Abstract
BACKGROUND AND PURPOSE:
The natural course of symptomatic carotid artery occlusion with hemodynamic impairment is poor. Surgical revascularization may improve the outcome; however, its efficacy has not been established yet. The goal of this study was to characterize the technical and clinical outcomes following endovascular recanalization of the ICA under cerebral circulatory protection.
MATERIALS AND METHODS:
Endovascular recanalization was attempted in 8 patients with symptomatic ICA occlusions. The duration of the occlusion ranged from 7 days to 7 months (mean, 2.5 months), and the mean length of the occlusion was 95 mm. Cerebral hemodynamics ipsilateral to the side of the occlusion were severely impaired in all patients. The endovascular procedure was performed under total cerebral circulatory protection, beginning with proximal protection with a subsequent switch to distal protection after successful guidewire passage.
RESULTS:
The occlusion was recanalized successfully in 7 of 8 patients (88%), resulting in improvement of ipsilateral cerebral hemodynamics without symptomatic stroke. Small asymptomatic ischemic lesions were detected in 6 of 8 patients (75%) on DWI, and 1 patient developed a mild groin hematoma. Ischemic episodes did not recur during the mean follow-up period of 19 months. However, 1 patient experienced asymptomatic reocclusion, which was re-treated successfully without complications, while another patient developed mild retinal hemorrhage at 3 months after the procedure due to the combination of antiplatelet and anticoagulant therapy.
CONCLUSIONS:
Endovascular revascularization of an ICA occlusion is feasible and well-tolerated in patients with subacute or chronic total occlusion of the ICA.
There is considerable interpatient variation in the natural course of carotid artery occlusion. The risk of ipsilateral stroke for patients with medically treated carotid artery occlusion is <3% per year.1 However, this figure increases to 10%–20% per year when considering the population subgroup with severe impairments in cerebrovascular hemodynamics,2,3 which provides a rationale for the revascularization of an occluded ICA well beyond the acute stage.
During the past several decades, several surgical procedures have been performed for the management of an occluded ICA, including thromboendarterectomy and extracranial-intracranial bypass. Thromboendarterectomy achieved successful results in selected cases, but the success rate was insufficient for the management of occlusive lesions beyond the acute phase.4 Similarly, although extracranial-intracranial bypass has potential as a revascularization procedure, it failed to yield clinical benefit in 1 study,5 likely because of the significant risk related to surgery and the lack of adequate patient-selection criteria.
Occlusive vascular lesions can also be managed with less invasive endovascular procedures. Indeed, successful revascularizations for coronary6 and iliac arteries7 have been documented, and these techniques continue to improve through the evolution of better guidewires and stent technologies to solve the initial issues of poor success rate and late restenosis.
Several technical problems must be overcome when considering the use of endovascular procedures for the recanalization of occluded carotid arteries. These include issues related to protecting the cerebral circulation from distal embolism during the procedure and how to pass a guidewire across a long occlusive lesion (eg, an occlusion extending from the CCA to an intracranial segment). Thus, the goal of this study was to refine the conventional procedures for carotid artery stent placement to achieve the recanalization of extensive occlusions of the ICA well after the acute phase under the total protection of the cerebral circulation. We report herein on the initial clinical outcomes.
Materials and Methods
From August 2006 to December 2008, 8 patients underwent endovascular revascularization for complete occlusion of the ICA beyond the acute phase (≥7 days after ischemic events). All patients were men, with a mean age of 68 years (range, 50–78 years). Diagnosis of complete occlusion of the ICA was based on conventional angiography with sufficient contrast medium and a prolonged run. The occlusion was located on the right side in 6 cases and on the left side in 2 cases. The distance in a straight line between the proximal and distal stumps was measured on the lateral projection of a carotid angiogram obtained before the procedure and was defined as the length of the occlusion in this study. The mean length of the occlusion was 95 mm (range, 22.7–124 mm). In 7 patients, the ICA occlusion extended >80 mm from the cervical segment to the intracranial segment, above which the ICA was opacified in an antegrade fashion via tiny anastomotic branches from the ECA. In 1 patient, the occlusion length was short (22.7 mm) because the occipital artery had an anomalous connection with the ICA at the cervical level.
