Abstract
BACKGROUND AND PURPOSE:
EPO has been shown to have beneficial effects in a variety of CNS injury models. The purpose of this study was to evaluate the effects of EPO on nerve regeneration and functional recovery in a rat model of peripheral nerve surgery.
MATERIALS AND METHODS:
The sciatic nerve of the rat with a 10-mm defect was bridged with a silicone rubber tube. Forty adult male Sprague-Dawley rats were assigned to the control or experimental groups to receive an intraperitoneal injection of NGF (2000 U/kg daily for 2 weeks) or EPO (5000 U/kg daily for 2 weeks), respectively. Macroscopic, functional, electrophysiologic, ultraminiature, and histologic assessments of nerves were performed 4–8 weeks after surgery.
RESULTS:
The results showed that in EPO-treated rats, there was a significant increase in the axon diameter, myelin thickness, and total number of nerve fibers as well as the degree of maturity of regenerated myelinated nerve fibers in comparison with those rats not treated with EPO. In addition, as measured by the SFI and MNCV, the motor function of the re-innervated hind limbs of rats with EPO treatment significantly improved at week 8, whereas there was no significant difference in the motor function between the 2 groups at 4 weeks.
CONCLUSIONS:
Our results demonstrated that EPO is able to enhance nerve regeneration and promote functional recovery after peripheral nerve injury in the rat, suggesting the potential clinical application of EPO for the treatment of peripheral nerve injury in humans.
Peripheral nerves are often damaged by crushing, compression, stretching, avulsion, or division, and despite the application of modern and sophisticated techniques of treatment and reconstruction, morphologic and functional regeneration is seldom complete1,2 due to the influence of factors like the nature and the level of the damage itself, the period of denervation, the type and diameter of the damaged nerve fibers, age, and other individual variables.3 Poor management of peripheral nerve injuries is associated with major functional deficits and can lead to painful neuroma when severed axons are unable to re-establish continuity with the distal nerve. Although peripheral nerves have the potential to regenerate after injury, this ability is strictly dependent on the regenerating axonal sprouts making appropriate contact with Schwann cell basal laminae in the distal nerve segment. After injuries to large nerve trunks, motoneurons are required to regenerate over a long distance, which they do at a very slow rate.4 Regenerating axons that fail to traverse the injury site and enter the basal lamina will degenerate, resulting in a progressive apoptosis of neurons and muscle atrophy.5 In addition, a slow rate of axonal regeneration constitutes a major deterrent to functional recovery after peripheral nerve injury.
EPO, a renal cytokine regulating hematopoiesis, is also produced by different cell types within the CNS, where it acts via the activation of EPOR. EPO activities are mediated by EPOR, expressed by neurons in both the central and peripheral nervous system.6–9 Central and peripheral glial cells also express EPOR.10,11 Brines et al showed that EPO exerts neurotrophic and neuroprotective activities in different in vivo and in vitro models of brain damage.12 These authors also demonstrated that the neuroprotective effects of EPO are associated with antiapoptotic activity of the cytokine on neuronal cells.13 Several independent research groups have reported that EPO protects cultured neurons against glutamate toxicity14,15 and reduces ischemic neuronal damage and neurologic dysfunction in rodent models of stroke.12,14,16,17 Moreover, the administration of EPO has a significant effect on axonal regrowth of peripheral nerve fibers, both after transection6,18 and in animals with established diabetic neuropathy.19 Recently, EPO has been demonstrated to reduce or prevent secondary inflammation and lipid peroxidation during oxidative stress,20 cerebral ischemia,21 and trauma.22 Recent work also has shown that EPO can stimulate postnatal neovascularization by increasing endothelial progenitor cell mobilization from the bone marrow.23 The presence and function of EPO/EPOR have not been established in the peripheral nervous system, but it is reasonable to expect that EPO/EPOR may provide trophic and/or neuroprotective support for neurons and Schwann cells.
Despite the beneficial effects of EPO in neuronal injury in a variety of CNS injury models, to our knowledge, no systematic data are available showing the beneficial effects of EPO in peripheral nerve injury. We report here that EPO improves regeneration and functional recovery of sciatic nerves after experimental surgery in rats.
Materials and Methods
All procedures were performed at the Experimental Animals Breeding and Research Center of Anhui Medical University. Animal care was conducted with the prior approval of the Animal Experimental Ethics Committee of this institution. The functional outcomes and electrophysiologic and histopathologic studies were performed by observers who were blinded to the experimental intervention that the rats had received.
