Abstract
BACKGROUND AND PURPOSE:
Endovascular options for therapy for patients with vasospasm after SAH include angioplasty and intra-arterial vasodilator infusion. Preliminary studies of the effects of the calcium channel antagonist verapamil on angiographic vasospasm have yielded mixed and/or qualitative results. In this study, improvement in angiographic vasospasm after intra-arterial verapamil administration is demonstrated with quantitative, blinded methods.
MATERIALS AND METHODS:
This retrospective observational case series includes 12 patients with vasospasm after SAH who collectively received 16 treatments with intra-arterial verapamil during a 2-year period at our institution. The exclusion criterion was concurrent treatment with angioplasty. Blinded reviewers quantitatively evaluated angiograms from each patient and/or treatment after presentation with SAH and before and after intra-arterial treatment of vasospasm.
RESULTS:
Patients were treated with intra-arterial verapamil for vasospasm 9 ± 4 days after SAH with a range from 1 to 16 days. For the 34 arterial distributions treated, the segment with the worst angiographic vasospasm from each arterial distribution averaged 51 ± 13% stenosis, which improved to 29 ± 18% stenosis (P < .001). There was no significant difference in treatment effect in proximal arterial segments, which may be amenable to angioplasty, compared with distal segments (P > .05). There was no significant difference in treatment effect in arterial segments previously subjected to angioplasty compared with other segments (P > .05).
CONCLUSIONS:
Intra-arterially administered verapamil improves angiographic vasospasm after SAH when administered at 10 ± 3 mg per arterial distribution. Optimal dose, infusion rate, and retreatment interval remain to be determined. Randomized controlled trials are needed to prove efficacy in the treatment of clinical vasospasm.
The incidence of SAH is approximately 9 cases per 100,000 patient-years and accounts for approximately 3%–5% of strokes.1 Mortality is higher than that in other causes of stroke, and half of cases ultimately result in fatality. Of patients who survive longer than a few weeks, approximately one-third remain dependent.2 Much of the morbidity and mortality associated with SAH in the days after the initial event is secondary to cerebral vasospasm, which can be divided into angiographic vasospasm and clinical vasospasm. “Angiographic vasospasm” refers to stenosis in the cerebral arteries, visualized by conventional angiography or CT angiography that is secondary to nearby subarachnoid blood products. The neurologic deficits attributable to this phenomenon are termed “clinical vasospasm” or “delayed ischemic neurologic deficits.”
The mainstays of vasospasm prevention and treatment include systemic administration of nimodipine and optimization of factors that affect cerebral blood flow, including hemodynamic therapy (ie, HHH).3–5 Endovascular therapies are typically used when conventional measures fail and include angioplasty and selective intra-arterial vasodilator infusion. Due to mechanical considerations, angioplasty is limited in application to the proximal arteries roughly ≥2 mm in diameter. Selective intra-arterial administration of pharmacologic agents allows simultaneous treatment of an entire arterial distribution, including distal vessels not accessible by angioplasty. The most studied intra-arterial pharmacologic agent until recent years was papaverine, but its use has decreased secondary to its short duration of action and reports of complications. Interest has since increased in the intra-arterial use of calcium channel antagonists, including verapamil, nimodipine, and nicardipine.6
Preliminary studies of the effects of intra-arterial verapamil on angiographic vasospasm have yielded mixed and often poorly quantified results.7–10 In 1 quantitative case series by Mazumdar et al9 using blinded reviewers, there was no statistically significant change in the diameters of vasospastic cerebral arteries after treatment. Three other case series reported increased diameters of treated vasospastic arteries, but these studies did not use blinded reviewers. One of the studies assigned subjective grades of angiographic vasospasm rather than applying specific measurements.8 Another study reported only binary results in which angiographic vasospasm was described as >50% before treatment in all cases, and treatment resulting in residual vasospasm of any degree <50% was reported as “completely effective.”10 The diagnostic method of grading angiographic vasospasm by eye has been found to be unreliable, and clear-cut definitions of vessel stenosis are essential.11 Bias in the data can be decreased by use of blinded reviewers. Certainly, quantitative data are requisite for any comparison between treatment methods or schedules with the goal of improving delivery of future care. In this report, blinded reviewers systematically analyzed DSA from 12 patients to quantify the effects of intra-arterial verapamil therapy on angiographic vasospasm.
