Abstract
BACKGROUND AND PURPOSE:
Cranial abnormalities, including CND, are common in children with ANSD. The purpose of this study was to assess whether CND is associated with brain or inner ear abnormalities in a cohort of children with ANSD.
MATERIALS AND METHODS:
Two neuroradiologists retrospectively reviewed cranial MR imaging examinations in 103 children with ANSD. Brain, cochlear nerve, and temporal bone abnormalities were described and tabulated. Findings were stratified on the basis of the presence and laterality of CND, and differences in the presence of associated inner ear or intracranial abnormalities were assessed by using 2-tailed Fisher exact tests.
RESULTS:
CND was identified in 33.0% of children and 26.9% of ears with ANSD. Significantly more patients with bilateral CND had intracranial abnormalities than those with unilateral CND (60.0% versus 15.8%; P = .012). Forty percent of patients with bilateral CND, 0% of patients with unilateral CND, and 10.1% of those without CND demonstrated hindbrain malformations. Patients with bilateral CND were more likely to demonstrate hindbrain malformations than patients with normal nerves (P = .01) or unilateral CND (P = .004). Labyrinthine abnormalities were significantly more common in patients with bilateral CND than in those without CND (P ≤ .001). Cochlear anomalies were more common in patients with bilateral versus unilateral CND (P = .01). IAC and cochlear aperture stenosis were more common in those with unilateral and bilateral CND than those without CND (both P < .001).
CONCLUSIONS:
Cochlear and hindbrain abnormalities are significantly more common among patients with ANSD with bilateral CND compared with those with at least 1 intact cochlear nerve.
ANSD is a relatively new term used to describe the auditory characteristics of hearing-impaired patients who exhibit normal cochlear outer hair cell function but aberrant or disordered neural conduction in other sites deep to the cochlea along the auditory pathway. Difficulty hearing noise, fluctuating hearing sensitivity, and speech perception performance which is not easily predicted on the basis of the level of residual hearing, are cited as findings.1–3 ANSD accounts for 10%–15% of newly identified cases of hearing loss among young children.3–5
It has been posited that lesions in the inner hair cells, the synapse between the inner hair cell and the auditory nerve, or the auditory nerve itself may account for the clinical findings of ANSD.2,6,7 In fact, lesions throughout the entire auditory stream have been identified in patients with the auditory characteristics of the disorder. These lesions can be congenital or acquired and can be part of a known clinical syndrome or can be seen in isolation. With the breadth of possible pathophysiologic mechanisms, ANSD can manifest with a wide variety and degree of symptoms and findings,8 but these various phenotypes may exhibit indistinguishable audiometric and electrophysiologic results (ie, the presence of otoacoustic emissions and/or a cochlear microphonic with absent or grossly abnormal auditory brain stem responses).9
Recently, we reported finding a high prevalence of brain and cochlear nerve pathology on cranial MR imaging in a large cohort of children with ANSD. Brain abnormalities were evident in approximately 40%, and cochlear nerve anomalies were present in 28%. Labyrinthine abnormalities were notably less common, occurring in roughly 10%.10 CND, defined as a small or absent cochlear nerve, represents a severe and literal form of ANSD. The reported prevalence of CND in children with ANSD is between 18% and 28%10–12 and is higher than that in CND described in children with SNHL not distinguished as ANSD (between 6% and 16.1%).13–16 Several studies have suggested that inner ear and brain abnormalities are common among patients with CND,13,14,16–20 but only 1 of these studies has looked specifically at patients with ANSD. In that study, substantially more patients with CND had concomitant inner ear anomalies than patients with normal-appearing cochlear nerves.12 This is not surprising given the intimate relationship between the developing inner ear and the cochlear nerve in fetal life. Furthermore, cochlear nerve development also appears to be influenced by trophic effects of the brain stem.21 In light of this observation and given the relatively high prevalences of both brain and cochlear nerve anomalies we have observed in patients with ANSD, we hypothesized that abnormalities of brain development (in particular, hindbrain formation) would also be associated with CND in this population of patients.
In this report, we attempted to expand the findings of our previous report of cranial imaging findings in a large cohort of children with ANSD.10 The specific purpose of this study was to investigate whether CND is associated with either intracranial abnormalities or inner ear malformations detected on MR imaging in children with ANSD.
Materials and Methods
Subjects
Approval of this study was obtained from the Biomedical Institutional Review Board of our institution. Retrospective review of the databases of the Division of Audiology and our Children's Communication Disorders Program in the Department of Otolaryngology-Head and Neck Surgery was undertaken to identify children with ANSD. The diagnosis of ANSD was established audiologically by the presence of otoacoustic emissions and/or a cochlear microphonic in conjunction with aberrant or absent auditory brain stem responses. The diagnostic protocol used at our institution has been described in detail elsewhere.11,22
A total of 147 children with ANSD were identified for initial screening. The patients' electronic medical records were screened to determine which of them had cranial MR imaging examinations archived on our PACS. Of note, 6 additional MR imaging examinations not included in our initial report10 were identified and considered for inclusion in this study. In all, 113 children with ANSD underwent cranial MR imaging between 2002 and 2008.