A history of the ischemic events was obtained and the evaluation of neurologic status was performed by the stroke neurologists and neurosurgeons in our institutions. All patients had recurrent ischemic episodes of the cerebral or retinal circulation after the initial diagnosis of ICA occlusion, despite medical therapy with antiplatelet agents. These patients also had cardiac disease or complications that prevented surgical revascularization under general anesthesia.
The date on which MR angiography, sonography, or conventional angiography established a diagnosis of ICA occlusion was considered the date of ICA occlusion for the purposes of this study. The mean duration of the occlusion was 2.5 months, ranging from 7 days to 7 months.
Iodine 123 N-isopropyl-p-iodoamphetamine or technetium Tc99m ethyl cysteinate dimmer SPECT with acetazolamide vasodilatory challenge (15 mg/kg) was performed before the interventions in all patients to evaluate CBF. Cerebral hemodynamics ipsilateral to the ICA occlusion were severely impaired in all cases. Three cases were established by qualitative analysis (ipsilateral decrease of CBF in the resting state and a further decrease after acetazolamide administration were visually inspected), and 5 cases were established by quantitative analysis (<80% resting CBF compared with the contralateral hemisphere and <10% increase after acetazolamide administration).
All patients underwent clinical follow-up every 1–3 months during a mean period of 19 months (range, 9–32 months). To detect ischemic lesions related to the procedure, whole-brain MR images with an echo-planar DWI sequence (section thickness, 5 mm; spacing, 1.7 mm; b-value, 1000 s/mm2) were obtained within 7 days after the procedures by using a 1.5T MR imaging scanner. All new bright spots on DWIs were summed as the number of the ischemic lesions related to the procedure. Conventional angiography, 3D-CTA, or carotid duplex sonography was performed to confirm patency of the recanalized lesion, and cerebral hemodynamics were re-evaluated by SPECT at least 6 months after the intervention.
Revascularization Procedure
A dual antiplatelet regimen with acetylsalicylic acid (100 mg) and clopidogrel (75 mg) or cilostazol (200 mg) was started at least 3 days before the procedure. All procedures were performed via the percutaneous transfemoral route under local anesthetic. After placement of sheath introducers, heparin was administered intravenously to keep the ACT >2.5 times the control ACT.
The procedure was started with proximal protection with flow reversal according to the method of Parodi et al (Fig 1).8 The CCA was occluded with an occlusion balloon on the tip of a 9F guiding catheter (Patlive; Terumo Clinical Supply, Gifu, Japan), and the ECA was occluded with a low-profile occlusion balloon (GuardWire; Medtronic, Minneapolis, Minnesota) that was passed through the 9F guiding catheter. The tail of the guiding catheter was connected to a filter device (Accessory Chamber Unit; Kawasumi Laboratories, Tokyo, Japan) through which the blood was returned into the venous circulation via a 4F sheath placed in the femoral vein.
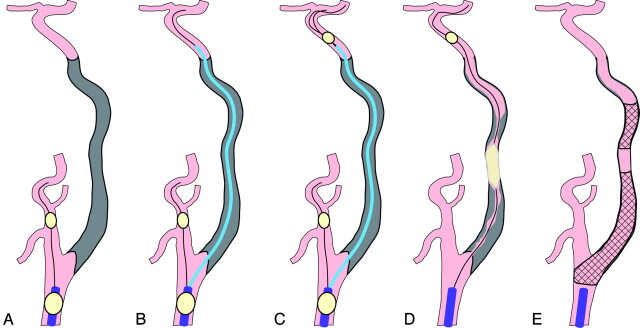
Fig 1.
Recanalization procedure schematic. A, The procedure is initiated with proximal protection with the occlusion balloon at the CCA and the ECA. B, The aspiration catheter is navigated along the guidewire, which is passed successfully across the occluded segment under proximal protection. C, The distal protection balloon is navigated beyond the occluded segment through the extraction port of the aspiration catheter. D, The occluded segment is dilated with the balloon under distal protection. E, The ICA is recanalized after the stents are deployed and the thrombi are aspirated.