Surgical Procedures
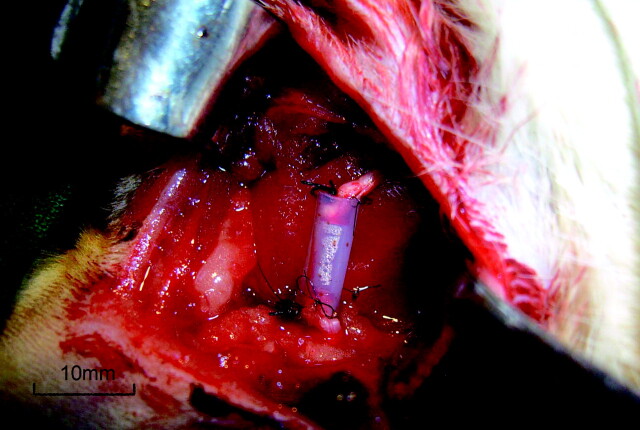
Forty adult male Sprague-Dawley rats weighing 200–230 g were used in this study. All animals were housed in plastic cages and allowed free access to laboratory feed and tap water. Anesthesia was induced with an intraperitoneal injection of thiopental sodium, 40 mg/kg. After hair shaving and local skin disinfection with povidone-iodine solution, the right sciatic nerve of each rat was exposed through a gluteal muscle−splitting incision. The sciatic nerve was transected in each rat by the excision of a 7-mm segment just proximal to the trifurcation of the nerve. A 10-mm silicone rubber tube (Phycon, outer diameter, 2.0 mm; inner diameter, 1.5 mm; Fuji Systems, Tokyo, Japan) was attached, by using 11–0 nylon interrupted microepineurial sutures, to the proximal and distal stumps, resulting in a 7-mm gap (Fig 1). Forty rats were randomly assigned to either the EPO (Kirin Brewery, Tokyo, Japan) group (n = 20) or the NGF (Genentech, South San Francisco, California) group (n = 20), and injected intraperitoneally with EPO and NGF, respectively for 2 weeks. The muscle was closed with 4–0 sutures, and the skin was sealed with wound clips. Animals were returned to standard housing.
Fig 1.
Photograph shows tubulation with a 7-mm-long interstump gap. The proximal and distal nerve stumps were joined to a 10-mm-long silicone rubber tube, leaving a 7-mm interstump gap. The ruler is graduated in millimeters.
Macroscopic Evaluation
A gross macroscopic assessment of nerve formation was made in all rats 4 and 8 weeks after surgery. At the end of 4 weeks, the rats were re-anesthetized and the surgical area was examined step by step by microdissection. Sciatic nerves were exposed and examined to determine the extent of fibrous tissue surrounding the silicone rubber tube. Skin closures, muscle fascia closure, nerve adherence to the surrounding muscle cavity tissue, and separability of nerves were assessed by the numeric grading scheme according to Petersen et al.24
Functional Evaluation
All animals were evaluated by a walking track test as described previously.25 Briefly, the animals were allowed to walk down a corridor, leaving blue footprints on the floor paper impregnated with a 0.5% solution of the anhydrous form of bromophenol blue in absolute acetone. The distance between the first and fifth toes (TS), the distance between the second and fourth toes (IT), and the print length (PL) were measured. The SFI was calculated according to the equation:
 |
In the formula, E refers to the experimental hind limb and N refers to the opposite normal hind limb. A total of 0 indicates normal nerve function and −100 represents complete dysfunction.
Electrophysiologic Evaluation
Functional outcome of nerve regeneration was measured electrophysiologically on the right sciatic nerve at 4 and 8 weeks after surgery by using the MEB-7102 instrument (Nihon Koden, Osaka, Japan). The rats were anesthetized, and the body temperature was kept constant at 37°C. They were fixed with tape on a smooth table to prevent movement artifacts due to the electric stimulation, and the lower limbs were gently stretched to make it easier to measure distances between distal and proximal points of stimulation. Conduction velocity was determined by measuring the latency of the response to electric stimulation of the receptive field at 5.0 mA, 0.1 ms, by using bipolar stainless steel electrodes (each 0.8 mm in diameter; interelectrode distance, 3 mm); this high-intensity stimulus was chosen in an attempt to avoid long-latency-coupled responses.26 The right sciatic nerve was stimulated at the sciatic notch, and the tibial nerve, at the ankle by bipolar electrodes at supramaximal stimuli. Distal and proximal latencies were measured from oscilloscope recordings. MNCV was calculated by dividing the distance between the stimulating and recording electrodes (measured with a fine caliper) by this latency.
