SUMMARY:
Molecular imaging is aimed at the noninvasive visualization of the expression and function of bioactive molecules that often represent specific molecular signatures in disease processes. Any molecular imaging procedure requires an imaging probe that is specific to a given molecular event, which puts an important emphasis on chemistry development. In MR imaging, the past years have witnessed significant advances in the design of molecular agents, though most of these efforts have not yet progressed to in vivo studies. In this review, we present some examples relevant to potential neurobiologic applications. Our aim was to show what chemistry can bring to the area of molecular MR imaging with a focus on the 2 main classes of imaging probes: Gd3+-based and PARACEST agents. We will discuss responsive probes for the detection of metal ions such as Ca, Zn, Fe, and Cu, pH, enzymatic activity, and oxygenation state.
Molecular imaging is an emerging science area aimed at noninvasive visualization of the expression and function of bioactive molecules that often represent specific molecular signatures in disease processes. It seeks the biochemical or physiologic abnormalities underlying the disease, rather than the structural consequences of these abnormalities. Potential clinical applications of molecular imaging have been widely recognized, though the limited sensitivity and specificity of current molecular imaging approaches are often a major roadblock for clinical applications. Besides clinical settings, drug development can also largely benefit from the integration of molecular imaging. Molecular imaging not only allows rationalization of the parameters related to disease processes but it can also replace the common invasive research techniques (such as histology, which requires sacrificing animals for each time point in the experiment) by time-effective repeatable real-time visualization of biologically relevant processes. Currently, progress in the molecular imaging area is simultaneously moving at 3 levels: 1) improving the imaging hardware for use in preclinical and clinical settings, 2) identifying and validating new biologically relevant imaging targets, and 3) developing innovative imaging probes. Unlike anatomic imaging, molecular imaging always requires a molecular imaging probe, and this places chemistry development in a central position.
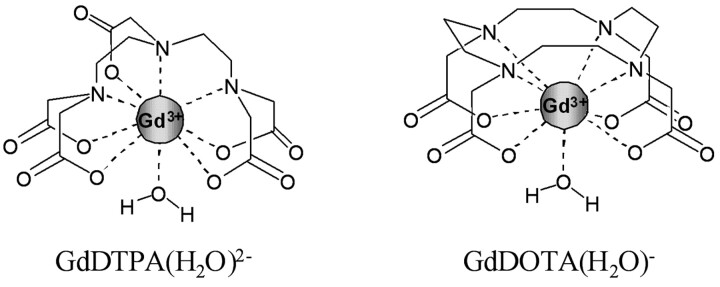
Among all molecular imaging technologies, MR imaging is particularly attractive. Its exceptional resolution allows acquiring anatomic images in conjunction with mapping the activity of biomarkers. Since the introduction of MR imaging into clinical practice, contrast-enhancing agents have been widely used to improve the relatively low sensitivity of the technique. Conventional MR imaging contrast agents are paramagnetic substances that enhance the water proton relaxation rates. Unlike optical or radiodiagnostic probes, the MR imaging agents do not directly generate a signal intensity but are detected via their effect on the water proton relaxation. Given the high paramagnetism of Gd3+ (it has 7 unpaired electrons), its poly(amino carboxylate) complexes are mostly used today in clinical practice. Gd3+ complexes have a more important effect on the longitudinal relaxation times (T1 agents) than on the transverse relaxation times (T2 agents). In the Gd3+ complexes, the poly(amino carboxylate) ligand, such as DTPA or DOTA (Fig 1), wraps around the Gd3+ ion to prevent its toxicity; however, it leaves enough room for 1 water molecule directly linked to the paramagnetic metal ion. The exchange of this water molecule with the surrounding water is extremely important because it will transmit the paramagnetic effect of the Gd to the bulk water to make the molecule detectable as a decreased proton relaxation time (Fig 2).
Fig 1.
Gd-DOTA (Dotarem; Guerbet, Aulnay-sous-Bois, France) and Gd-DTPA (Magnevist; Schering, Berlin, Germany). Two examples of clinically used Gd3+ poly(amino carboxylate) complexes. All other approved Gd3+-based contrast agents are derivatives of Gd-DOTA or Gd-DTPA.
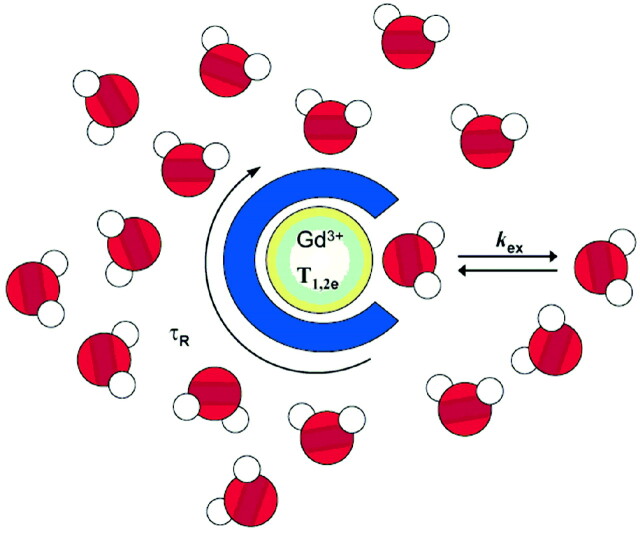
Fig 2.
In Gd3+-based contrast agents, the paramagnetic Gd3+ ion is coordinated by an organic ligand. The water molecules directly linked to the metal exchange with bulk water, and this exchange transmits the paramagnetic effect of Gd3+ to the surrounding environment. The efficacy of the agent is mainly related to the number of water molecules bound to Gd3+ (the hydration number), q (the exchange rate of the coordinated water molecule[s] with the surrounding water), kex, and the rotational correction time τR.
The currently used clinical agents are nonspecific. While they help delineate the structural anatomic consequences of diseases, they are not capable of indicating the biochemical or physiologic abnormalities underlying them. The efficacy of the agent to accelerate water proton relaxation, defined as relaxivity, has to be selectively influenced by the particular parameter/biomarker that we want to detect, to create a “smart” (activatable or responsive) contrast agent. The relaxation behavior of paramagnetic complexes and the underlying mechanisms have been extensively discussed elsewhere.1–3 Here we only summarize the various modulation pathways for the relaxivity of Gd3+-based agents. The relaxivity (paramagnetic relaxation enhancement of water proton relaxation per millimolar concentration of Gd3+) is inherently related to the microscopic properties of the Gd3+ complex. The most important microscopic factors are the following: 1) the number of water molecules directly coordinated to the metal ion (hydration number), 2) the exchange rate of these water molecules with the surrounding water, and 3) the motion dynamics of the molecule (how fast the complex tumbles), usually characterized by the reorientational correlation time of the agent (Fig 2). In principle, any of these parameters could be modulated; however, most of the smart contrast agents function on the basis of changes in the hydration number or in the reorientational correlation time of the molecule because they are the easiest to predict and modulate by chemical changes. The relaxivity is linearly proportional to the hydration number. The reorientational correlation time of the agent is determined by the size and the flexibility of the molecule. Generally speaking, slower rotation (larger size and reduced flexibility) translates to higher relaxivity of the Gd3+ complex. However, this effect is strongly dependent on the magnetic field, and vanishes at fields above ∼4T.