A 0.035-inch standard guidewire with an angled tip (Radifocus Guidewire M; Terumo, Tokyo, Japan) was used to enter the occluded segment with the support of a 4F catheter (Tempo 4; Cordis, Miami Lakes, Florida). The tip of the guidewire was rotated in a deliberate fashion at the stump of the ICA. After the guidewire entered the occluded segment, the tip of the 4F catheter was wedged into the occluded segment, and a 0.014-inch guidewire (Runthrough NS; Terumo) in combination with a microcatheter (Echelon 10; ev3 Neurovascular, Irvine, California) was used to advance further into the occluded segment. IVUS (Eagle Eye Gold; Volcano, San Diego, California) was performed to confirm that the guidewire was in the true lumen before the guidewire entered the petrous segment of the ICA.
The IVUS probe was advanced smoothly into the occluded segment along the guidewire, and there were no specific problems using IVUS in our patients. If the guidewire was in the false lumen, the true lumen was probed with another guidewire by using the first guidewire as a landmark (parallel-wire technique6). After confirmation of the location of the guidewire within the true lumen in the cervical segment of the ICA, we advanced the guidewire and the microcatheter further into the petrous segment and navigated them beyond the occluded segment. After confirming blood return through the microcatheter, we performed a microcatheter run to confirm that the occluded segment was penetrated properly.
A low-profile aspiration catheter with a large extraction port (Rebirth 7F; Goodman, Nagoya, Japan) was guided beyond the occluded segment along the successfully penetrated guidewire. Another GuardWire was delivered beyond the occluded segment through the extraction port to achieve distal protection at the cavernous segment of the ICA. Then, proximal protection was released, and the GuardWire in the ECA was removed.
The occluded segment was dilated with a 3.0- to 4.0-mm balloon (Gateway; Boston Scientific, Natick, Massachusetts) according to measurements yielded on IVUS. Debris was aspirated with 60–120 mL of blood. The segments that should be treated with stents were determined according to the IVUS and the run from the aspiration catheter, and the segments with a large protruding plaque or a dissected flap in the cervical segments were treated with self-expanding stents (Wallstent RP, Boston Scientific; or Precise, Cordis). IVUS was performed again, and postdilation was performed in case of suboptimal expansion. When the dissections were observed in the intracranial segments, they were usually observed without stents because self-expandable stents for intracranial arteries are not available at present in our country. However, coronary stents (S-stent; Biosensors Interventional Technologies, Singapore) were deployed in case of impending occlusion. Debris was again aspirated. Finally, distal protection was terminated to allow blood to pass through the recanalized ICA.
After the procedure, oral anticoagulant was started to maintain the prothrombin time between 1.5 and 2.5 IU. Heparin was continued for at least 3 days until oral anticoagulation reached therapeutic levels. The anticoagulation regimen was stopped 3 months after the procedure. The dual antiplatelet regimen was transitioned to a single antiplatelet therapy at the 3-month time point.
Results
Initial and follow-up results are summarized in the Table.
Initial and follow-up results
| Case No. | Initial Results |
Follow-Up Results |
||||
|---|---|---|---|---|---|---|
| Recanalized | DW | Complication | Period (mo) | Ischemic Events and Complication | Patency | |
| 1 | Yes | 4 | None | 32 | None | Patent at 1 year (CA) |
| 2 | Yes | 11 | None | 26 | None | Patent at 1 year (CA) |
| 3 | Yes | 0 | None | 22 | None | Patent at 1 year (CTA) |
| 4 | Yes | 14 | None | 20 | Retinal hemorrhage | Patent at 1 year (CA) |
| 5 | Yes | 5 | None | 18 | None | Patent at 1 year (CA) |
| 6 | No | 2 | None | 14 | None | Not recanalized |
| 7 | Yes | 3 | Groin hematoma | 14 | None | Reoccluded 2 months after 1st procedure (CTA) |
| Patent 4 months after 2nd recanalization (CTA) | ||||||
| 8 | Yes | 0 | None | 9 | None | Patent at 1 month (duplex sonography) |
Initial Success Rate
Revascularization of the occluded ICA was successful in 7 patients (88%), with smooth flow through the entire ICA and residual stenosis <50% (Figs 2 and 3). The time required for the procedure ranged from 145 to 290 minutes (mean, 220 minutes), and the mean volume of contrast medium used per procedure ranged from 198 to 400 mL (mean, 245 mL). In 1 patient, endovascular recanalization was abandoned after 290 minutes because the true lumen could not be secured with the guidewire (case 6). Stent placement for the intracranial segment of ICA was required in 4 patients among 7 recanalized patients.
