SUMMARY:
The recently emerged novel influenza A(H1N1) virus continues to spread globally. The clinical disease generally appears mild, but unfavorable outcomes have been reported. We describe a case of a 3-year-old Italian girl infected with influenza A(H1N1) virus presenting with neurologic deterioration. CT findings were negative, but MR imaging findings were consistent with ANE. To our knowledge, this is the first case reported in Europe and the second in worldwide pediatric radiology literature.
Influenza-associated encephalopathy is a clinical entity primarily described in the Japanese literature1 and also reported in the United States and Western countries, though less frequently.2,3 High fever, convulsions, and rapid neurologic decline within 2 or 3 days after onset of symptoms are typically documented in these patients and are related to a high mortality rate.
Mizuguchi et al4 reported a possible novel subset of acute encephalopathy affecting Japanese children, characterized by the presence of symmetric thalamic lesions associated with abnormal liver function. They termed it “ANEC.”
ANEC is a disease entity almost exclusively described in East Asian children and typically characterized by multifocal symmetric lesions involving the thalami, brain stem, cerebellum, and white matter. A history of a mild antecedent illness (fever and upper respiratory infection) is elicited in more than 90% of affected patients. Clinical symptoms often resemble those of influenza encephalopathy and include sudden onset of high fever, severe convulsions, and dramatic neurologic deficits rapidly progressing to coma.5
Serum antibody titer to the influenza A virus is elevated in some cases, but the precise role of influenza virus infection in the pathogenesis of ANEC still remains unknown. In this study, we report the case of a patient with influenza A(H1N1) virus encephalopathy with symmetric hemorrhagic lesions in the thalami and deep cerebellar nuclei documented on MR imaging.
Case Report
A 3-year-old Italian girl was referred to our child unit care because of high fever (maximum temperature of 41.2 C°) and severe convulsions following a 2-day prodromal illness with cough and diarrhea.
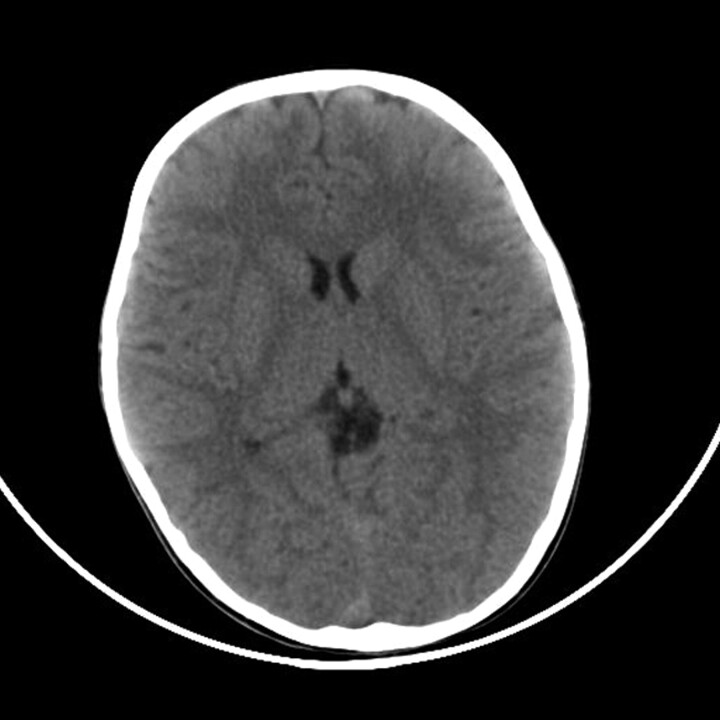
There was no previous history of influenza immunization or recent travel, and she had taken no acetylsalicylic acid. Her 5-year-old sister had experienced fever and mild signs of upper respiratory infection approximately 1 week before. During hospitalization, she gradually developed altered consciousness, rapidly progressing to coma within 2–3 days. Laboratory tests at this time revealed severe liver dysfunction (aspartate aminotransferase level, 16,852 U/L; alanine aminotransferase level, 10,300 mm3/L; lactate dehydrogenase level, 27,878 U/L) and abnormal coagulation. Complete blood count demonstrated thrombocytopenia (54,000 μ/L). Blood ammonia and glucose levels were normal. Brain CT yielded no abnormalities (Fig 1).