Histomorphometry and Electron Microscopy
Twenty sciatic nerves per group were prepared for histologic analysis of peripheral nerve regeneration at 4 and 8 weeks after surgery. The regenerated sciatic nerve in the silicone rubber tube was removed entirely and fixed by immersion in 10% neutral formalin. After fixation, each specimen was embedded in paraffin. Five-micrometer-thick cross-sections of regenerated nerve were obtained and stained with hematoxylin-eosin and with the modified Bielschowsky silver method. Nerve regeneration was examined with the aid of light microscopy. Myelinated axon counts were obtained at ×400 magnification by using the following sampling technique: Of 100 squares with a surface area of 0.01 mm2 each, 20 were randomly selected in an ocular grid and used to count myelinated axons. These counts were then normalized regarding surface area. Seventy-nanometer-thick cross-sections of nerve originally located 10 mm distal to the silicone rubber tube were obtained. For electron microscopy, ultrathin sections were cut by using a LKB III ultramicrotome (Diversified Equipment Co., Lorton, Virginia) and stained with uranyl acetate-lead acetate. Myelin thickness and axon diameter were measured by using an electron microscope (JEM-1230, JEOL, Tokyo, Japan).
Immunohistochemistry and Assessment of Immunostaining
The sections from the nerve segment in the silicone rubber tube were washed thoroughly twice with PBS (pH 7.4), then fixed for 30 minutes in 4% buffered paraformaldehyde and incubated at room temperature with antibody diluted in PBS with 0.3% Triton X-100% and 1% normal goat serum for 3 hours (polyclonal rabbit anti-PGP 9.5, 1:100; Abzoom, Wuppertal, Germany). Following incubation with primary antibody, the sections were washed in PBS (3 × 5 minutes) and incubated for 3 hours with secondary antibody at a 1:100 dilution (goat-IgG anti-rabbit-IgG, SP-9001; Zymed, San Francisco, California). The specimens were examined by using light microscopy (Axiophot, Zeiss, Jena, Germany). The specificity of the immunoreactions was controlled by the application of the buffer instead of the primary antiserum. All control sections were free of immunostaining.
Statistical Analysis
All results are reported as mean ± SD. Statistical comparisons among groups involved the use of the Student t test with the software SPSS 13.0 (SPSS, Chicago, Ill). Statistical significance was ascribed to the data when P < .05.
Results
Macroscopic Evaluation
Skin sutures were removed and sciatic nerves were exposed through the original incision. There was no sign of infection or inflammatory reaction. Nerves treated with NGF exhibited an attenuated collagenous scar formation surrounding the regenerated sciatic nerve, whereas nerves treated with EPO were surrounded by only a very thin lucent membrane.
Functional Evaluation
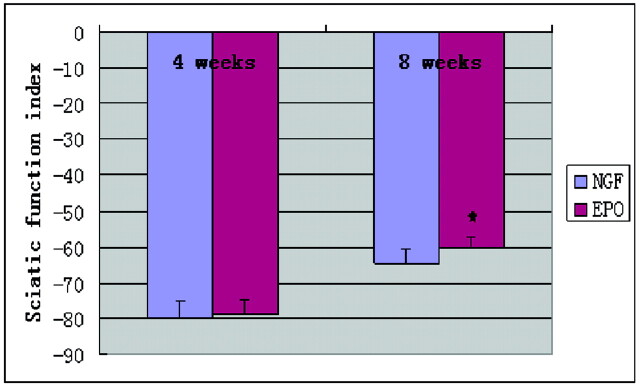
Preoperatively, the SFI in rats in both groups was approximately zero, indicating normal function. After injury, the SFI decreased to approximately −100, indicating complete loss of function. The SFI values as an indicator of functional recovery are listed in Table 1. No statistical difference was found between the NGF group and EPO group in the initial recordings at 4 weeks after the operation; the values were similar in the 2 groups. At the eighth week, the values were significantly better than those at the fourth week for both groups; the SFI value in the EPO group was significantly greater than that in the NGF group (Fig 2).
Table 1:
Results of functional and electrophysiologic evaluation (mean ± SEM)
| 4 Weeks | 8 Weeks | |
|---|---|---|
| SFIa | ||
| NGF | −79.98 ± 4.58 | −64.65 ± 4.11 |
| EPO | −78.85 ± 3.87, P > .05 | −60.26 ± 2.91, P < .05 |
| MNCV (m/s)b | ||
| NGF | 9.20 ± 1.07 | 16.37 ± 3.40 |
| EPO | 10.60 ± 1.36, P < .05 | 20.56 ± 4.18, P < .05 |
The SFI studies at 8 weeks postsurgery demonstrated better improvement in nerve function in rats that received EPO.
Rats treated with EPO showed better results than those of the NGF group at the 2 time points.
Fig 2.