MR Imaging Protocol
MR imaging was performed with a dedicated pediatric vestibulocochlear nerve (CNVIII) protocol, on either a 1.5T scanner (Sonata, Avanto, Vision, or Symphony; Siemens Medical Solutions, Malvern, Pennsylvania) or a 3T scanner (Magnetom Trio, Siemens Medical Solutions) by using a single- or 12-channel head coil. The protocol includes axial and sagittal unenhanced T1-weighted images, axial T2-weighted images, and axial fluid-attenuated inversion recovery images through the entire brain, as well as high-resolution 3D CISS or RESTORE (Siemens Medical Solutions, Malvern, Pennsylvania) images through the temporal bones. Parameters for the CISS sequence varied by scanner (TR/TE/NEX, 5.42–12.25 ms/2.42–5.9 ms/1–2; FA, 50°–80°; FOV, 120–180 mm; matrix size, 256), with resultant near-isotropic voxel sizes ranging from 0.5 to 0.7 mm in length. The RESTORE sequence, which was performed only on the Sonata, was acquired with the following parameters: TR/TE/NEX, 1000 ms/136 ms/1; echo-train length, 21; FA, 180°; FOV, 140 mm; matrix size, 192, resulting in a voxel size of 0.7 mm. In most cases, patients were scanned under conscious sedation or general anesthesia. Total scanning time for each examination was approximately 20 minutes. The temporal bone sequences were reconstructed in the axial plane as well as in an oblique sagittal plane oriented perpendicular to the long axis of each IAC for viewing.
Image Review and Data Collection
MR images were consensus-reviewed on a clinical PACS station (Impax 5.0; Agfa, Ridgefield Park, New Jersey) by 2 board-certified neuroradiologists with Certificates of Added Qualification (B.Y.H. and M.C.), who were blinded to the side of hearing loss. Diagnostic quality was assessed for all studies, and scans were excluded if they were not performed by using the CNVIII protocol or if technical factors such as excessive patient motion resulted in nondiagnostic images. The reviewers examined the temporal bones for the presence of cochlear, vestibular, or SCC abnormalities as well as for enlargement of the endolymphatic duct and sac. These were classified as labyrinthine abnormalities.
Each IAC was then measured at the level of the porus acousticus from its posterior margin to the anterior wall of the IAC along a line orthogonal to the long axis of the IAC on the axial image giving the largest measurement. IACs with a diameter <2 mm were considered stenotic.23,24 Each IAC was then assessed for the presence of a cochlear nerve, which was subjectively categorized as normal, small, or absent. We designated the nerve as absent when it could not be identified on either the axial or oblique sagittal temporal bone images. The cochlear nerve was considered small if it was clearly visible but substantially smaller than the other nerves in the ipsilateral IAC or the cochlear nerve in the contralateral IAC. In those cases in which an IAC was too small to allow reliable assessment of its intracanalicular contents, the cochlear nerve was assumed to be absent. Finally, the readers subjectively assessed the status of the cochlear aperture (henceforth referred to as the BCNC) to determine whether it appeared normal, small, or atretic.
Cranial MR images were reviewed for concomitant brain or CSF space abnormalities, which were described and categorized into 4 subgroups: cerebral (forebrain) abnormalities, brain stem or cerebellar (mid- and hindbrain) abnormalities, CSF and ventricular abnormalities, and abnormalities of white matter and myelination. Abnormalities included in each of these subgroups are summarized in Table 1.
Table 1:
Categorization of intracranial anomalies seen on MR imaging studies
| Type of Brain Abnormality | |||
|---|---|---|---|
| Forebrain Developmental | Mid- or Hindbrain Developmental | CSF-Related | WM |
| Callosal dysgenesis | Pontine hypoplasia | Ventriculomegaly | Patchy foci of abnormal WM signal (nonspecific) |
| Cortical malformations (pachygyria) | Dandy-Walker malformation | Enlarged extra-axial CSF spaces | Delayed myelination |
| Gray matter heterotopias | Cerebellar hypoplasia | Arachnoid cysts | PVL |
| Porencephalic cysts | Vermian hypoplasia | ||
| Septo-optic dysplasia | Pontine tegmental cap dysplasia | ||
Data Analysis
For statistical analyses, the patients were divided into those with normal cochlear nerves by MR imaging and those with CND (defined as an absent or small cochlear nerve). The latter group was further subdivided into those with unilateral or bilateral CND. The proportion of patients with intracranial abnormalities, inner ear malformations, IAC stenosis, or BCNC stenosis was calculated for each group, and between-group differences were assessed by using 2-tailed Fisher exact tests. Statistical analyses were performed by using the Statistical Package for the Social Sciences, Version 16.0 (SPSS, Chicago, Illinois). A P value < .05 indicated a significant result. For post hoc tests, a Bonferroni correction was used to account for multiple comparisons with a resultant significant P value threshold of < .017.
Results
Of the 113 patients who underwent cranial MR imaging evaluation, 10 were excluded because their MR imaging studies were not performed by using the CNVIII protocol or because of excessive motion on the scans, leaving 103 patients for inclusion in the study. Patients ranged in age at the time of MR imaging from 11 weeks to 13.5 years, with a mean age of 2.31 ± 2.58 years. Forty-six (44.7%) patients were female. Eight patients were diagnosed with specific genetic or syndromic disorders, including 2 patients with CHARGE syndrome, 1 with Turner syndrome, 1 with Rett syndrome, 1 with Peters anomaly, 1 with Cornelia de Lange syndrome, 1 with congenital 21-hydroxylase deficiency, and 1 with possible Usher syndrome.
Seventy-nine children (76.7%) had bilateral ANSD. Of the remaining 24 children, 14 had right-sided ANSD, and 10 had left-sided ANSD.
CND was identified in 34 patients, of whom 15 (14.6%) had bilateral CND and 19 (18.4%) had unilateral CND (12 right and 7 left). Four cochlear nerves were characterized as small (1 in a patient with bilateral CND, and 3 in patients with unilateral CND), and 45 cochlear nerves were absent (Fig 1). Two-thirds (16/24) of patients with unilateral ANSD had unilateral CND, which always corresponded to the side of hearing loss; none of the patients with unilateral ANSD had bilateral CND. Among those with bilateral ANSD, 15 (19.0%) had bilateral CND, and 3 (3.8%) had unilateral CND. Overall, 26.9% of ears affected by ANSD demonstrated CND. CND was observed in both patients with CHARGE syndrome (1 unilateral and 1 bilateral) and in the patient with Rett syndrome (bilateral).