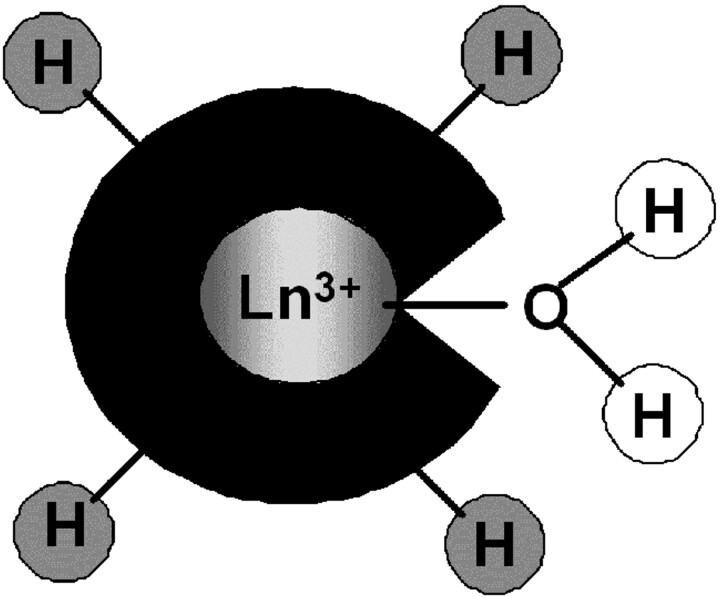
Recently, a new class of potential paramagnetic MR imaging contrast agents, based on CEST, has been explored as a promising alternative to classic relaxivity agents.4–8 In particular, they have generated a great enthusiasm as activatable probes. PARACEST agents are complexes of lanthanide ions other than gadolinium, which possess paramagnetically shifted exchangeable protons. Those protons (−NH of amides, amines, or −OH of water or alcohols) are in slow exchange with bulk water protons (Fig 3). They can be selectively saturated by radio-frequency pulses applied at the appropriate frequency, and due to the chemical exchange between these and the water protons, their saturation will result in a decrease of the water proton signal intensity, which becomes observable on an MR image. PARACEST sensors are based on modulation of the exchange rate or the resonance frequency of those mobile protons on the agent. The concept of chemical exchange saturation transfer can also be applied to nonparamagnetic endogenous molecules; however, the signals of their protons are located close to the bulk water signal intensity, which hampers their selective saturation. PARACEST probes are ideally suited for molecular imaging because contrary to Gd3+-based MR imaging agents, the contrast can be switched on and off at will by simply adjusting the pulse sequence parameters. Another advantage of PARACEST with respect to relaxivity agents is the possibility of applying ratiometric approaches that eliminate the problem of unknown local concentration of the agent and allow quantitative determination. Such ratiometric approaches involve the use of a cocktail of 2 isostructural complexes: one as a reporter of a physiologic parameter and the other as a simple concentration marker, whereas both have an identical biodistribution.
Fig 3.
Schematic representation of a PARACEST agent with exchangeable protons on the ligand (amine, amide, alcohol…) (gray) or on the coordinated water (white).
In recent years, significant progress has been made in the design of novel molecular MR imaging probes, though very few of them have entered in vivo studies. With respect to neuroimaging, many pathologies are associated with an altered permeability or disruption of the BBB; however, for a contrast agent, crossing the intact BBB still remains a challenge. In this review, we present some examples of recent development of molecular MR imaging probes relevant to neurobiologic applications. It is not intended to be an exhaustive review but rather an illustration of what chemistry can bring to molecular MR imaging. Other reviews related to molecular neuroimaging, not restricted to MR imaging, have been recently published.9–11 Here we will discuss paramagnetic lanthanide complexes involving Gd3+ agents and PARACEST probes and will focus on responsive agents designed to detect metal ions, such as Ca2+, Zn2+, Cu2+, and Fe2+/3+, pH, enzymatic activity, and oxygenation state. We have included the above metal ions because disruption of their homeostasis is associated with various neurodegenerative diseases. Changes in the extracellular pH and enzymatic activities can also be the signature of specific pathology processes. The in vivo noninvasive detection of abnormalities in the concentration of these metal ions, in pH, or in enzymatic activities might serve in the future as an important diagnostic tool of the underlying diseases. Information on the redox status, which is an important factor governing tumor aggressiveness, can help choose the adapted tumor treatment. We should note that many of these abnormalities are observable in the extracellular media, which largely facilitates the chemical design of the imaging probes (no need for intracellular delivery).
Imaging Probes Responsive to Metal Ions
Ca Sensing
Understanding how the brain works involves both a comprehension of the physiologic functioning of its individual elements (neurons and glia cells) and a description of their functional architecture and the underlying networks. The activity of networks can be studied with in vivo neuroimaging, in particular with BOLD functional MR imaging. The BOLD technique has, however, physiologic limitations linked to the vascular origin of the signal intensity.12 The temporal resolution is in the range of several seconds, and the relationship between the BOLD signal intensity and the neural activity is still not fully understood. One step further could be the use of bioactivated contrast agents as functional markers for processes directly linked to neural signaling. For tracking the neural activity, several specific markers might be envisaged, being responsive to the concentration of certain ions, neurotransmitters, or transmembrane potentials. Very important work in this direction has been performed during the past years by imaging fluorescence signals reporting intracellular Ca2+ fluctuations.13–15 However, the intrinsic depth limitations associated with optical imaging restrict these applications to superficial regions.
The extracellular concentrations of Ca2+ play an important role in both physiologic and pathologic processes. Ca2+ ions are not only crucial in several steps in neuronal signaling during normal brain activity but are also associated with a variety of pathologies such as ischemia, hypoglycemia, and seizures.16 The involvement of Ca2+ has been implicated in neurometabolic disorders such as in Parkinson disease,17 Huntington disease,18 amyotropic lateral sclerosis,19 schizophrenia,20,21 muscular dystrophy,22 etc, suggesting a broad spectral role of Ca in neuropsychiatric disorders. While Ca2+ has been an important target for MR imaging probes, so far, to our knowledge, no agents responsive to neurotransmitters or transmembrane potentials have been reported.