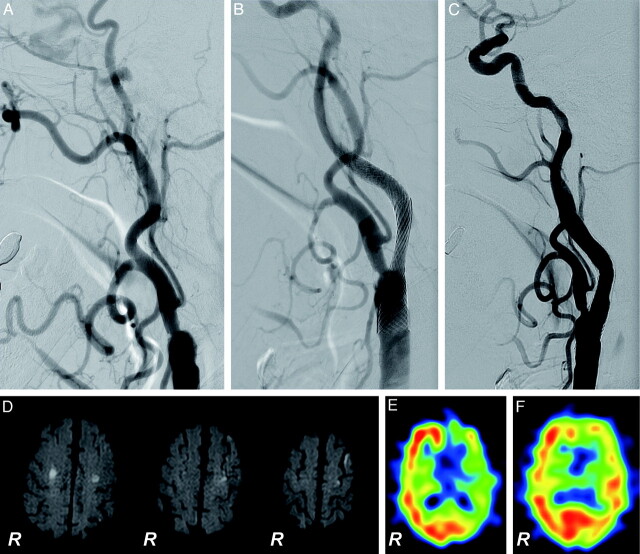
Fig 2.
Successful recanalization of a left chronic ICA occlusion (case 4). A 78-year-old man with recurrent episodes of transient right hemiparesis underwent recanalization at 6 months after the initial diagnosis of left ICA occlusion. A–C, Lateral projections of the left carotid angiogram. A, Before treatment, the occlusive lesion extends from the cervical segment to the petrous segment of the ICA. B, Just after the recanalization. C, One-year follow-up. D, DWI obtained 1 day after the procedure. Asymptomatic small ischemic lesions ≤9 mm are detected in the ipsilateral side of the recanalized ICA and in the contralateral side. E and F, SPECT images with acetazolamide vasodilatory challenge before and 13 months after the procedure, respectively. Severe impairment of the left cerebral hemispheric vascular reserve improved after successful recanalization of the ipsilateral ICA.
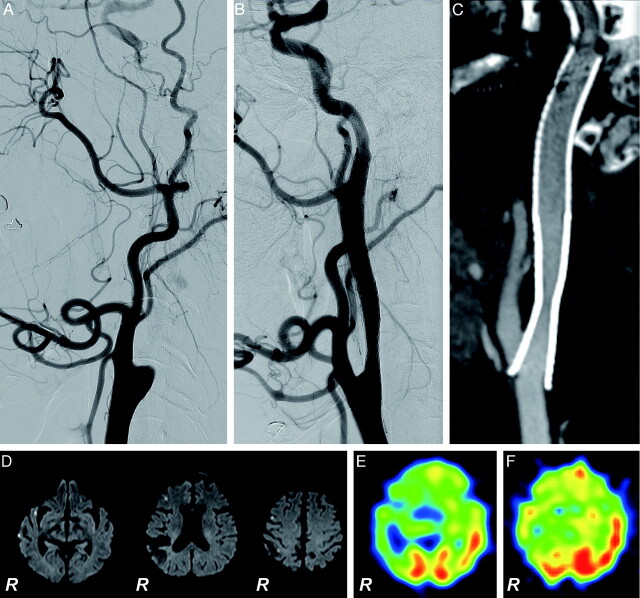
Fig 3.