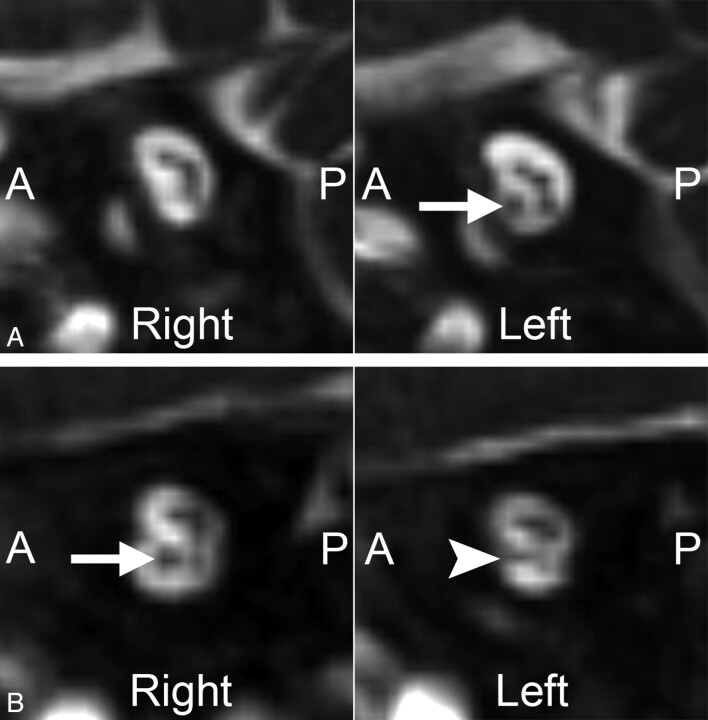
Fig 1.
Two cases of unilateral CND. Magnified oblique sagittal CISS images through the IACs in 2 different patients with ANSD. A, An 8-month-old boy with complete absence of the right cochlear nerve. Compare these findings with the normal-appearing cochlear nerve on the left (arrow). B, A 10-month-old girl with a hypoplastic left cochlear nerve. The left cochlear nerve (arrowhead) is present but is noticeably smaller than the normal-appearing right cochlear nerve (arrow).
Inner Ear Abnormalities
Abnormalities of the labyrinth (cochlea, vestibule, SCCs, or endolymphatic duct and sac) were present in 15 patients (14.6%). Cochlear malformations were noted in 11 patients (Table 2); these were all mild abnormalities of cochlear partitioning, such as partial modiolar deficiency or hypoplasia of the apical turn (Figs 2A and 3A). Three patients—2 of whom had CHARGE syndrome—demonstrated complete aplasia of the SCCs in their affected ears (Fig 4), and 3 patients demonstrated absent or severely malformed lateral SCCs only. All SCC abnormalities were associated with dysplastic vestibules—in 4 cases, the vestibules were enlarged, and in 2 cases, the vestibules were small. One patient demonstrated an enlarged vestibule with normal-appearing SCCs.
Table 2:
Inner ear/IAC abnormalities in children with ANSDa
| Type of Temporal Bone Abnormality | Cochlear Nerve Status |
||
|---|---|---|---|
| Normal (n = 69) | Unilateral CND (n = 19) | Bilateral CND (n = 15) | |
| None | 68 (98.6%)1,2 | 2 (10.5%)1 | 1 (6.7%)2 |
| Any labyrinthine abnormality | 1 (1.4%)3 | 3 (15.8%)4 | 11 (73.3%)3,4 |
| Cochlear malformations | 1 (1.4%)5 | 2 (10.5%)6 | 8 (53.3%)5,6 |
| Vestibular malformations | 0 (0.0%)7 | 2 (10.5%) | 5 (33.3%)7 |
| SCC malformations | 0 (0.0%)8 | 2 (10.5%) | 4 (26.7%)8 |
| Large endolymphatic duct/sac | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) |
| IAC stenosis/atresia | 0 (0.0%)9,10 | 5 (26.3%)9 | 7 (46.7%)10 |
| BCNC stenosis/atresia | 0 (0.0%)11,12 | 15 (78.9%)11 | 13 (86.7%)12 |
Superscripts denote significant group pair differences: 1–5, 7–12, P ≤ .001; 6, P =.010.
Fig 2.