Although strictly speaking, it is not a Ca2+-responsive probe, Mn2+ has been widely used to detect neural activity and to obtain information between interconnected groups of neurons in highly distributed networks.23,24 The Mn2+ ion works as a paramagnetic mimic of Ca2+; therefore, it accumulates in neurons in an activity-dependent manner. However, Mn-enhanced MR imaging has several drawbacks. The MR imaging signal intensity is proportional to the Mn2+ concentration in tissue; thus, significant amounts of Mn2+ are required to produce detectable contrast. Elevated Mn2+ concentrations can perturb the circuits under study and lead to toxic effects. On the other hand, the very slow uptake of Mn2+ (from hours to days) prevents real-time Ca imaging.
Attempts toward Ca2+-sensitive MR imaging probes involve mainly 2 different approaches: 1) Gd-complexes with a T1 response on interaction with Ca2+ ions, and 2) a T2 agent based on the Ca2+-related aggregation of superparamagnetic iron nanoparticles and calmodulin.25 The main limitation of this second approach is the relatively long time course of the Ca-dependent aggregation (a few seconds), preventing sensing of fast Ca-concentration changes.
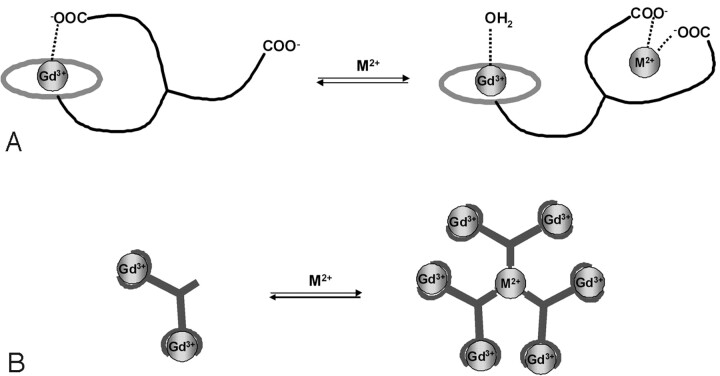
It is much easier to achieve a fast magnetic response by intramolecular interactions. Therefore, T1 agents (Gd3+ complexes) are more favorable with respect to the time course of the Ca-dependent MR imaging response.26 The design of all Gd3+-based Ca2+ ion−sensitive probes reported so far involved changes in the coordination sphere of the Gd3+ ion following coordination of Ca2+. The probes integrate 2 coordinating units that selectively chelate Gd3+ and Ca2+ ions. In the absence of the sensed Ca2+ ion, 1 (or more) of the donor groups of the Ca chelating center is weakly coordinated to the Gd3+ ion. On interaction with Ca2+, this donor group will switch from Gd3+ to participate in the Ca2+ coordination, thus liberating 1 coordination position on the Gd3+ ion. The coordination position becoming free around the Gd3+ is immediately occupied by a water molecule (Fig 4A), and the increase of the hydration number results in a relaxivity increase. This approach was used in the design of a series of compounds with 2 macrocyclic chelators connected by a spacer containing a Ca-selective chelating unit. The first example was reported by Li et al.27 Their sensor responded to Ca2+ in the micromolar range, corresponding to intracellular concentrations. However, not only the intracellular delivery of the agent is problematic, but given the low relaxivity of the agent, it is not MR imaging−detectable at micromolar concentrations, which would be needed to sense Ca2+ in the micromolar range.
Fig 4.
Illustration of 2 different strategies to modulate relaxivity of Gd3+ complexes by cation recognition. A and B, Change in the q (A) and change in the size, hence the τR (B). The binding site for the sensed metal ion has to be highly selective with respect to other endogenous cations.
Targeting the extracellular Ca2+ seems more reasonable for MR imaging applications. The concentration of free extracellular Ca2+ is in the millimolar range and drops up to 30%–35% from the resting state during intense stimulation (typically from 1.2 to 0.8 mmol/L).9 Sensing Ca2+ changes at millimolar concentrations is more compatible with the relatively high concentrations of the magnetic imaging probe needed to detect contrast changes. Furthermore, targeting extracellular Ca2+ also simplifies the chemical design, because additional requirements for cell internalization can be neglected. A series of Gd3+ complexes have been reported for extracellular Ca2+ sensing28–31; the best compounds show ∼10% relaxivity change under biologically relevant conditions (in brain extracellular fluid, for a Ca2+-concentration change between 0.8 and 1.2 mmol/L) with a high selectivity for Ca2+, which could already be detectable on MR images.
Zn Sensing
Zn is the second most abundant transition metal in the body, and its highest concentrations occur in the brain. Neuronal zinc is partitioned into 2 main classes: a static pool of Zn that is tightly bound to various metalloproteins and a labile pool of Zn that is mobile. More than 90% of the Zn2+ found in the brain and the body is classified as static, playing structural roles in transcription factors and related proteins and structural and catalytic roles in enzymes. The primary source of labile Zn2+ in the brain is from Zn2+ release into the synapse during transmission and can reach concentrations of ≤200–300 μmol/L. Exposure to uncontrolled concentrations of a labile pool of Zn2+ can lead to excitotoxic neuronal death, particularly during epileptic seizures, head trauma, cerebral ischemia and reperfusion, and related situations of overintense neural activity.11 In addition to these, long-term disturbances in Zn2+ homeostasis have also been implicated in aging and age-related neurodegenerative diseases such as Alzheimer disease. Indeed, the Zn2+ ion can reach ∼1 mmol/L concentration in amyloid plaques and is implicated in their formation by aggregation of the amyloid-β peptide.32
As for Ca2+, the main strategy to detect Zn2+ ions is based on a change in the number of water molecules directly bound to the Gd3+ center of a bipartite responsive agent (Fig 4A). Hanaoka et al studied a system based on the association of a DTPA core and TPEN as a specific Zn2+ chelator. The main drawback of this system is that it can form 1:1 and 2:1 Zn:Gd-complex adducts leading respectively to a decrease and a further increase in relaxivity on Zn2+ complexation. This situation is problematic because one can reach the same relaxivity value for 2 different amounts of Zn2+.33 The system has been further modified to replace 1 pyridine unit of each TPEN by a carboxylate function, to solve this problem.34 Similarly, Major et al studied a series of macrocyclic complexes associated with various Zn2+ chelators such as TPEN or a diaminoacetate arm. They showed that at least 1 acetate arm involved in Zn2+ chelation was necessary to observe an increase in relaxivity of about 100% on Zn2+ coordination.35 These probes have not been tested in vivo, but the in vitro MR images showed that the diaminoacetate system is sensitive enough to detect physiologically relevant concentrations of Zn2+ (∼100 μmol/L).36 One highly sensitive PARACEST agent responsive to Zn2+ has been reported by Trokowski et al.37 The Zn2+ chelator consists of 2 TPEN units linked to a cyclen core for Eu3+ complexation. On Zn2+ binding, the PARACEST behavior undergoes dramatic changes.37 It was explained by an acceleration of the proton exchange, though the underlying mechanism has not been identified. The system is selective for Zn2+ over Mg2+ or Ca2+ ions and is estimated to be able to detect changes in concentration of labile Zn ranging from 5 nmol/L to 0.12 μmol/L.37
Fe Sensing
Fe is the most abundant transition metal in the body and in the brain. Indeed, this organ has the highest rate of oxidative metabolism in the body and uses Fe as a key component of enzymes involved in oxygen transport and metabolism. Biologic iron is most commonly found in the +2 (ferrous) and +3 (ferric) oxidation states. Fe2+/3+ concentrations in the extracellular environment are in the low micromolar range in the CSF and are typically between 20 and 30 μmol/L in the blood serum of a healthy adult. Intracellular Fe concentrations in neurons range from 0.5 to 1 mmol/L. The major part of Fe in the brain is tightly held by the storage protein ferritin. Exchangeable Fe in the brain has been proposed to exist in the intracellular environment, in a store termed the “labile iron pool,” containing Fe bound by small anions (PO43−, CO32−, citrate), polypeptides, and surface components of membrane. However, the existence of this pool is still under active debate.11 Fe undoubtedly accumulates in the brain with age and is associated with neurodegenerative diseases, including Alzheimer and Parkinson diseases.
