA, Qayyum A, et al. Fetal MRI: a developing technique for the developing patient. AJR Am J Roentgenol 2004;182:243–52 [DOI] [PubMed] [Google Scholar]
- 9. Glenn OA, Barkovich J. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis: part 2. AJNR Am J Neuroradiol 2006;27:1807–14 [PMC free article] [PubMed] [Google Scholar]
- 10. Dubois J, Benders M, Cachia A, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 2008;18:1444–54 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong E, Schleicher A, Omran H, et al. The ontogeny of human gyrification. Cereb Cortex 1995;5:56–63 [DOI] [PubMed] [Google Scholar]
- 12. Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210:411–17 [DOI] [PubMed] [Google Scholar]
- 13. Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385:313–18 [DOI] [PubMed] [Google Scholar]
- 14. Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 1995;18:383–88 [DOI] [PubMed] [Google Scholar]
- 15. Rakic P. Neuroscience: genetic control of cortical convolutions. Science 2004;303:1983–84 [DOI] [PubMed] [Google Scholar]
- 16. Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain 2008;131:2028–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb Cortex 2005;15:1900–13 [DOI] [PubMed] [Google Scholar]
- 18. Tang PH, Bartha AI, Norton ME, et al. Agenesis of the corpus callosum: an MR imaging analysis of associated abnormalities in the fetus. AJNR Am J Neuroradiol 2009;30:257–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris JE, Rickard S, Paley MN, et al. The value of in-utero magnetic resonance imaging in ultrasound diagnosed foetal isolated cerebral ventriculomegaly. Clin Radiol 2007;62:140–44 [DOI] [PubMed] [Google Scholar]
- 20. Barkovich AJ, Kuzniecky RI, Dobyns WB, et al. A classification scheme for malformations of cortical development. Neuropediatrics 1996;27:59–63 [DOI] [PubMed] [Google Scholar]
- 21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 22. Moore DS, McCabe GP. Introduction to the Practice of Statistics, 3rd ed. New York: W.H. Freeman; 1999 [Google Scholar]
- 23. Hetts SW, Sherr EH, Chao S, et al. Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. AJR Am J Roentgenol 2006;187:1343–48 [DOI] [PubMed] [Google Scholar]
- 24. Larroche JC. Morphological criteria of central nervous system development in the human foetus. J Neuroradiol 1981;8:93–108 [PubMed] [Google Scholar]
- 25. Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol 1977;1:86–93 [DOI] [PubMed] [Google Scholar]