Comparison of the mean functional recovery of each group after sciatic nerve transection and repair. Functional analysis of neural regeneration was assessed at 4 and 8 weeks after surgery by using walking-track analysis. Measurements made from walking-track prints were then submitted to an SFI. Data are the mean ± SEM. The asterisk indicates P < .05 compared with the NGF group significance.
Electrophysiologic Evaluation
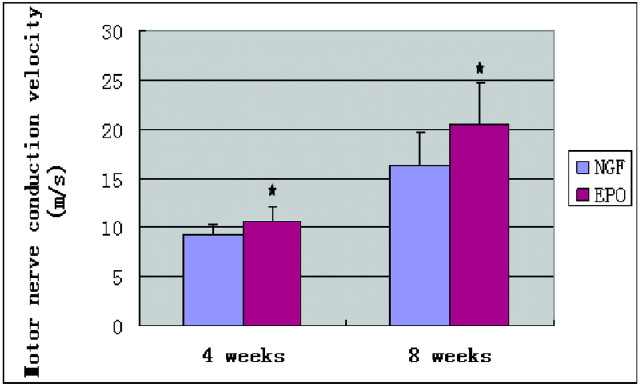
Electrophysiologic measurements reflect the functional behavior of the regenerated nerve, with the MNCV being one of the most relevant of the these measurements studied for regenerative capacity. MNCV was measured to confirm the recovery-promoting effect of EPO. The results of MNCV measurement at 4 and 8 weeks after the operation are listed in Table 1. MNCV was 9.20 ± 1.07 m/s for the NGF group and 10.60 ± 1.36 m/s for the EPO group at 4 weeks. Differences in MNCV between the 2 groups were statistically significant (P < .05). At week 8, the MNCV was also significantly lower in the NGF group (16.37 ± 3.40 m/s) than in the EPO group (20.56 ± 4.18 m/s) (Fig 3). EPO treatment significantly inhibited a MNCV decrease.
Fig 3.
Effect of EPO administration on MNCV. At weeks 4 and 8 after surgery, MNCV was significantly lower in the NGF group than in the EPO group. There are statistically significant differences in MNCV between the 2 groups. Data are mean ± SEM. The asterisk indicates P < .05 compared with the NGF group.
Histomorphometry and Electron Microscopy
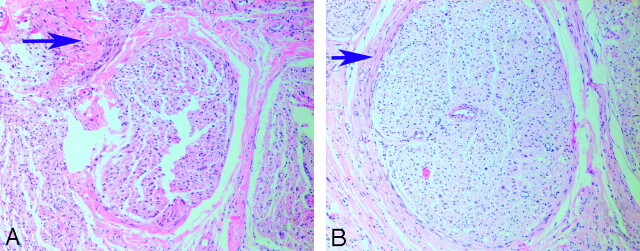
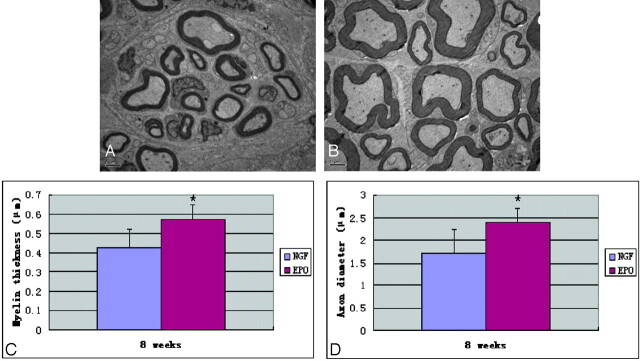
Nerves treated with NGF demonstrated a thick band of scar tissue surrounding nerve fiber bundles. In contrast, nerves treated with EPO were surrounded by thin bands of scar tissue (Fig 4). Significant statistical difference was found between the 2 groups with respect to the myelinated fiber counts (Table 2 and Fig 5). In addition, electron micrographs demonstrated multiple large robust myelinated fibers in the EPO group. In contrast, nerves treated with NGF had fewer myelinated fibers, and nerves treated with EPO had significantly higher myelin thickness and axon diameter than nerves treated with NGF (Table 2). Analysis of the distal sciatic nerve tissue harvested from each group revealed significant differences, summarized in Fig 6. The most remarkable differences were seen in the total number of myelinated fibers, myelin thickness, and axon diameter, which all showed much greater regeneration in the EPO-treated group.
Fig 4.
The arrows demonstrate the thickness of scar tissue of nerve fiber bundles 8 weeks after surgery. A, A rat treated with NGF shows a very thick band of scar tissue surrounding fiber bundles. B, A rat treated with EPO shows very thin bands of scar tissue surrounding fiber bundles (hematoxylin-eosin stain, original magnification ×100).