Because the coordination chemistry of Fe2+ and Fe3+ is very different, sensors have to be developed specifically for each oxidation state. Fe2+ is well known for forming stable complexes with phenanthroline, bpy, and tpy ligands in 1:3 and 1:2 metal:ligand ratios. When attached to Gd3+ chelates, such ligands can promote the formation of supramolecular assemblies in the presence of Fe2+. Despite the fact that these systems were mainly studied as contrast agents for imaging at high magnetic fields, they could potentially be used for sensing Fe2+ ions. Indeed, these assemblies formed around the Fe2+ core have a larger size than the monomer Gd3+ complex alone (Fig 4B), which results in a reduced rotational mobility leading to a relaxivity enhancement. In the case of the phenanthroline derivatives, an increase of 90% in the relaxivity is observed on the formation of the heterobimetallic tris complex.38 The same phenomenon was observed for bpy and tpy derivatives. The former shows an increase of about 200% at 40 MHz on the formation of the heterobimetallic complex (metallostar), giving rise to an exceptionally high relaxivity confined to a small molecular space.39,40 This high in vitro relaxivity is retained under in vivo conditions in mice. The pharmacokinetics of this metallostar, injected intravenously, were found to be similar to that of Gd-DOTA, involving fast renal clearance and a leakage to the extracellular space in the muscle tissue.41 Recently, another high-molecular-weight tetrametallic supramolecular complex [(Gd-DTPA-phenantroline)3Fe] has been obtained on self-assembly around 1 Fe2+ ion. The slower elimination of the compound from rats as compared with Gd-DTPA was found to correlate with a reduced volume of distribution and suggests that the complex is confined to the blood compartment.42
Another strategy to detect Fe2+ ions uses a molecule including 3 Gd3+-containing moieties linked together by a tris-hydroxamate unit, which is a strong binding agent for Fe2+. This approach relies on a concept similar to that schematized in Fig 4B; however, here the Gd3+ complexes can be found in 1 molecular entity. This molecule is very flexible, resulting in a limited relaxivity. On chelation of Fe by the tris-hydroxamate, the whole system becomes highly rigid, thereby restricting flexibility of the entire assembly. This results in about 60% of the relaxivity increase at 20 MHz, without changing the molecular weight of the molecule.43
Concerning detection of Fe3+, only 1 system has been described on the basis of DTPA−bis salicylamide. The relaxivity increases on the formation of the Fe3+-bound complex due to the increase in the molecular weight. The main drawback of this system is its pH-dependency.44
Cu Sensing
Cu is the third most abundant transition metal in the body and in the brain, with average neural Cu concentrations on the order of 0.1 mmol/L. Like Fe, Cu can be found in 2 different oxidation states, +1 (cuprous) and +2 (cupric); the former is more common in the reductive intracellular environment, whereas the latter is dominant in the more oxidative extracellular environment. Cu is partitioned into tightly bound and labile pools, and free Cu can be released into the synapse on presynaptic excitation, reaching ≤15–30 μmol/L in the synaptic cleft.11 The normal brain extracellular concentrations are in the range of 0.2–1.7 μmol/L, but like Zn, Cu was found in high concentrations (∼400 μmol/L) in amyloid plaques that are involved in Alzheimer disease.32 Disruption of Cu homeostasis is implicated in other neurodegenerative diseases, including Parkinson disease, familial amyotrophic lateral sclerosis, Menkes disease, and Wilson disease.45
No MR imaging contrast agent has been proposed for Cu+ sensing. For Cu2+ detection, a single MR imaging contrast agent has been described in the literature. This Gd3+ complex also functions on the basis of a change in the hydration number on Cu2+ coordination (Fig 4A). The ligand is composed of a macrocyclic core of complex Gd3+ coupled with a pendant iminodiacetate arm for Cu2+ binding. In the presence of Cu2+, this arm de-coordinates from Gd3+, leading to a higher hydration number and a subsequent increase in relaxivity.46 In a typical experiment, the addition of 30-μmol/L Cu2+, the estimated concentration released during neural activity, to a 0.21-mmol/L solution of complex gave a 9% relaxivity increase, a value which might be suitable for in vivo imaging applications. However, so far, to our knowledge, no in vivo MR imaging experiments have been reported for Cu sensing.
pH-Sensitive Probes
The potential use of pH-sensitive probes is vast, though so far pH mapping of tissues has been mainly intended to facilitate cancer detection and assess the tumor status. Indeed, the pH on the surface of tumors is ∼0.4 units lower than that in normal tissue, but in some cases, it can be as low as 6.0.47 On the other hand, pH-sensitive probes could also indicate neuronal activity because they induce a slight acidification of the extracellular medium (pH 7.2–7.4).48 One could also imagine the use of pH-responsive probes to determine if the brain environment is suitable for a drug that functions in a pH-sensitive environment.
The design of pH-sensing probes has been an intensive field in MR imaging contrast agent research. Nonexhaustive examples are provided.49–55 Some of the pH probes also proved useful for in vivo application. The combination of the pH-sensitive amido-phosphonate derivative of Gd-DOTA and a pH-insensitive analog was used in a dual injection to image renal pH in mice.56,57 The assumption was made that both compounds have comparable pharmacokinetics; hence, the concentration of the former can be inferred from the concentration of the latter. This mixture has been also used to obtain extracellular pH MR imaging maps in a rat glioma model, with improved spatial resolution compared with spectroscopic methods.58 Differences of the order of 1 pH unit could be detected; the absolute pH values have been calculated by using a calibration method.