Successful recanalization of right chronic ICA occlusion (case 5). A 69-year-old man with a minor completed stroke in the right parietal lobe underwent recanalization at 7 months after the initial diagnosis of right ICA occlusion. A–C, Lateral projections of the right carotid angiogram. A, Before treatment, the occlusive lesion extends from the cervical segment to the cavernous segment of the ICA. B, Just after the recanalization. C, Sagittal reconstruction of 3D-CTA obtained 4 months after the procedure shows no restenosis along the recanalized ICA. D, DWI obtained 1 day after the procedure. Small asymptomatic ischemic lesions are detected in the ipsilateral cerebral hemisphere. E and F, SPECT images with acetazolamide vasodilatory challenge before and 6 months after the procedure, respectively. Severe impairment of the vascular reserve of the right cerebral hemisphere improved after successful recanalization of the right ICA.
Procedure-Related Complications
There were no wire-induced vascular perforations and no vessel ruptures during the procedures.
New postprocedure neurologic deficits did not occur in any of the patients. However, asymptomatic ischemic lesions, as demonstrated on DWIs, attributed to the procedure, occurred in 6 of 8 patients (75%). The mean number of the bright spots on whole-brain DWI was 4.9 per patient (range, 0–14 per patient). Ischemic lesion size was <3 mm on DWIs in all cases except case 4, in which the size of the ischemic lesion reached 9 mm. Mild groin hematoma occurred in 1 patient during anticoagulation with intravenous heparin. This patient did not require a blood transfusion or surgical evacuation. Hyperperfusion syndrome was not observed in any patient.
An increase in serum creatinine levels of ≥0.5 mg/dL or ≥25% above the baseline was not observed in any patient after the procedure.
Follow-Up Results
Ischemic episodes within the cerebral and retinal circulation ipsilateral to the occluded ICA resolved and did not recur during the follow-up period in all 7 patients who underwent successful recanalization.
One patient (case 4) experienced mild retinal hemorrhage in the ipsilateral eye at 3 months after the recanalization; this resulted in a subtle peripheral visual field defect. This patient had diabetic mellitus and was receiving both anticoagulation therapy and dual antiplatelet therapy after the procedure, which likely contributed to this complication.
The recanalized ICAs were patent without restenosis in 5 patients on conventional angiography performed 1 year after the procedure. In 1 patient (case 7), CTA performed 2 months after the procedure showed reocclusion of the ICA, though the patient remained asymptomatic. This patient underwent repeat endovascular recanalization with successful results and no new ischemic lesions on DWIs. The recanalized ICA was patent on CTA obtained 4 months after the second procedure. In another patient (case 8), ICA patency was only confirmed by carotid duplex sonography at 1 month after the procedure.
Cerebral hemodynamics were evaluated with SPECT at least 6 months after the procedure in 4 of 7 recanalized patients. In these 4 patients, ipsilateral CBF was improved relative to preprocedural levels.
Discussion
Protected revascularization of a completely occluded ICA was successfully performed in 7 of 8 patients (88%) in the present study. This resulted in improved ipsilateral cerebral perfusion and resolution of ischemic symptoms in the retinal and cerebral circulation without recurrence during the 19-month follow-up period.
Endovascular recanalization of the occluded ICA is an accepted treatment technique for acute stroke,9 but it is rarely performed beyond the acute phase. However, since initial reports from Terada et al,10 in which a patient underwent successful endovascular recanalization of a chronic ICA occlusion with proximal protection, several other successful reports have followed11–15; for example, Kao et al13 were successful in recanalizing 22 short-length (mean, 27.9 mm) chronic ICA occlusions among 30 cases (73%).
Because extensive ICA occlusion from the cervical to the intracranial segment is associated with the presence of thrombi within the lumen, cerebral protection is indicated during any recanalization procedure. Although proximal protection alone can reduce the risk of thromboembolic complications,10,15 insufficient reverse flow through long lesions can mitigate this effect by preventing elimination of debris. Thus, the present study used distal protection in combination with proximal protection to minimize the risk of thromboembolic complications. To achieve distal protection successfully, we adopted a low-profile occlusion balloon (GuardWire) rather than the filters. This occlusion balloon has a shorter length than the filter (10 mm versus 20–30 mm), which allowed placement of the protection device in the curvature of the cavernous segment of the ICA. Furthermore, its lower profile allowed delivery through the extraction port of the aspiration catheter rather than through the potentially dissected lumen after dilation, and this resulted in a higher success rate (100%) to achieve distal protection than that in the previous report,16 in which distal protection was achieved in 73%. Distal protection has an additional advantage in that the dilated lumen with potential dissections can be opacified by injecting contrast media through the aspiration catheter to determine the segments to be treated with stents.