Table 2:
Results of histomorphometry and electron microscopy and immunohistochemistry (mean ± SEM)
| 4 Weeks | 8 Weeks | |
|---|---|---|
| Myelin thickness (μm)a | ||
| NGF | 0.43 ± 0.09 | |
| EPO | 0.57 ± 0.08, P < .05 | |
| Axon diameter (μm)a | ||
| NGF | 1.73 ± 0.51 | |
| EPO | 2.39 ± 0.32, P < .05 | |
| Myelinated axon counts (at original magnification ×400)b | ||
| NGF | 17.56 ± 4.19 | 49.30 ± 9.56 |
| EPO | 22.90 ± 5.24, P < .05 | 58.00 ± 7.73, P < .05 |
| MOD (at original magnification ×400)b | ||
| NGF | 0.2173 ± 0.0257 | 0.3089 ± 0.0343 |
| EPO | 0.2419 ± 0.0204, P < .05 | 0.3611 ± 0.0547, P < .05 |
| IOD (at original magnification ×400)b | ||
| NGF | 15.8494 ± 3.0825 | 16.6742 ± 1.7156 |
| EPO | 19.4382 ± 4.1861, P < .05 | 22.4111 ± 4.3418, P < .01 |
At 8 weeks after surgery, nerves treated with EPO had significantly larger myelin thickness and axon diameter compared with those in the NGF group, and there were statistically significant differences between the 2 groups.
In addition, there were also statistically significant differences in MOD and IOD as well as myelinated axon counts between the 2 groups at the 2 time points.
Fig 5.
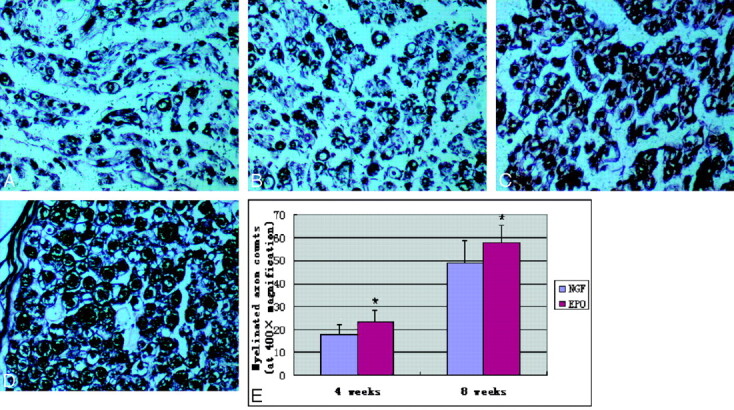
Representative photomicrographs of regenerated sciatic nerve cross-sections. The modified Bielschowsky silver stain was used to visualize myelinated axons. A and B, Regeneration of myelinated axons at 4 weeks. C and D, Regeneration at 8 weeks. A−D, These photomicrographs demonstrated significantly better regeneration in the EPO group (B and D) compared with the NGF group (A and C). E, Quantitation of myelinated axon counts in regenerated sciatic nerve cross-sections is shown. There are statistically significant differences in myelinated axon counts between the 2 groups at 4 and 8 weeks after surgery. Data are the mean ± SEM. The asterisk indicates P < .05 compared with the NGF group significance (uranyl acetate-lead stain, original magnification ×400).
Fig 6.
A and B, Representative ultrastructural imaging of cross-sections of nerve originally located 10-mm distal to the silicone rubber tube in the NGF and EPO groups respectively at week 8 after surgery. A, The NGF group shows lower myelin thickness and axonal diameter and fewer myelinated fibers. B, The EPO group shows higher myelin thickness and axonal diameter and more myelinated fibers. C and D, There are statistically significant differences in myelin thickness (C) and axonal diameter (D) between the 2 groups at 8 weeks after surgery. Data are the mean ± SEM. The asterisk indicates P < .05 compared with the NGF group significance.
Expression of PGP 9.5
In all cases of immunopositive nerve fibers, the immunohistochemistry reaction was localized within the axoplasm. Quantitative analysis by using MOD and IOD revealed that the intensity of PGP 9.5 immunoreactivity in the sciatic nerve of the operated side was significantly increased in both groups at 4 and 8 weeks following sciatic nerve axotomy. The optical attenuation of PGP 9.5-immunoreactive nerve fibers as evaluated by image analysis is presented in detail in Table 2. The expression of PGP 9.5 was significantly higher in the EPO group at 4 and 8 weeks, with the value being significantly higher than that in the NGF group. There were statistically significant differences in MOD and IOD between the 2 groups. The results of this analysis are shown in Fig 7. EPO treatment significantly enhanced the expression of PGP 9.5 in the regenerated sciatic nerve.