A key factor determining the effectiveness of an agent is the difference between the relaxivity of the “on” state compared with that of the “off” state. Recently, the amplitude of the relaxivity response to pH variation of this low-molecular-weight probe has been largely improved (more than doubled) by conjugating it to a macromolecular dendrimeric scaffold.59 However, improving the relaxivity response to pH through increased molecular weight may also negatively impact the effectiveness of such agents. Large molecules, such as dendrimers, remain in the vasculature longer than discrete agents, which are better able to diffuse into all extracellular space. Furthermore, large molecules also tend to clear more slowly from the body as a result of increased liver uptake. This extends the retention time of Gd3+ in the body. Further studies into the in vivo behavior of dendrimer-based MR imaging contrast media will be required to establish whether this approach, which is successful for increasing the relaxivity response, will yield agents that can actually be applied in vivo.
PARACEST agents have a great potential to indicate pH. The PARACEST effect originates from proton exchange between the paramagnetic probe and water, and the magnitude of the observed effect is intrinsically dependent on the exchange rate, itself related to pH.60 Unlike traditional Gd3+-based agents, it is possible to administer and independently visualize 2 PARACEST agents in the same experiment and thus develop a concentration-independent ratiometric method for pH determination. For instance, one can acquire the PARACEST pH profiles for a cocktail of 2 isostructural probes, including an identical ligand but 2 different paramagnetic metal ions. The individual PARACEST response of the 2 probes to pH will be different, whereas their distribution in tissue should be identical. Consequently, the ratio of the 2 PARACEST profiles is concentration-independent and can be used to determine pH directly without knowing the analytic concentration of either agent. In some cases, the 2 proton pools giving rise to the 2 PARACEST effects can be found within a single molecule, which further simplifies the pH measurement.4,61,62
Enzyme-Activated Contrast Agents
Among all responsive agents, enzyme targeting represents a specific advantage, which is particularly valid for MR imaging detection, given its low sensitivity. A small concentration of the enzyme can catalytically convert a relatively high amount of the enzyme-responsive magnetic probe, which markedly decreases the limit of detection for the enzyme compared with other biomolecules. In addition, the high specificity of enzymatic reactions means that the observed change in the MR imaging properties can be unambiguously attributed to the targeted enzyme. The presence of certain enzymatic reactions indicates the cellular state and can provide the signature of a given pathology. Therefore, the real-time noninvasive in vivo detection of specific enzymatic activities would have invaluable diagnostic impact. As an example, the importance of enzymatic activity has been established in the processes of tumor formation, growth, and metastasis. Molecular biology studies have defined enzymatic steps of the apoptotic response to anticancer therapies in vitro and in vivo. The detection of gene markers (such as β-galactosidase) could be another important field of application.
The first enzymatically responsive potential MR imaging contrast agent was a Gd-DOTA−derivative bearing a galactopyranose residue that prevents water coordination.63,64 This sugar moiety is a substrate for the enzyme β-galactosidase. Its enzymatic cleavage by β-galactosidase opens the access of water to the first coordination sphere of Gd3+, resulting in a relaxivity increase, thus irreversibly activating the agent. It has been successfully used for the in vivo detection of β-galactosidase messenger ribonucleic acid expression in living Xenopus laevis embryos.
Among other examples, Anelli et al65 functionalized Gd(DTPA)2− with sulfonamide, which is known as a specific carbonic anhydrase inhibitor. The agent reacts with carbonic anhydrase, and thus targets enzyme-rich tissues. Nivorozhkin et al66 reported the enzymatic transformation of a prodrug Gd3+ complex with poor affinity to HSA and low relaxivity to a species with improved HSA affinity and enhanced relaxivity. In this approach, the origin of the relaxivity increase was the slower rotation for protein-bound chelates with respect to the small-molecular-weight prodrug. The same concept was applied for the detection of alkaline phosphatase.67 Mazooz et al68 described a Gd-DTPA peptide acting as a transglutaminase substrate, which was used to monitor transglutaminase activity. Shiftan et al reported MR imaging visualization of hyaluronidase in ovarian carcinoma,69 related to the aggressiveness of ovarian cancer metastasis. Chen et al70 visualized plaque rupture in atherosclerosis with a Gd-DOTA−serotonin derivative, which polymerizes in the presence of neutrophil myeloperoxidase, resulting in a remarkable relaxivity increase. Tyramido or tryptamido derivatives of Gd-DTPA were proposed as enzyme-activated agents for peroxidase imaging.71
Another concept for enzyme detection is based on the self-immolative mechanism. Duimstra et al72 reported a Gd3+ complex with a self-immolative moiety, designed for the detection of β-glucuronidase. PARACEST agents with a response to enzymatic activity have also been reported. Yoo and Pagel73 and Yoo et al74 used a thulium-DOTA-monoamide complex containing a peptide chain, which is hydrolyzed by the caspase-3 enzyme. Following enzymatic cleavage, the PARACEST effect originating from the amide proton disappears due to the hydrolysis of the amide bond.
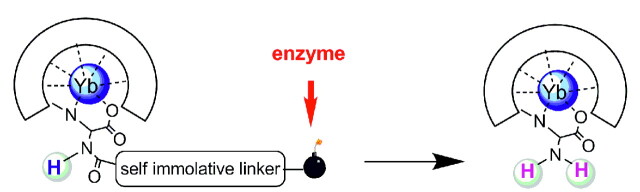
In another example, the lanthanide complex has been linked to an enzyme-specific substrate via a self-immolative spacer (Fig 5).75 With benzyloxycarbamates as a self-immolative unit, the substrate can be any enzyme-recognized moiety capable of transitionally reducing the electron donor capabilities of the phenyl substituent. The enzymatic cleavage of the substrate initiates an electron cascade and leads to the spontaneous elimination of the spacer. This results in the appearance of a PARACEST effect attributed to the exchange of the amine protons. This platform represents several positive features: the substrate is at the extremity of a spacer, which facilitates the enzymatic cleavage, and the PARACEST properties will not be affected by the variation of the substrate. Because the PARACEST effect is observed after enzymatic activation, it can be optimized once for the whole family. The same lanthanide chelate and spacer can be applied for the detection of diverse enzymes, by varying the substrate. The system works as a switch off-on probe, which can be a further advantage in practical in vivo or in vitro applications.
Fig 5.