Fig 1.
Axial noncontrast CT image obtained on admission shows normal attenuation in the thalami.
Lumbar puncture showed slightly increased pressure (170 mm H2O). CSF contained 6 cells/mm3 and revealed increased protein content (232 mg/L), numerous red cells, and normal lactate and pyruvate levels.
MR images were obtained on a 1.5T Achieva system (Philips Healthcare, Best, the Netherlands). Study protocol included sagittal T1-weighted; coronal, sagittal and axial T2-weighted; axial fluid-attenuated inversion recovery; and T2-weighted gradient echo images.
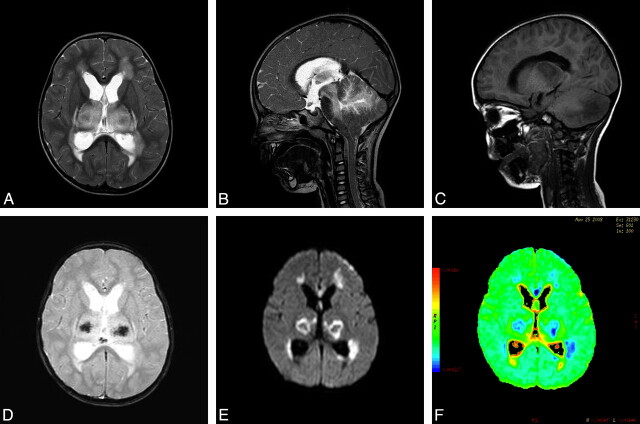
MR imaging demonstrated abnormal signal intensity in the supratentorial white matter, splenium of the corpus callosum, dorsal aspect of pons, bilateral thalami, and cerebellar hemispheres with mass effect on the surrounding parenchyma (Fig 2A–C). There were signs of increased intracranial pressure including hydrocephalus and tonsillar herniation (Fig 2B). Petechial hemorrhage within the central portion of the thalami was observed on T2-weighted gradient- echo sequences (Fig 2D). DWI showed corresponding regions of restricted diffusion (Fig 2E, -F).
Fig 2.
MR imaging in the acute stage. A, Axial T2-weighted image shows symmetric increased signal intensity in the thalami and supratentorial frontal white matter. B, Sagittal T2-weighted image demonstrates hyperintensity in the cerebellum and the brain stem, including the midbrain and dorsal pons. Note tonsillar herniation and hydrocephalus before shunt surgery. C, Sagittal T1-weighted MR image shows hypointensity in the thalami and cerebellar hemisphere. D, T2-weighted gradient-echo image reveals decreased signal intensity in the central portion of the thalami, indicating hemorrhagic necrosis. E and F, Axial DWI and an apparent diffusion coefficient color-coded map reveal restricted diffusion, with a concentric pattern, symmetrically involving the thalami.
The patient had a significant increase in serum antibody titer to influenza A(H1N1) virus, which was demonstrated on a nasopharyngeal swab but was not found in CSF specimens by using virus isolation and a polymerase chain reaction assay.
Findings of routine CSF, blood, urine, and nasal secretion cultures were negative for other pathogens including HSV, human cytomegalovirus, influenza B virus, Japanese Encephalitis virus, and West Nile virus.
She was treated with intravenous acyclovir and oseltamivir; vancomycin, ampicillin, and meropenem were also administered.