Fig 7.
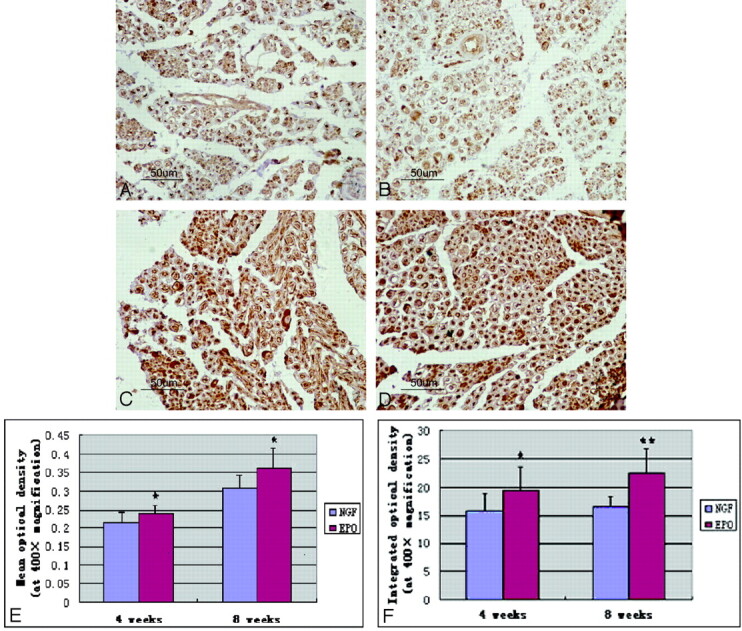
Representative photomicrographs of immunohistochemical staining of PGP 9.5 in regenerated sciatic nerve cross-sections. Axons and the periphery of the axons show strong immunoreactivity. A and B, Expression of PGP 9.5 in immunopositive nerve fibers at 4 weeks. C and D, Expression of PGP 9.5 at week 8. A−F, Quantitative analysis by using MOD and IOD reveals that nerves treated with EPO (B and D) have significantly higher MOD (E) and IOD (F) compared with those in the NGF group (A and C), and there are statistically significant differences between the 2 groups at the 2 time points. Data are the mean ± SEM. The asterisk indicates P < .05. Double asterisks indicate P < .01 compared with the NGF group significance.
Discussion
Retrograde regeneration has been estimated to increase the proximal fiber count by 40%–50% in the first several months after nerve repair.27,28 Axonal sprouts that grow in a retrograde direction originate from the proximal stump near the suture line and can extend considerable distances along the parent nerve and may even backtrack to proximal branches before innervating novel targets.29,30 In the last few years, evidence has accumulated showing that EPO exerts neurotrophic and neuroprotective effects in various in vivo and in vitro models of brain injury12 and has a significant effect on axonal regrowth of peripheral nerve fibers.6,18,19 In fact, an early study that indicated a role for EPO in the nervous system showed that the administration of EPO had a neurotrophic effect on transected nerve tracts and on the growth of neuronal processes in culture.31 Our research was performed to examine the outcome of EPO treatment on axonal growth and recovery of function. The results of this study demonstrate that EPO provides neuroprotective and neurotrophic actions in a rat model of peripheral nerve injury. Our findings show that EPO promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats compared with controls and those treated with NGF.
For effectiveness of EPO treatment, a functional evaluation was preferred. The SFI gives general information about nerve regeneration and functional recovery because it represents the outcome of the repair process. It has been proved to be reliable, repeatable, economical, and highly useful for determining motor function following compression and stretch injury,32 nerve graft or conduit,33 and surgical repair.34 The degree of recovery of sciatic nerve function was assessed by measuring the SFI, which provided a noninvasive and easily quantifiable method in the rat model.35 Because SFI compared the experimental and the normal prints, each animal acted as its own control. Our data showed that at 4 weeks after surgery, the function was still poor in both groups. At the eighth week, an increasing ability to walk became evident. A better and faster functional recovery was shown in the EPO group. In contrast, the functional recovery progressed more slowly in the NGF group. As a result, although the SFI was no better than that in the NGF-treated group at 4 weeks, functional outcome was better in the EPO-treated group at week 8, with statistically significant differences between the 2 groups (Fig 2 and Table 1). The recovery of the SFI for the EPO group was superior to that in the NGF group, indicating that EPO has the effect of promoting functional recovery after transection of sciatic nerve fibers, though there was no significant difference in the SFI between the EPO group and the NGF group at 4 weeks after surgery.