An example of an enzyme-activated PARACEST contrast agent: The enzyme specifically reacts with the substrate leading to the spontaneous elimination of the self-immolative linker. This gives rise to a change in the PARACEST properties.
Redox Responsive Agents
It is widely believed that hypoxia is an important factor governing tumor aggressiveness and that hypoxic tissue is more resistant to conventional therapeutics.76–78 Methods for imaging tumor redox status would allow the noninvasive application of this potential biomarker of tumor sensitivity to existing and novel chemotherapies, as well as radiation therapy. The utility of such methods could also extend to other pathologies, such as cardiovascular disease, because free radical formation is associated with deleterious effects on the coronary microcirculation during recovery from myocardial infarction.79
Hypoxia is mostly detected by imaging techniques including positron-emission tomography and BOLD MR imaging. Reports on redox-sensitive MR imaging contrast agents have been rather scarce. The simplest design of a redox-responsive MR imaging contrast agent could be based on metal complexes whose metal ion can be reduced or oxidized depending on the biologic environment in which the 2 oxidation forms have different relaxation properties. Thus, the 2 redox states influence the proton relaxation of the surrounding protons to a different extent, resulting in different image intensities. In this perspective, the adducts formed between tpps complexes of Mn (III) and Mn (II) and poly-β-cyclodextrin have been reported to have considerably different relaxivities depending on the redox state of the metal, itself determined by the partial oxygen pressure of the solution.80 More recently, DOTA-based complexes of Gd bearing a thiol moiety were synthesized, and it was hypothesized that these complexes would form reversible covalent linkages with HSA, which contains a reactive thiol at cysteine-34. This redox-sensitive reversible binding of Gd complexes to plasma albumin could be exploited for imaging the tissue-redox state.81
Recently, the first example of a redox-active responsive PARACEST agent has been reported by Ratnakar et al.82 The system is based on a DOTA-derivative Eu complex bearing a nitro group, which can be reduced under hypoxic conditions to an amine. The authors demonstrated that there is a clear difference in the PARACEST signal intensity between the oxidized nitro and the reduced amino derivative. They suggest that the PARACEST difference generated by the chemically reducible probe could be exploited to design biologically applicable redox-responsive imaging agents.
Conclusions
A large number of activatable smart Gd3+-based or PARACEST MR imaging probes with potential utility for molecular neuroimaging applications have been reported in the literature. So far only a very few of them have progressed to in vivo tests. Future work will likely focus on extending applications of these agents to living animals, as well as on exploring new ways of creating molecular MR imaging probes to meet requirements such as higher specificity or lower detection limits. Ultimately these advances may allow spotting disease-causing abnormalities before any symptoms surface. Molecular imaging agents could speed up the advent of personalized medicine, in which therapies are tailored to individual patients and doctors can monitor the impact of treatment on a molecular scale.
Abbreviations
- BBB
blood-brain barrier
- BOLD
blood oxygen level−dependent
- bpy
bipyridine
- C
carbon
- Ca
calcium
- CEST
chemical exchange saturation transfer
- CO3
carbonate
- Cu
copper
- DTPA
diethylene-triamine pentaacetic acid
- DOTA
1,4,7,10-tetraaza-1,4,7,10-tetrakis(carboxymethyl)cyclododecane
- Eu
europium
- Fe
iron
- Gd
gadolinium
- H
hydrogen
- HSA
human serum albumin
- kex
exchange rate
- M
metal ion
- Mn
manganese
- N
nitrogen
- O
oxygen
- OH
hydroxide
- PARACEST
paramagnetic CEST
- PO4
phosphate
- q
number of water molecules coordinated to Gd3+
- T1,2e
electron spin relaxation times
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylene-diamine
- tpps
5,10,15,20-tetrakis-(p-sulfonatophenyl porphinate
- tpy
terpyridine
- Yb
ytterbium
- Zn
zinc
- tauR
reorientational correlation time of the molecule
Footnotes
This work was supported by the National Institute of Cancer, La Ligue contre le Cancer and Agence Nationale de la Recherche, France; as well as the European Cooperation in Science and Technology Action D38 “Metal-Based Systems for Molecular Imaging Applications.”
References
- 1. Merbach AE, Toth E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. Chichester, UK: John Wiley & Sons; 2001. [Google Scholar]
- 2. Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 1999;99:2293–352 [DOI] [PubMed] [Google Scholar]
- 3. Allen MJ, Meade TJ. Magnetic resonance contrast agents for medical and molecular imaging. Met Ions Biol Syst 2004;42:1–38 [PubMed] [Google Scholar]
- 4. Aime S, Delli Castelli D, Terreno E. Novel pH-reporter MRI contrast agents. Angew Chem Int Ed Engl 2002;41:4334–36 [DOI] [PubMed] [Google Scholar]
- 5. Zhang S, Winter P, Wu K, et al. A novel europium(III)-based MRI contrast agent. J Am Chem Soc 2001;123:1517–18 [DOI] [PubMed] [Google Scholar]
- 6. Woods M, Woessner DE, Sherry AD. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem Soc Rev 2006;35:500–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woessner DE, Zhang S, Merritt ME, et al. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med 2005;53:790–99 [DOI] [PubMed] [Google Scholar]
- 8. Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng 2008;10:391–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammoud DA, Hoffman JM, Pomer MG. Molecular neuroimaging: from conventional to emerging techniques. Radiology 2007;245:21–42 [DOI] [PubMed] [Google Scholar]
- 10. Jasanoff A. MRI contrast agents for functional molecular imaging of brain activity. Curr Op Neurobiol 2007;17:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Que LE, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 2008;108:1517–49 [DOI] [PubMed] [Google Scholar]
- 12. Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008;453:869–78 [DOI] [PubMed] [Google Scholar]