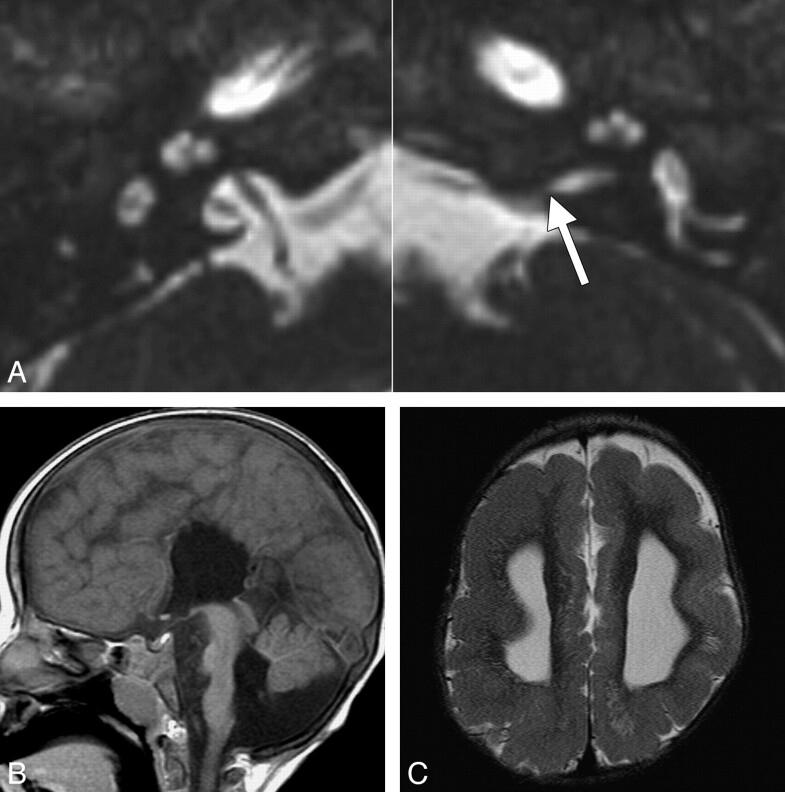
Inner ear and brain abnormalities in a 2-year-old girl with ANSD and bilateral CND. A, Axial CISS images through the bilateral inner ears and IACs. The left IAC is stenotic, particularly at the level of the porus acousticus (arrow), while the right IAC is normal in caliber. Both cochleae are isolated and dysplastic, with truncated apical turns, and the right modiolus is deficient. B, Midsagittal T1-weighted image demonstrates callosal agenesis, pontine hypoplasia, and inferior vermian hypoplasia. There is a prominent ventral cleft at the level of the pontomedullary junction. C, Axial T2-weighted image through the lateral ventricles demonstrates ventriculomegaly and diffuse pachygyria with relatively few sulci.
Fig 3.
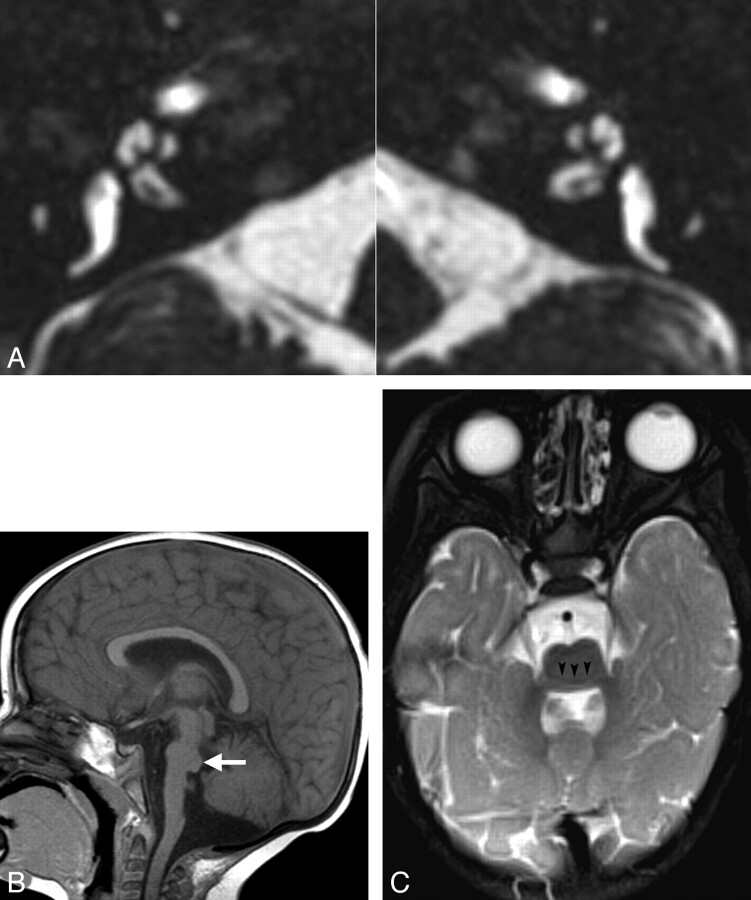
Inner ear and brain abnormalities in patients with bilateral CND and pontine segmental cap dysplasia. A, Axial CISS images through the inner ears in a 1-year-old girl. Both cochleae demonstrate deficient modioli and incomplete partitioning. The BCNCs are also small and possibly atretic. B, Sagittal midline T1-weighted image in a 2-year-old boy demonstrates characteristic pontine hypoplasia and an abnormal exophytic masslike band along the dorsal superior surface of the pons (white arrow). The vermis is also hypoplastic inferiorly. C, Axial T2-weighted image at the level of the MCPs in the same patient as A. The pons and MCPs are small, and there is a transversely oriented band along the dorsal pons, separated from the remainder of the pons by a hypointense horizontal cleft (black arrowheads).
Fig 4.
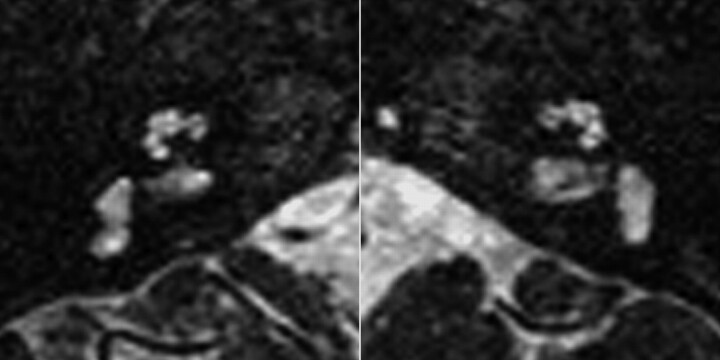
Vestibular and SCC abnormalities in a patient with bilateral CND. Axial CISS images in a 1-year-old girl with CHARGE syndrome. No semicircular canals are present, and both vestibules are enlarged and dysplastic. The right BCNC appears narrowed and the left BCNC is atretic.