In addition, to further understand the specific process during functional recovery, MNCV will be considered in our next step because there is significant correlation between electrophysiologic and morphologic parameters (ie, conduction velocity and axon diameters/myelination).36 In this study, the MNCV of the regenerating nerves was faster in the EPO group than in the NGF group at weeks 4 and 8 (Table 1). There were statistically significant differences between the 2 groups in MNCV after axotomy, and the MNCV values were consistent with previously reported values. This outcome suggests that the recordings were reliable and reproducible (Fig 3). Our findings indicate that administration of EPO significantly inhibits the decrease of MNCV and promotes functional recovery following sciatic nerve transection in adult rats. Electrophysiologic studies revealed higher MNCV values in rats treated with EPO, reflecting a higher number of normally functioning axons. Taken together, these data indicate that administration of EPO promotes functional recovery and enhances nerve regeneration after sciatic nerve transection in the rat. In the present study, we observed that the improvement in functional recovery was accompanied by significant increases in myelin thickness and axon diameter as well as myelinated fiber counts (Figs 5 and 6 and Table 2). The observed improvement in the axon regeneration is in agreement with the results of the previous experimental studies and shows that EPO reduces neurologic deficits in a variety of peripheral nerve injury models.37
To quantify axonal outgrowth from peripheral nerve-dorsal root ganglions, we labeled preparations by using panaxonal antibodies to PGP 9.5 and visualized them by using peroxidase-conjugated avidin as described previously.38 PGP 9.5 appears to be one of the earliest neuron-specific genes to be expressed in the developing nervous system. The expression pattern of PGP 9.5 closely matches the degree of maturity of regenerated nerve fibers. After 4 and 8 weeks of regeneration, one of the most striking observations was the abundance of PGP 9.5 expression and the significant differences between the NGF group and EPO group, indicating continuing axonal sprouting and growth. The polyclonal PGP 9.5 antibody showed, in sciatic nerve cross-sections from the silicone rubber tube segment, specific strong pan-neuronal immunoreactivity, with low background staining. Quantitative analysis by using MOD and IOD revealed that nerves treated with EPO had significantly higher MOD and IOD compared with those in the NGF group at the 2 time points (Fig 7 and Table 2). These histologic and quantitative studies support the data obtained from our tests on functional recovery. The promotion of SFI and MNCV recovery by the treatment of EPO also confirmed these findings.
The mechanism underlying the observed improvement in regeneration and function in the transected sciatic nerves is presently not investigated in our study. Clearly, further investigations are needed to understand the mechanism of the protective action of EPO in peripheral nerve injury.
In conclusion, data from the present study show that EPO promotes functional recovery and enhances nerve regeneration of the surgically transected sciatic nerves in rats. Further studies are needed to define the mechanisms and optimal dose of EPO in peripheral nerve injuries in experimental animals and humans.
Acknowledgments
We thank Yi Yang, MD, for help with the PGP 9.5 immunohistochemistry, and Xiangyang Hu, MD, for help in analyzing the histologic sections.
Abbreviations
- CNS
central nervous system
- EPO
erythropoietin
- EPOR
EPO receptor
- IgG
immunoglobulin G
- IOD
integrated optical attenuation
- MNCV
motor nerve conduction velocity
- MOD
mean optical attenuation
- NGF
nerve growth factor
- PBS
phosphate buffered solution
- PGP 9.5
protein gene product 9.5
- SEM
standard error of the mean
- SFI
sciatic function index
- SPSS
Statistical Package for the Social Sciences
Footnotes
This work was supported by grants from the Key Project of Chinese Ministry of Education and Anhui Science & Technology Department.