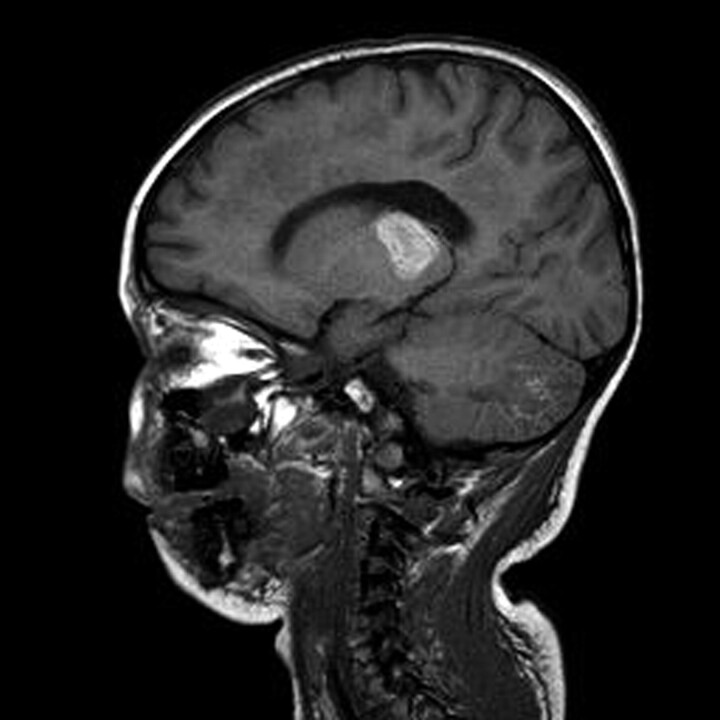
One week following presentation her level of consciousness constantly improved, but voluntary movements and speech were still impaired. A 10-day follow-up MR imaging demonstrated ring enhancement of the thalamic lesions after administration of contrast medium with an interval decrease in the mass effect. Moreover, we observed conspicuous T1 hyperintensity of the thalami, probably related to the presence of methemoglobin (Fig 3). Hemosiderin foci within thalamic lesions were still noted on T2-weighted gradient-echo sequences.
Fig 3.
MR image obtained 10 days after admission. Sagittal T1-weighted MR image shows hyperintensity in the thalami and cerebellum, consistent with subacute hemorrhagic changes.
A follow-up MR imaging performed 40 days after admission demonstrated well-defined regions of increased T2 signal intensity in the cerebellar hemispheres, bilateral thalami, frontal white matter, and splenium of the corpus callosum with corresponding areas of decreased T1 signal intensity consistent with cavitation (Fig 4A, -B). DWI was measured along 9 noncollinear directions by using a diffusion-weighted b factor in each direction, with 700 s/mm2.
Fig 4.


MR imaging in the chronic stage, performed 40 days after admission demonstrates severe sequelae. A and B, Axial and coronal T2-weighted images reveal sharply marginated bilateral cavitation in the cerebellum and shrunken thalami. C–E, 3D views on a frontotemporal reconstruction depict fractional anisotropy in the pyramidal tracts (C) cerebellar hemispheres (D), and frontal white matter (E), showing destruction of the normal anisotropy.
Additionally, by means of View Forum, (Philips Achieva Healthcare, Best, the Netherlands), we created DTI fiber tracks, visualized the tracks in 3D, and performed quantitative analyses on the delineated tracts (Fig 4C–E).
DTI revealed lower anisotropy, lower parallel diffusion, higher transverse diffusion, and slightly higher mean diffusivity in the previously described affected regions.
Discussion
Influenza A viruses represent a continuous pandemic threat. In April 2009, a novel influenza A virus, the so-called swine-origin influenza A(H1N1) virus, was isolated in Mexico.6 Although unfavorable outcome was mostly related to pulmonary complications, to our knowledge, at least 1 case of ANE has been recently reported in the medical literature.7
In our case, the symmetric brain lesions involving the thalami, cerebral periventricular white matter, brain stem tegmentum, and cerebellar hemispheres together with clinical and biochemical findings were consistent with ANE as previously reported by Mizuguchi et al.2,4
The patient presented with high fever, seizures, and progressive neurologic decline. Laboratory tests demonstrated elevated liver transaminase levels and abnormal coagulation. There was a significant increase in CSF antibody serum titer to influenza A(H1N1) virus and a nasopharyngeal swab was positive for influenza A(H1N1) virus, thus indicating that she was infected with the virus.
Initially our MR imaging study showed edema of the frontal white matter, splenium of the corpus callosum, brain stem, bilateral thalami, and cerebellar hemispheres, reflecting T2 increased signal intensity in the affected regions and mass effect on the surrounding parenchyma together with signs of increased intracranial pressure. A T2-weighted gradient-echo sequence documented decreased signal intensity in the thalami, indicating petechial hemorrhage. Serial MR imaging, performed 10 and 40 days after admission, revealed gradual resolution of edema and cavitation of the thalami as typically described in patients with ANE.