Significantly more patients with CND demonstrated labyrinthine abnormalities compared with patients without CND (41.2% versus 1.4%, P < .001). Of the 49 CND-affected ears, 25 (51.0%) showed concomitant labyrinthine anomalies. Prevalences of inner ear abnormalities are summarized in Table 2.
IAC stenosis was present in 12 patients (11.7%) and was only seen in patients with CND (35.3% of patients with CND). When CND was classified by side, it was associated with a stenotic IAC in 16/49 ears (32.7%) because 3 patients with bilateral CND had IAC stenosis on only 1 side (Fig 2A). Four ears demonstrated an essentially atretic IAC with no discernible porus acousticus and only a small canal for the facial nerve.
Stenosis or atresia of the BCNC (Figs 2A, 3A, and 4) was by far the most common inner ear anomaly seen and was only seen in patients with CND (Table 2). Of the 49 ears with deficient cochlear nerves, 40 (81.6%) demonstrated small or atretic BCNCs (Figs 2A and 3A).
Only 1 patient with bilateral CND had normal temporal bones; the remaining 14 (93.3%) had bilateral labyrinthine, IAC, or BCNC abnormalities. In the group with unilateral CND, 15 (78.9%) had a unilateral abnormality, which was always ipsilateral to the side of CND, and 2 (10.5%) had bilateral abnormalities. Of the 49 ears with CND, 45 (91.8%) demonstrated an abnormality of the labyrinth, IAC, or BCNC.
Patients with bilateral CND were significantly more likely to demonstrate cochlear, vestibular, SCC, IAC, and BCNC abnormalities than patients without CND and were also significantly more likely to have cochlear malformations than patients with unilateral CND (all P values ≤ .01). Only IAC and BCNC stenoses were significantly more common in patients with unilateral CND than in patients without CND (both P < .001) (Table 2).
Intracranial Abnormalities
A summary of the intracranial abnormalities stratified by cochlear nerve status is provided in Table 3. When patients with CND (irrespective of laterality) were compared with those without CND, no significant difference was seen in the prevalence of intracranial abnormalities (35.3% versus 36.2%, respectively; P = 1.0). A significantly larger percentage of patients with bilateral CND had an intracranial abnormality on MR imaging compared with those with only 1 deficient nerve (P = .012). Although intracranial abnormalities were seen more often in patients with bilateral CND than in those with 2 normal cochlear nerves, the difference was not significant (P = .15).
Table 3:
Intracranial abnormalities in children with ANSDa
| Type of Intracranial Abnormality | Cochlear Nerve Status |
||
|---|---|---|---|
| Normal (n = 69) | Unilateral CND (n = 19) | Bilateral CND (n = 15) | |
| None | 44 (63.8%) | 16 (84.2%) | 6 (40.0%) |
| Any intracranial abnormality | 25 (36.2%) | 3 (15.8%)1 | 9 (60.0%)1 |
| Forebrain malformations | 3 (4.3%) | 0 (0.0%) | 4 (26.7%) |
| Mid-/hindbrain malformations | 7 (10.1%)2 | 0 (0.0%)3 | 6 (40.0%)2,3 |
| CSF abnormalities | 12 (17.4%) | 3 (11.7%) | 4 (26.7%) |
| Abnormalities of WM | 16 (23.2%) | 0 (0.0%) | 2 (13.3%) |
Superscripts denote significant group pair differences: 1, P = .012; 2, P = .010; 3, P = .004.
Among patients with 2 normal cochlear nerves, abnormal cerebral white matter was the most common finding, occurring in 23.2% of patients. Three of these patients (4.3%) had forebrain abnormalities, which included callosal dysgenesis in 2 patients and subependymal gray matter heterotopia in 1 patient. Hindbrain malformations were seen in 7 patients with normal cochlear nerves and included 5 cases of pontine hypoplasia, 5 cases of cerebellar or vermian hypoplasia, and 1 Chiari I malformation.
Only CSF-related abnormalities were seen in the patients with unilateral CND, with ventriculomegaly noted in 1 case and prominence of the extra-axial CSF-containing spaces in the other 2.
Abnormalities of hindbrain development were the most common intracranial findings among those with bilateral CND, occurring in 40%. All of these patients demonstrated brain stem malformations, which typically included some degree of pontine hypoplasia (Figs 2 and 3). Two patients had features consistent with the recently described entity known as “pontine tegmental cap dysplasia,” which is characterized by a hypoplastic pons with an exophytic masslike band of white matter coursing along its dorsal superior aspect (Fig 3).25,26 Three patients had hypoplasia of the cerebellar hemispheres or vermis, including 1 patient with a Dandy-Walker malformation associated with significant hypoplasia of the entire brain stem and cerebellum. Supratentorial abnormalities in patients with bilateral CND included 2 cases of callosal dysgenesis/agenesis—1 of which was associated with diffuse pachygyria (Fig 2), 1 case of septo-optic dysplasia, and 1 case of gross microcephaly without other obvious abnormalities in a patient diagnosed with Rett syndrome. White matter abnormalities were seen in only 2 patients with bilateral CND—1 with PVL and the second with greater than expected symmetric nonspecific periatrial white matter hyperintensities on T2-weighted images.
Hindbrain abnormalities were significantly more prevalent in patients with bilateral CND than in both patients with normal cochlear nerves (P = .01) and patients with unilateral CND (P = .004).
The combination of both inner ear and intracranial abnormalities was seen in 60.0% of patients with bilateral CND, 15.8% of patients with unilateral CND, and none of the patients with 2 normal cochlear nerves.