References
- 1. Azze RJ, Mattar R, Jr. Lesões dos nervos periféricos. In: Pardini AG., Jr ed. Traumatismos da Mão. 3rd ed. Rio de Janeiro, Brazil: Medsi; 2000. [Google Scholar]
- 2. Ogard WK, Stockert BW. Neuropatias periféricas. In: Umphred DA. ed. Fisioterapia Neurological. 2nd ed., São Paulo, Brazil: Manole; 1994. [Google Scholar]
- 3. Sunderland S. Nerve and Nerve Injury. 2nd ed. London, UK: Churchill Livingstone: 1985. [Google Scholar]
- 4. Fu S, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 1997;14:67–116 [DOI] [PubMed] [Google Scholar]
- 5. Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci 1990;13:43–60 [DOI] [PubMed] [Google Scholar]
- 6. Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci 2003;18:1497–506 [DOI] [PubMed] [Google Scholar]
- 7. Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A 1995;92:3717–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuda S, Nagao M, Takahata K, et al. Functional erythropoietin receptor of cells with neural characteristics: comparison with receptor properties of erythroid cells. J Biol Chem 1993;268:11208–16 [PubMed] [Google Scholar]
- 9. Sekiguchi Y, Kikuchi S, Myers RR, et al. ISSLS prize winner: erythropoietin inhibits spinal neuronal apoptosis and pain following nerve root crush. Spine (Phila Pa 1976) 2003;28:2577–84 [DOI] [PubMed] [Google Scholar]
- 10. Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for EPO in peripheral nerve injury. Glia 2005;51:254–65 [DOI] [PubMed] [Google Scholar]
- 11. Sugawa M, Saakurai Y, Ishikawa-Ieda Y, et al. Effects of erythropoietin on glial development: oligodendrocyte maturation and astrocyte proliferation. Neurosci Res 2002;44:391–403 [DOI] [PubMed] [Google Scholar]
- 12. Brines ML, Grezzi P, Keenan S, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 2000;97:10526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A 2001;98:4044–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 1999;19:643–51 [DOI] [PubMed] [Google Scholar]
- 15. Morishita E, Masuda S, Nagao M, et al. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1997;76:105–16 [DOI] [PubMed] [Google Scholar]
- 16. Sadamoto Y, Igase K, Sakanaka M, et al. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun 1998;253:26–32 [DOI] [PubMed] [Google Scholar]
- 17. Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A 1998;95:4635–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. FASEB J 2001;15:1804–06 [DOI] [PubMed] [Google Scholar]
- 19. Bianchi R, Buyukakilli B, Brines M, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A 2004;101:823–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol 2003;138:1107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villa P, Bigini P, Mennini T, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med 2003;198:971–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaptanoglu E, Solaroglu I, Okutan O, et al. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev 2004;27:113–20 [DOI] [PubMed] [Google Scholar]
- 23. Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003;102:1340–46 [DOI] [PubMed] [Google Scholar]
- 24. Petersen J, Russell L, Andrus K, et al. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery 1996;38:976–983 [DOI] [PubMed] [Google Scholar]
- 25. Chen LE, Seaber AV, Glisson RR, et al. The functional recovery of the peripheral nerve following defined acute crush injuries. J Orhop Res 1992;10:657–64 [DOI] [PubMed] [Google Scholar]
- 26. Lisney SJ. Coupling between chorda tympani fibres in the cat. Brain Res 1981;233:413–416 [DOI] [PubMed] [Google Scholar]
- 27. Aitken JT. The effect of peripheral connexions on the maturation of regenerating nerve fibres. J Anat 1949;83(pt 1):32–43 [PMC free article] [PubMed] [Google Scholar]
- 28. Mackinnon SE, Dellon AL, O'Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 1991;14:1116–22 [DOI] [PubMed] [Google Scholar]
- 29. Scadding JW, Thomas PK. Retrograde growth of myelinated fibres in experimental neuromas. J Anat 1983;136:793–99 [PMC free article] [PubMed] [Google Scholar]
- 30. Leis AA, Lancon JA, Stokic DS. Retrograde regeneration following neurotmesis of the ulnar nerve. Muscle Nerve 2003;28:512–514 [DOI] [PubMed] [Google Scholar]
- 31. Konishi Y, Chui DH, Hirose H, et al. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res 1993;609:29–35 [DOI] [PubMed] [Google Scholar]
- 32. Spiegel DA, Seaber AV, Chen LE, et al. Recovery following stretch injury to the sciatic nerve of the rat: an in vivo study. J Reconstr Microsur 1993;9:69–74 [DOI] [PubMed] [Google Scholar]
- 33. Chen LE, Seaber AV, Urbaniak JR, et al. Denatured muscle as a nerve conduit: a functional, morphological and electrophysiological evaluation. J Reconstr Microsurg 1994;10:137–44 [DOI] [PubMed] [Google Scholar]
- 34. De Medinaceli L, Seaber AV. Experimental nerve reconnection: importance of initial repair. Microsurgery 1989;10:56–70 [DOI] [PubMed] [Google Scholar]
- 35. Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 1989;83:129–38 [DOI] [PubMed] [Google Scholar]
- 36. Dellon AL, Mackinnon SE. Selection of the appropriate parameter to measure neural regeneration. Ann Plast Surg 1989;23:197–202 [DOI] [PubMed] [Google Scholar]
- 37. Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol 2005;174:2060–64 [DOI] [PubMed] [Google Scholar]
- 38. Tonge D, Edström A, Ekström P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol 1998;54:459–80 [DOI] [PubMed] [Google Scholar]