- 13. Waters J, Larkum M, Sakmann B, et al. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo. J Neurosci 2003;23:8558–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerr JND, Greenberg D, Helmchen F. From the cover: imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci U S A 2005;102:14063–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohki K, Chung S, Ch'ng YH, et al. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 2005;433:597–603. Epub 2005 Jan 19 [DOI] [PubMed] [Google Scholar]
- 16. Somjen GG. Ions in the Brain: Normal Function, Seizures, and Stroke. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 17. Koller CW. Neuroprotective therapy for Parkinson's disease. Exp Neurol 1997;144:24–28 [DOI] [PubMed] [Google Scholar]
- 18. Panov AV, Burke JR, Strittmatter WJ, et al. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch Biochem Biophys 2003;410:1–6 [DOI] [PubMed] [Google Scholar]
- 19. Yan HD, Lim W, Lee KW, et al. Sera from amyotrophic lateral sclerosis patients reduce high-voltage activated Ca2+ currents in mice dorsal root ganglion neurons. Neurosci Lett 1997;235:69–72 [DOI] [PubMed] [Google Scholar]
- 20. Jimerson DC, Post RM, Carman JS, et al. CSF calcium: clinical correlates in affective illness and schizophrenia. Biol Psychiatry 1979;14:37–51 [PubMed] [Google Scholar]
- 21. Yarlagadda A. Role of calcium regulation in pathophysiology model of schizophrenia and possible interventions. Med Hypotheses 2002;58:182–86 [DOI] [PubMed] [Google Scholar]
- 22. Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta 2002;1600:38–44 [DOI] [PubMed] [Google Scholar]
- 23. Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med 1997;38:378–88 [DOI] [PubMed] [Google Scholar]
- 24. Koretsky AP, Silva AC. Manganese-enhanced magnetic resonance imaging (MEMRI). NMR Biomed 2004;17:527–31 [DOI] [PubMed] [Google Scholar]
- 25. Atanasijevic T, Shusteff M, Fam P, et al. Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc Natl Acad Sci U S A 2006;103:14707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shapiro MG, Atanasijevic T, Faas H, et al. Dynamic imaging with MRI contrast agents: quantitative considerations. Magn Res Imaging 2006;24:449–62 [DOI] [PubMed] [Google Scholar]
- 27. Li W, Fraser SE, Meade TJ. A calcium-sensitive magnetic resonance imaging contrast agent. J Am Chem Soc 1999;12:1413–14 [Google Scholar]
- 28. Dhingra K, Fousková P, Angelovski G, et al. Towards extracellular Ca2+ sensing by MRI: synthesis and calcium-dependent 1H and 17O relaxation studies of two novel bismacrocyclic Gd3+ complexes. J Biol Inorg Chem 2008;13:35–46. Epub 2007 Sep 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mishra A, Fousková P, Angelovski G, et al. Facile synthesis and relaxation properties of novel bispolyazamacrocyclic Gd(3+) complexes: an attempt towards calcium-sensitive MRI contrast agents. Inorg Chem 2008;47:1370–81. Epub 2008 Mar 28 [DOI] [PubMed] [Google Scholar]
- 30. Angelovski G, Fousková P, Mamedov I, et al. Smart MRI agents sensing extracellular calcium fluctuations. Chembiochem 2008;9:1729–34 [DOI] [PubMed] [Google Scholar]
- 31. Dhingra K, Maier ME, Beyerlein M, et al. Synthesis and characterization of a smart contrast agent sensitive to calcium. Chem Commun (Camb) 2008;3444–46. Epub 2008 Jun 4 [DOI] [PubMed] [Google Scholar]
- 32. Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans 2009:1080–94. Epub 2008 Nov 26 [DOI] [PubMed] [Google Scholar]
- 33. Hanaoka K, Kikuchi K, Urano Y, et al. Selective sensing of zinc ions with a novel magnetic resonance imaging contrast agent. J Chem Soc Perkin Trans 2 2001:1840 [Google Scholar]
- 34. Hanaoka K, Kikuchi K, Urano Y, et al. Design and synthesis of a novel magnetic resonance imaging contrast agent for selective sensing of zinc ion. Chem Biol 2002;9:1027–32 [DOI] [PubMed] [Google Scholar]
- 35. Major JL, Boiteau RM, Meade TJ. Mechanisms of ZnII-activated resonance imaging agents. Inorg Chem 2008;47:10788–95. Epub 2008 Oct 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Major JL, Parigi G, Luchinat C, et al. The synthesis and in vitro testing of a zinc-activated MRI contrast agent. Proc Natl Acad Sci U S A 2007;104:13881–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trokowski R, Ren J, Kalman FK, et al. Selective sensing of zinc ions with a PARACEST contrast agent. Angew Chem Int Ed Engl 2005;44:6920–23 [DOI] [PubMed] [Google Scholar]
- 38. Paris J, Gameiro C, Humblet V, et al. Auto-assembling of ditopic macrocyclic lanthanide chelates with transition-metal ions: rigid multimetallic high relaxivity contrast agents for magnetic resonance imaging. Inorg Chem 2006;45:5092–102 [DOI] [PubMed] [Google Scholar]
- 39. Livramento JB, Toth E, Sour A, et al. High relaxivity confined to a small molecular space: a metallostar-based, potential MRI contrast agent. Angew Chem Int Ed Engl 2005;44:1480–84 [DOI] [PubMed] [Google Scholar]
- 40. Livramento JB, Sour A, Borel A, et al. A starburst-shaped heterometallic compound incorporating six densely packed Gd(3+) ions. Chemistry 2006;12:989–1003 [DOI] [PubMed] [Google Scholar]
- 41. Livramento JB, Weidensteiner C, Prata MI, et al. First in vivo MRI assessment of a self-assembled metallostar compound endowed with a remarkable high field relaxivity. Contrast Media Mol Imaging 2006;1:30–39 [DOI] [PubMed] [Google Scholar]
- 42. Parac-Vogt TN, Vander Elst L, Kimpe K, et al. Pharmacokinetic and in vivo evaluation of a self-assembled gadolinium(III)-iron(II) contrast agent with high relaxivity. Contrast Med Mol Imaging 2006;1:267–78 [DOI] [PubMed] [Google Scholar]
- 43. Jacques V, Desreux JF. New classes of MRI contrast agents. Top Curr Chem 2002;221:125–64 [Google Scholar]
- 44. Aime S, Botta M, Fasano M, et al. Paramagnetic GdIII-FeIII heterobimetallic complexes of DTPA-bis-salicylamide. Spectrochimica Acta 1993;9:1315–22 [Google Scholar]
- 45. Gaggelli E, Kozlowsky H, Valensin D, et al. Copper homeostasis and neurodegenerative disorders (Alzheimer's prion, and Parkinson's diseases and amyotrophic lateral sclerosis). Chem Rev 2006;106:1995–2044 [DOI] [PubMed] [Google Scholar]
- 46. Que EL, Chang CJ. A smart magnetic resonance contrast agent for selective copper sensing. J Am Chem Soc 2006;128:15942–43 [DOI] [PubMed] [Google Scholar]
- 47. Gillies RJ, Raghunand N, Karczmar GS, et al. MRI of the tumor microenvironment. J Magn Reson Imaging 2002;16:430–50 [DOI] [PubMed] [Google Scholar]