Discussion
In this study, we report a significant association between bilateral CND and the presence of labyrinthine and hindbrain malformations in children with ANSD. Glastonbury et al19 reported temporal bone abnormalities in 100% of CND-affected ears; this rate is similar to that of abnormalities (91.8%) in CND-affected ears in our cohort. However, narrowing or atresia of the BCNC made up a larger proportion of the abnormalities we observed, and we found a much lower prevalence of other labyrinthine anomalies among CND ears (51%) than did Glastonbury et al (94%). This discrepancy may be due to the fact that we looked exclusively at patients with ANSD, who, by definition, have audiometric or electrophysiologic evidence of preserved cochlear function and would, therefore, be more likely to have a normal-appearing cochlear apparatus. In fact, only 14.6% of our total sample had labyrinthine anomalies; by comparison, previous studies on imaging in SNHL not distinguished as ANSD report malformations in up to 32% of patients.13,16 Furthermore, cochlear abnormalities in our sample were limited to only mild defects of partitioning. It is possible that our threshold for diagnosing inner ear pathology on MR imaging was higher that used by Glastonbury et al, which could account for a lower rate of cochlear anomalies, but we do not believe that this alone would explain the discrepancy we observed.
Walton et al12 specifically examined patients with ANSD and found inner ear abnormalities (not including abnormalities of the BCNC) in 93.3% of CND-affected ears. Their cohort, unlike ours, included only patients with bilateral ANSD; by comparison, 19/34 CND-affected patients in our cohort had unilateral CND. This apparent difference in patient selection may explain our lower observed rates of inner ear abnormalities. Notably, 73.3% of patients with bilateral CND in our cohort had concomitant inner ear anomalies, compared with only 4.5% of patients with at least 1 normal-appearing cochlear nerve. Like Walton et al, we found a low prevalence of inner ear anomalies (1.4%) in patients who did not have CND, which suggests that in the absence of CND, inner ear anomalies are rare among patients with ANSD.
Perhaps most puzzling was the observation that both cochlear and brain stem malformations were significantly less common in patients with unilateral CND compared with those with bilateral CND. In fact, no hindbrain abnormalities were seen among patients in our sample with unilateral CND. We speculate that bilateral CND may result from an early developmental insult that affects both hindbrain and cochlear formation, while unilateral CND might result from a later localized insult limited to the cochlear inner hair cells, spiral ganglion, or the cochlear nerve itself. Our observations that a substantial proportion (84.2%) of patients with unilateral CND also had only ipsilateral hearing loss and that the combination of both inner ear and intracranial abnormalities was substantially more likely to be seen among the bilateral CND group seem to support this premise.
In human embryos, the otic placode begins to develop at the same time that rhombencephalic segmentation occurs, and the hindbrain likely plays a crucial role in otic placode specification. The cells giving rise to CNVIII delaminate from the otocyst by the fourth fetal week27 and undergo a sequence of events, which include cell proliferation, differentiation into cochlear and vestibular ganglion neurons, establishment of polarity with development of axonal processes toward the inner ear and the brain stem, and synaptogenesis.21 Experiments in insects have shown that path-finding properties of sensory neurons are determined both by global and local patterning processes and are mediated by transcription factors that underlie the developmental program of a specific sensory organ.21 Expression of certain neurotrophic factors by the otocyst—chief among these being brain-derived neurotrophic factor and neurotrophin-3—is required for early cell migration, neurite outgrowth, and overall survival of vestibular and cochlear ganglion cells; absence of these factors has been shown to result in loss of ganglion cells.28–31
The hindbrain also appears to play a role in CNVIII development, though to a lesser extent, and the exact signaling mechanisms underlying the trophic effects of the brain stem on cochlear ganglion cells and their axons are not understood.32 Centrally directed processes of cochlear ganglion cells arrive and contact the brain stem by the fifth-to-sixth gestational week,27 and cochlear nerve fibers begin to invade the cochlear nucleus by 16 weeks' gestation, subsequently forming synaptic connections.21 It is possible that cochlear nerve axons require the presence of an appropriate population of target cells (ie, the cochlear nuclei) and that the developing afferent neurons of CNVIII undergo apoptosis if synaptic connections with the cell bodies of the cochlear nuclei are not established.
It is likely that the spectrum of brain and inner ear abnormalities seen in patients with bilateral CND reflects a wide range of pathophysiologic processes of variable penetrance and severity that alters ≥1 of the early embryologic interactions described above. Special note should be made of the 2 patients in our cohort with features of pontine tegmental cap dysplasia, both of whom had bilateral CND. Individuals with this malformation present with multiple cranial neuropathies, including SNHL. Cranial MR imaging will show a hypoplastic pons with a variably shaped exophytic band of white matter composed of abnormal transversely oriented axons coursing along its dorsal superior aspect and absence of the normal transverse pontine fiber tract. It has been hypothesized that the malformation results from abnormal neuronal migration or axonal guidance.25,26 Of the patients in our cohort with pontine tegmental cap dysplasia, 1 demonstrated mild bilateral modiolar deficiency (Fig 3A) and stenotic bilateral IACs and BCNCs. The other patient had normal-appearing labyrinths but extremely small or atretic BCNCs; the IACs in this patient also appeared narrowed but measured greater than the 2-mm cutoff we set for IAC stenosis.