Symptomatic ischemic events did not occur in our patients and in the cases described in the previous case reports10,11,14,15; however, 2 symptomatic ischemic events in 54 procedures (4%) were documented by Lin et al.16 There are no available data as to the asymptomatic ischemic lesion related to endovascular recanalization of the occluded ICA beyond the acute phase. In our study, 75% of patients did develop small asymptomatic ischemic lesions (4.9 per patient), as demonstrated by DWI. This rate is higher than that observed during standard carotid artery stent placement procedures (25%–55% of patients).17,18
Nonischemic adverse events can also develop after the procedure. Komiyama et al15 reported a mild blue toe syndrome that developed 1 month after the endovascular recanalization of the occluded ICA, and Lin et al16 described 1 pseudoaneurysm, 1 iatrogenic carotid cavernous fistula, and 1 cervical extravasation after their 54 endovascular procedures for chronic total occlusion of ICA. In the present study, minor complications (groin hematoma and retinal hemorrhage) occurred in 2 of 8 patients. Although contrast-induced nephropathy did not occur in our patients, attention should be paid to the renal function because the complex procedure demands a considerable amount of contrast medium and the candidates for this procedure are usually aged and have multiple medical risks.
Despite the potential risk of the procedure, the natural course of symptomatic ICA occlusion with hemodynamic impairment is considered poor, with an ipsilateral subsequent stroke rate of 10%–20% per year.2,3 Furthermore, cerebral metabolic disorders associated with carotid artery occlusion result in cognitive deterioration.19 Thus, the complication rate in the present study may be comparatively favorable.
The recanalization procedure used for the management of ICA occlusion in the present study is associated with some technical challenges. First, success of the procedure is dependent on passing the guidewire through the occluded segment, which is arguably the most difficult step of the procedure.6,7 The success rate may be optimized by careful selection of patients in whom more facile passage of the guidewire is anticipated. Indeed, a tapering appearance of the stump and antegrade flow within the cavernous segment of the ICA due to the anastomosis from the ECA may be indications of successful revascularization.20 Alternatively, additional imaging devices, such as IVUS, could be used to visualize the occluded lumen to capture the true lumen by using the parallel-wire technique.6 However, this technique cannot be used in the intracranial segment of the ICA due to the tortuosity of the vessel and the stiffness of the device. As described in the context of the coronary and iliac lesions,6,7 occlusive lesions have a higher propensity for restenosis and reocclusion compared with stenotic lesions. As to the carotid lesions, 3 cases of restenosis and reocclusion are documented among the 35 recanalized ICAs (8.6%).16 In the present study, there were no cases of restenosis; however, 1 of 7 recanalized patients experienced reocclusion. The use of drug-eluting stents may help mitigate this problem in the future.
This study has several limitations. Our number of cases is small, the technical success rate might be overestimated, and the potential catastrophic adverse events might be underestimated. In addition, the protective effects of the procedure against ischemic events are not proved by our study on the basis of a small number of subjects. Also, the procedure described here is considerably complex compared with the standard procedure used for carotid artery stenosis. Our procedure demands dexterous guidewire manipulations and multiple expensive devices such as IVUS and occlusion balloons, which might limit the generalizability of our method.
Conclusions
Protected endovascular revascularization of an ICA occlusion is feasible and well-tolerated in patients with subacute or chronic total occlusion of the ICA. Further studies are required to elucidate fully the risks and the benefits of this procedure.