- 48. Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 2003;83:1183–221 [DOI] [PubMed] [Google Scholar]
- 49. Kalman FK, Woods M, Caravan P, et al. Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH. Inorg Chem 2007;46:5260–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aime S, Crich SG, Gianolio E, et al. High sensitivity lanthanide(III) based probes for MR-medical imaging. Coord Chem Rev 2006;250:1562–79 [Google Scholar]
- 51. Mikawa M, Miwa N, Brautigam M, et al. A pH-sensitive contrast agent for functional magnetic resonance imaging (MRI). Chem Lett 1998;7:693–94 [Google Scholar]
- 52. Aime S, Crich SG, Botta M, et al. A macromolecular Gd(III) complex as pH-responsive relaxometric probe for MRI applications. Chem Commun 1999:1577–78 [Google Scholar]
- 53. Lowe MP, Parker D, Reany O, et al. pH-dependent modulation of relaxivity and luminescence in macrocyclic gadolinium and europium complexes based on reversible intramolecular sulfonamide ligation. J Am Chem Soc 2001;123:7601–09 [DOI] [PubMed] [Google Scholar]
- 54. Hovland R, Glogard C, Aasen AJ, et al. Gadolinium DO3A derivatives mimicking phospholipids; preparation and in vitro evaluation as pH responsive MRI contrast agents. J Chem Soc Perkin Trans 2 2001;6:929–33 [Google Scholar]
- 55. Toth E, Bolskar RD, Borel A, et al. Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. J Am Chem Soc 2005;127:799–805 [DOI] [PubMed] [Google Scholar]
- 56. Zhang S, Wu K, Sherry AD. A novel pH-sensitive MRI contrast agent. Angew Chem Int Ed Engl 1999;38:3192–94 [PubMed] [Google Scholar]
- 57. Raghunand N, Howison C, Sherry AD, et al. Renal and systemic pH imaging by contrast-enhanced MRI. Magn Reson Med 2003;49:249–57 [DOI] [PubMed] [Google Scholar]
- 58. Garcia-Martin ML, Martinez GV, Raghunand N, et al. High-resolution pH(e) imaging of rat glioma using pH-dependent relaxivity. Magn Reson Med 2006;55:309–15 [DOI] [PubMed] [Google Scholar]
- 59. Ali MM, Woods M, Caravan P, et al. Synthesis and relaxometric studies of a dendrimer-based pH-responsive MRI contrast agent. Chemistry 2008;14:7250–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn Reson Med 2000;44:799–802 [DOI] [PubMed] [Google Scholar]
- 61. Aime S, Barge A, Delli Castelli D, et al. Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn Reson Med 2002;47:639–48 [DOI] [PubMed] [Google Scholar]
- 62. Terreno E, Delli Castelli D, Cravotto G, et al. Ln(III)-DOTAMGly complexes: a versatile series to assess the determinants of the efficacy of paramagnetic chemical exchange saturation transfer agents for magnetic resonance imaging applications Invest Radiol 2004;39:235–43 [DOI] [PubMed] [Google Scholar]
- 63. Moats RA, Fraser SE, Meade TJ. A “smart” magnetic resonance imaging agent that reports on specific enzymatic activity. Angew Chem Int Ed Engl 1997;36:726–28 [Google Scholar]
- 64. Louie AY, Hüber MM, Ahresn ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol 2000;18:321–25 [DOI] [PubMed] [Google Scholar]
- 65. Anelli PL, Bertini I, Fragai M, et al. Sulfonamide-functionalized gadolinium DTPA complexes as possible contrast agents for MRI: a relaxometric investigation. Eur J Inorg Chem 2000:625–30 [Google Scholar]
- 66. Nivorozhkin AL, Kolodziej AF, Caravan P, et al. Enzyme-activated Gd(III) magnetic resonance imaging contrast agents with a prominent receptor-induced magnetization enhancement. Angew Chem Int Ed Engl 2001;40:2903–06 [DOI] [PubMed] [Google Scholar]
- 67. Lauffer RB, McMurry TJ, Dunham SO, et al. Epix Medical Inc. Bioactivated diagnostic imaging contrast agents. Patent publication number WO9736619. October 9, 1997.
- 68. Mazooz G, Mehlman T, Lai TS, et al. Development of magnetic resonance imaging contrast material for in vivo mapping of tissue transglutaminase activity. Cancer Res 2005;65:1369–75 [DOI] [PubMed] [Google Scholar]
- 69. Shiftan L, Israely T, Cohen M, et al. Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma. Cancer Res 2005;65:10316–23 [DOI] [PubMed] [Google Scholar]
- 70. Chen JW, Pham W, Weissleder R, et al. Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn. Reson Med 2004;52:1021–28 [DOI] [PubMed] [Google Scholar]
- 71. Querol M, Chen JW, Weissleder R, et al. DTPA-bisamide-based MR sensor agents for peroxidase imaging. Org Lett 2005;7:1719–22 [DOI] [PubMed] [Google Scholar]
- 72. Duimstra JA, Femia FJ, Meade TJ. A gadolinium chelate for detection of beta-glucuronidase: a self-immolative approach. J Am Chem Soc 2005;127:12847–55 [DOI] [PubMed] [Google Scholar]
- 73. Yoo B, Pagel MD. A PARACEST MRI contrast agent to detect enzyme activity. J Am Chem Soc 2006;128:14032–33 [DOI] [PubMed] [Google Scholar]
- 74. Yoo B, Raam MS, Rosenblum RM, et al. Enzyme-responsive PARACEST MRI contrast agents: new biomedical imaging approach for studies of the proteasome. Contrast Media Mol Imaging 2007;2:189–98 [DOI] [PubMed] [Google Scholar]
- 75. Chauvin T, Durand P, Bernier M, et al. Detection of enzymatic activity by PARACEST MRI: a general approach to target a large variety of enzymes. Angew Chem Int Ed Engl 2008;47:4370–72 [DOI] [PubMed] [Google Scholar]
- 76. Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol 2003;76:S11–S22 [DOI] [PubMed] [Google Scholar]
- 77. Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol 2000;76:589–605 [DOI] [PubMed] [Google Scholar]
- 78. Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat 2000;3:39–47 [DOI] [PubMed] [Google Scholar]
- 79. Matsumoto H, Inoue N, Takaoka H, et al. Depletion of antioxidants is associated with no-reflow phenomenon in acute myocardial infarction. Clin Cardiol 2004;27:466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aime S, Botta M, Gianolio E, et al. A pO2-responsive MRI contrast agent based on the redox switch of manganese (II/III)-porphyrin complexes. Angew Chem Int Ed 2000;39:747–50 [DOI] [PubMed] [Google Scholar]
- 81. Raghunand N, Jagadish B, Trouard TP, et al. Redox-sensitive contrast agents for MRI based on reversible binding of thiols to serum albumin. Magn Reson Med 2006;55:1272–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ratnakar SJ, Woods M, Lubag AJ, et al. Modulation of water exchange in europium(III) DOTA-tetraamide complexes via electronic substituent effects. J Am Chem Soc 2008;130:6–7. Epub 2007 Dec 8 [DOI] [PMC free article] [PubMed] [Google Scholar]