IAC and BCNC stenoses were common among patients with CND. It has been suggested that IAC formation is dependent on the presence of axons from CNVIII, which seem to inhibit cartilage formation at the medial aspect of the otic vesicle during the process of otic capsule chondrification.33 Glastonbury et al19 further hypothesized that the size of the IAC may depend on the volume of CNVIII fibers that traverse the canal during its formation. Along these lines, it is possible that the presence and size of the BCNC may depend on the degree to which the cochlear nerve develops in utero. We were able to identify the vestibular nerve in at least two-thirds of CND-affected ears; furthermore, in 75% of ears in which neither division of CNVIII could be identified, a patent (though frequently small) IAC was still present. These observations suggest that in most cases of ANSD with congenital CND, at least some portion of CNVIII is developed during fetal life. In the cases in which only the vestibular nerve is seen, the cochlear division either never forms (atretic BCNC) or degenerates (stenotic BCNC). In the cases in which both divisions of CNVIII are absent but the IAC is at least partially patent, some portion of the nerve was likely present during the completion of IAC formation but subsequently atrophied before or shortly after birth. Even in the 4 ears demonstrating an atretic porus acousticus, a fluid-filled fundal portion of the IAC was identified in all cases, suggesting that some vestibulocochlear ganglion cells initially developed but that the process of axonal growth was arrested before contact with the brain stem and completion of IAC canalization.
In both patients with ANSD and those with cochlear SNHL, preoperative identification of intact cochlear nerves is critical because cochlear implantation is contraindicated in those with bilateral CND.12,34,35 For these patients, ABI is now being investigated as a treatment option.36 Experience with ABI for CND is limited, and to date, to our knowledge, no studies have examined the influence of concomitant structural brain abnormalities on implant outcomes. We posit that the presence of brain stem malformations may predict poorer ABI outcomes, particularly if the abnormality involves the circuitry of the auditory pathway. Unfortunately, our study focused solely on gross morphologic changes identifiable on conventional MR imaging sequences. It would be interesting to examine patients with CND with diffusion tensor imaging to determine if these patients, and, in particular the patients with gross brain stem abnormalities, demonstrate significant alterations of the auditory pathway fibers. Recent studies have demonstrated decreased fractional anisotropy in the lateral lemnisci and inferior colliculi of patients with SNHL37 and patients with unilateral congenital CND.38
Several limitations of this study should be noted. First, biases inherent in the retrospective nature of the study could have influenced our results. Second, although MR imaging readers were blinded to the patients' clinical presentations, for obvious reasons, they could not be blinded to cochlear nerve status. Despite the readers' efforts to view each aspect of the MR imaging examinations independently, knowledge of cochlear nerve status could have affected their interpretations, especially given the subjective nature of the inner ear and brain assessments. Finally, because our analysis focused only on children with ANSD, our findings should not be generalized to all children with SNHL.
Conclusions
Patients with ANSD with bilateral CND are significantly more likely to demonstrate both cochlear and hindbrain malformations than other patients with ANSD. Therefore, MR imaging examinations of patients with ANSD with bilateral CND should be carefully scrutinized for concomitant anomalies. Although the exact mechanisms underlying these associations are not entirely known, alterations in the normal interactions between the developing inner ears, cochlear nerves, and rhombencephalon during early fetal life likely play major roles in the etiology of bilateral CND, while cases of unilateral CND may be due to more localized processes affecting only CNVIII. These findings may have clinical implications, particularly for individuals being considered for ABI, but further investigation into the effects of congenital brain malformations on implant outcomes is necessary.
Abbreviations
- A
anterior
- ABI
auditory brain stem implantation
- ANSD
auditory neuropathy spectrum disorder
- BCNC
bony cochlear nerve canal (cochlear aperture)
- CISS
constructive interference in steady state
- CNVIII
vestibulocochlear nerve (eighth cranial nerve)
- CND
cochlear nerve deficiency
- FA
flip angle
- IAC
internal auditory canal
- MCP
middle cerebellar peduncle
- P
posterior
- PVL
periventricular leukomalacia
- SCC
semicircular canal
- SNHL
sensorineural hearing loss
- WM
white matter
References
- 1. Buss E, Labadie RF, Brown CJ, et al. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol 2002;23:328–32 [DOI] [PubMed] [Google Scholar]
- 2. Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain 1996;119:741–53 [DOI] [PubMed] [Google Scholar]
- 3. Rance G, Beer DE, Cone-Wesson B, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 1999;20:238–52 [DOI] [PubMed] [Google Scholar]
- 4. Madden C, Rutter M, Hilbert L, et al. Clinical and audiological features in auditory neuropathy. Arch Otolaryngol Head Neck Surg 2002;128:1026–30 [DOI] [PubMed] [Google Scholar]
- 5. Kraus N, Ozdamar O, Stein L, et al. Absent auditory brain stem response: peripheral hearing loss or brain stem dysfunction? Laryngoscope 1984;94:400–06 [DOI] [PubMed] [Google Scholar]
- 6. Berlin CI, Hood L, Morlet T, et al. Auditory neuropathy/dys-synchrony: diagnosis and management. Ment Retard Dev Disabil Res Rev 2003;9:225–31 [DOI] [PubMed] [Google Scholar]