Abbreviations
- ACT
activated clotting time
- CA
catheter angiography
- CBF
cerebral blood flow
- CCA
common carotid artery
- CTA
CT angiography
- DW
the number of ischemic lesions on the whole-brain DWI
- DWI
diffusion-weighted imaging
- ECA
external carotid artery
- ICA
internal carotid artery
- IVUS
intravascular sonography
- R
right
- SPECT
single-photon emission tomography
References
- 1. Klijn CJ, Kappelle LJ, Tulleken CA, et al. Symptomatic carotid artery occlusion: a reappraisal of hemodynamic factors. Stroke 1997;28:2084–93 [DOI] [PubMed] [Google Scholar]
- 2. Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–60 [DOI] [PubMed] [Google Scholar]
- 3. Kuroda S, Houkin K, Kamiyama H, et al. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001;32:2110–16 [DOI] [PubMed] [Google Scholar]
- 4. Hafner CD, Tew JM. Surgical management of the totally occluded internal carotid artery: a ten-year study. Surgery 1981;89:710–17 [PubMed] [Google Scholar]
- 5. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial—the EC/IC Bypass Study Group. N Engl J Med 1985;313:1191–200 [DOI] [PubMed] [Google Scholar]
- 6. Stone GW, Colombo A, Teirstein PS, et al. Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and results. Catheter Cardiovasc Interv 2005;66:217–36 [DOI] [PubMed] [Google Scholar]
- 7. Scheinert D, Schroder M, Ludwig J, et al. Stent-supported recanalization of chronic iliac artery occlusions. Am J Med 2001;110:708–15 [DOI] [PubMed] [Google Scholar]
- 8. Parodi JC, Ferreira LM, Sicard G, et al. Cerebral protection during carotid stenting using flow reversal. J Vasc Surg 2005;41:416–22 [DOI] [PubMed] [Google Scholar]
- 9. Jovin TG, Gupta R, Uchino K, et al. Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke 2005;36:2426–30 [DOI] [PubMed] [Google Scholar]
- 10. Terada T, Yamaga H, Tsumoto T, et al. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage: case report. J Neurosurg 2005;102:558–64 [DOI] [PubMed] [Google Scholar]
- 11. Thomas AJ, Gupta R, Tayal AH, et al. Stenting and angioplasty of the symptomatic chronically occluded carotid artery. AJNR Am J Neuroradiol 2007;28:168–71 [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatt A, Majid A, Kassab M, et al. Chronic total symptomatic carotid artery occlusion treated successfully with stenting and angioplasty. J Neuroimaging 2009;19:68–71 [DOI] [PubMed] [Google Scholar]
- 13. Kao HL, Lin MS, Wang CS, et al. Feasibility of endovascular recanalization for symptomatic cervical internal carotid artery occlusion. J Am Coll Cardiol 2007;49:765–71 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi N, Miyachi S, Hattori K, et al. Carotid angioplasty with stenting for chronic internal carotid artery occlusion: technical note. Neuroradiology 2006;48:847–51 [DOI] [PubMed] [Google Scholar]
- 15. Komiyama M, Yoshimura M, Honnda Y, et al. Percutaneous angioplasty of a chronic total occlusion of the intracranial internal carotid artery: case report. Surg Neurol 2006;66:513–18, discussion 18 [DOI] [PubMed] [Google Scholar]
- 16. Lin MS, Lin LC, Li HY, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Intervent 2008;1:119–25 [DOI] [PubMed] [Google Scholar]
- 17. Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion-weighted magnetic resonance imaging in carotid angioplasty and stenting with balloon embolic protection devices. Neuroradiology 2006;48:100–12 [DOI] [PubMed] [Google Scholar]
- 18. Schluter M, Tubler T, Steffens JC, et al. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol 2003;42:1007–13 [DOI] [PubMed] [Google Scholar]
- 19. Bakker FC, Klijn CJ, Jennekens-Schinkel A, et al. Cognitive impairment is related to cerebral lactate in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. Stroke 2003;34:1419–24 [DOI] [PubMed] [Google Scholar]
- 20. Ahn HS, Rosenbaum AE, Allen GS, et al. Occluded but nonthrombosed internal carotid artery: an indication for endarterectomy. AJNR Am J Neuroradiol 1983;4:286–88 [PMC free article] [PubMed] [Google Scholar]