Currently, >110 cases, predominantly reported in children younger than 5 years of age of East Asian descent, including Japan, Taiwan, and Korea, have been described.8 Sporadic cases have been documented worldwide.2
To our knowledge, this is the first case of ANE reported in Europe during the 2009 influenza A(H1N1) pandemia and the only one resulting in patient survival.
ANE is often associated with influenza A infection but has also been described in Human herpesvirus-6, HSV, Mycoplasma, and measles infections.9 At the present time, many authors have speculated that ANE may be an immune-mediated or metabolic disease entity.5
Nonetheless, the etiology and pathogenesis of this disease still remain unknown.
The early onset of ANE following a prodromal illness suggests that the pathogenesis of ANE may be different from that of postinfectious encephalitis or acute disseminated encephalomyelitis, though Yagishita et al10 consider ANE a postviral or postinfectious brain disorder.
A wide range of disorders affecting deep gray matter should be considered in the differential diagnosis, including inborn errors of metabolism, hypoxic-ischemic encephalopathy, viral encephalitis, acute disseminated encephalomyelitis, and Reye syndrome. ANE demonstrates clinical and laboratory test findings different from these diseases; nonetheless, recognition of typical neuroradiologic features is extremely important to correctly diagnose this rare encephalopathy frequently associated with high mortality and morbidity.11
Radiologists should become aware of the central role of MR imaging in aiding clinicians to formulate a presumptive diagnosis of ANE and, therefore, initiate appropriate treatment. The importance of this imaging technique, eventually associated with DTI, should be advocated as well in the follow-up of these patients to guide the clinical approach and predict the final outcome.3,8
Abbreviations
- ANE
acute necrotizing encephalopathy
- ANEC
acute necrotizing encephalopathy of childhood
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- HSV
herpes simplex virus
References
- 1. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 1997;19:81–92 [DOI] [PubMed] [Google Scholar]
- 2. Spring MD, Wright PF. Influenza-associated acute necrotizing encephalopathy in a 2-year-old American child. Int Congr Series 2004;1263:346–49 [Google Scholar]
- 3. Wong AM, Simon EM, Zimmerman RA, et al. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol 2006;27:1919–23 [PMC free article] [PubMed] [Google Scholar]
- 4. Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotizing encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry 1995;58:555–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sugaya N. Influenza-associated encephalopathy in Japan: pathogenesis and treatment. Pediatr Int 2000;42:215–18 [DOI] [PubMed] [Google Scholar]
- 6. Michaelis M, Doerr HW, Cinatl J, Jr. Novel swine-origin influenza A virus in humans: another pandemic knocking at the door. Med Microbiol Immunol 2009;198:175–83. Epub 2009 Jun 20 [DOI] [PubMed] [Google Scholar]
- 7. Lyon JB, Remigio C, Milligan T, et al. Acute necrotizing encephalopathy in a child with A H1N1 influenza infection. Pediatr Radiol 2010;40:200–05. Epub 2009 Dec 18 [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Kim IO, Lim MK, et al. Acute necrotizing encephalopathy in Korean infants and children: imaging findings and diverse clinical outcome. Korean J Radiol 2004;5:171–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skelton BW, Hollinshead MC, Sledd AT, et al. Acute necrotizing encephalopathy of childhood: typical findings in atypical disease. Pediatr Radiol 2008;38:810–13. Epub 2008 Apr 16 [DOI] [PubMed] [Google Scholar]
- 10. Yagishita A, Nakano I, Ushioda T, et al. Acute encephalopathy with bilateral thalamotegmental involvement in infants and children: imaging and pathology findings. AJNR Am J Neuroradiol 1995;16:439–47 [PMC free article] [PubMed] [Google Scholar]
- 11. Weitkamp JH, Spring MD, Brogran T, et al. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J 2004;23:259–63 [DOI] [PubMed] [Google Scholar]