- 7. Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol 2003;13:452–58 [DOI] [PubMed] [Google Scholar]
- 8. Berlin CI, Morlet T, Hood LJ. Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatr Clin North Am 2003;50:331–40, vii–viii [DOI] [PubMed] [Google Scholar]
- 9. Rapin I, Gravel J. “Auditory neuropathy”: physiologic and pathologic evidence calls for more diagnostic specificity. Int J Pediatr Otorhinolaryngol 2003;67:707–28 [DOI] [PubMed] [Google Scholar]
- 10. Roche JP, Huang BY, Castillo M, et al. Imaging characteristics of children with auditory neuropathy spectrum disorder. Otol Neurotol 2010;31:780–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buchman CA, Roush PA, Teagle HF, et al. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear 2006;27:399–408 [DOI] [PubMed] [Google Scholar]
- 12. Walton J, Gibson WP, Sanli H, et al. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol 2008;29:302–09 [DOI] [PubMed] [Google Scholar]
- 13. Parry DA, Booth T, Roland PS. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otol Neurotol 2005;26:976–82 [DOI] [PubMed] [Google Scholar]
- 14. Simons JP, Mandell DL, Arjmand EM. Computed tomography and magnetic resonance imaging in pediatric unilateral and asymmetric sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 2006;132:186–92 [DOI] [PubMed] [Google Scholar]
- 15. Lapointe A, Viamonte C, Morriss MC, et al. Central nervous system findings by magnetic resonance in children with profound sensorineural hearing loss. Int J Pediatr Otorhinolaryngol 2006;70:863–68 [DOI] [PubMed] [Google Scholar]
- 16. McClay JE, Booth TN, Parry DA, et al. Evaluation of pediatric sensorineural hearing loss with magnetic resonance imaging. Arch Otolaryngol Head Neck Surg 2008;134:945–52 [DOI] [PubMed] [Google Scholar]
- 17. Carner M, Colletti L, Shannon R, et al. Imaging in 28 children with cochlear nerve aplasia. Acta Otolaryngol 2009;129:458–61 [DOI] [PubMed] [Google Scholar]
- 18. Casselman JW, Offeciers FE, Govaerts PF, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology 1997;202:773–81 [DOI] [PubMed] [Google Scholar]
- 19. Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol 2002;23:635–43 [PMC free article] [PubMed] [Google Scholar]
- 20. Westerhof JP, Rademaker J, Weber BP, et al. Congenital malformations of the inner ear and the vestibulocochlear nerve in children with sensorineural hearing loss: evaluation with CT and MRI. J Comput Assist Tomogr 2001;25:719–26 [DOI] [PubMed] [Google Scholar]
- 21. Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 2002;25:51–101 [DOI] [PubMed] [Google Scholar]
- 22. Buchman CA, Roush PA, Teagle HF, et al. Clinical management of children with auditory neuropathy. In: Eisenberg L. ed. Clinical Management of Children with Cochlear Implants. San Diego: Plural Publishing; 2009:633–54 [Google Scholar]
- 23. Baek SK, Chae SW, Jung HH. Congenital internal auditory canal stenosis. J Laryngol Otol 2003;117:784–87 [DOI] [PubMed] [Google Scholar]
- 24. Valvassori GE, Pierce RH. The normal internal auditory canal. Am J Roentgenol Radium Ther Nucl Med 1964;92:1232–41 [PubMed] [Google Scholar]
- 25. Barth PG, Majoie CB, Caan MW, et al. Pontine tegmental cap dysplasia: a novel brain malformation with a defect in axonal guidance. Brain 2007;130:2258–66 [DOI] [PubMed] [Google Scholar]
- 26. Jissendi-Tchofo P, Doherty D, McGillivray G, et al. Pontine tegmental cap dysplasia: MR imaging and diffusion tensor imaging findings of impaired axonal navigation. AJNR Am J Neuroradiol 2009;30:113–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore JK, Linthicum FH, Jr. The human auditory system: a timeline of development. Int J Audiol 2007;46:460–78 [DOI] [PubMed] [Google Scholar]
- 28. Hossain WA, Brumwell CL, Morest DK. Sequential interactions of fibroblast growth factor-2, brain-derived neurotrophic factor, neurotrophin-3, and their receptors define critical periods in the development of cochlear ganglion cells. Exp Neurol 2002;175:138–51 [DOI] [PubMed] [Google Scholar]
- 29. Bernd P. The role of neurotrophins during early development. Gene Expr 2008;14:241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fritzsch B, Silos-Santiago I, Bianchi LM, et al. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci 1997;20:159–64 [DOI] [PubMed] [Google Scholar]
- 31. Lefebvre PP, Leprince P, Weber T, et al. Neuronotrophic effect of developing otic vesicle on cochleo-vestibular neurons: evidence for nerve growth factor involvement. Brain Res 1990;507:254–60 [DOI] [PubMed] [Google Scholar]
- 32. Ard MD, Morest DK, Hauger SH. Trophic interactions between the cochleovestibular ganglion of the chick embryo and its synaptic targets in culture. Neuroscience 1985;16:151–70 [DOI] [PubMed] [Google Scholar]
- 33. McPhee JR, Van de Water TR. Epithelial-mesenchymal tissue interactions guiding otic capsule formation: the role of the otocyst. J Embryol Exp Morphol 1986;97:1–24 [PubMed] [Google Scholar]
- 34. Gray RF, Ray J, Baguley DM, et al. Cochlear implant failure due to unexpected absence of the eighth nerve: a cautionary tale. J Laryngol Otol 1998;112:646–49 [DOI] [PubMed] [Google Scholar]
- 35. Maxwell AP, Mason SM, O'Donoghue GM. Cochlear nerve aplasia: its importance in cochlear implantation. Am J Otol 1999;20:335–37 [PubMed] [Google Scholar]
- 36. Sennaroglu L, Ziyal I, Atas A, et al. Preliminary results of auditory brainstem implantation in prelingually deaf children with inner ear malformations including stenosis of the cochlear aperture and aplasia of the cochlear nerve. Otol Neurotol 2009;30:708–15 [DOI] [PubMed] [Google Scholar]
- 37. Lin Y, Wang J, Wu C, et al. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J Magn Reson Imaging 2008;28:598–603 [DOI] [PubMed] [Google Scholar]
- 38. Wu CM, Ng SH, Wang JJ, et al. Diffusion tensor imaging of the subcortical auditory tract in subjects with congenital cochlear nerve deficiency. AJNR Am J Neuroradiol 2009;30:1773–77 [DOI] [PMC free article] [PubMed] [Google Scholar]