Materials and Methods
This retrospective observational case series included all patients treated with intra-arterial verapamil for cerebral vasospasm between January 1, 2006, and December 31, 2008, at our institution, where follow-up angiography was performed ≥20 minutes after treatment. The exclusion criterion was treatment of vasospasm with angioplasty during the same procedure. There was no control patient population. All verapamil infusions were performed through catheters with tips placed in the distal cervical ICAs or cervical VAs.
For each patient and/or intra-arterial verapamil treatment, cerebral DSAs were analyzed at 3 time points, including time of presentation with SAH, time of diagnosis of angiographic vasospasm immediately before treatment with intra-arterial verapamil, and 20–35 minutes after treatment with intra-arterial verapamil. For internal carotid DSA, artery diameters were measured in ≤11 predetermined segments. Segments measured on the anteroposterior view included A1 and A2 ACA segments and M1, M2, and M3 MCA segments. Segments measured on the lateral view included the supraclinoid ICA, A3 of the ACA, and the angular branch of the MCA. If a fetal origin of the PCA was present, the PcomA and P2 and P3 PCA segments were measured on the lateral view. For vertebral DSA, artery diameters were measured at ≤7 predetermined segments on the anteroposterior view. Segments measured included the basilar artery and right and left P1, P2, and P3 PCA segments. Because DSA is subject to variability in magnification between angiograms, all artery measurements were scaled to skull diameters.
A team of 3 reviewers composed of 1 diagnostic neuroradiology fellow and 2 interventional neuroradiology fellows analyzed each DSA. The first reviewer compared the DSA from the time of presentation with that from the time of angiographic vasospasm diagnosis before treatment. The first reviewer then marked the site of the worst angiographic vasospasm within each predetermined arterial segment described above. In any segment in which the first reviewer did not subjectively identify vasospasm, a representative site was still marked. The first reviewer was blinded to the results of treatment when choosing sites. After all sites were marked, the same sites were marked on the angiograms from the other time points. The second and third reviewers were then presented with the DSAs from all 3 time points in each patient and/or treatment in random order during the course of many days. These 2 reviewers, blinded to patient information and DSA time points, measured the artery diameters at each site marked. All images were analyzed at 400% magnification on a PACS, and measurements were recorded to the nearest 0.1 mm. Measurements from these 2 reviewers were averaged.
This project was reviewed and approved by the Human Research Protection Office at our institution, which granted a waiver of informed consent.
Results
Between January 2006 and December 2008, 12 patients (2 men and 10 women) underwent a total of 16 procedures for treatment of cerebral vasospasm with intra-arterial verapamil that fulfilled the inclusion and exclusion criteria for this study (Table 1). Patient ages were a mean of 45 ± 11 years, ranging from 19 to 59 years. At presentation, head CTs demonstrated Fisher grade 3 SAH in 11 patients and Fisher grade 4 SAH in 1 patient. At DSA, 10 patients had 1 aneurysm, 1 patient had 2 aneurysms, and 1 patient had no angiographic etiology for hemorrhage. In patient 6, the right superior hypophyseal aneurysm was a 1-mm blood-blister-type aneurysm and was not thought to be the cause of the SAH. All of the other aneurysms were either surgically clipped or endovascularly coiled. The patients were treated with intra-arterial verapamil for vasospasm 9 ± 4 days after SAH, with a range from 1 to 16 days. For the 34 treated arterial distributions, verapamil was administered at 10 mg for 31 arteries, 5 mg for 1 artery, and 20 mg for 2 arteries. The average total dose for each patient per procedure was 22 ± 8 mg.
Table 1:
Demographics, SAH grade, aneurysm management, and intra-arterial vasospasm treatment in 12 patients who collectively underwent 16 procedures
| Pt/Procedure No. | Age (yr)/Sex | HH Grade | Fisher Grade | Aneurysm Location, Treatment | Findings Prompting Treatment | PHD When Treated | Vessel, Verapamil Infused (mg) |
|---|---|---|---|---|---|---|---|
| 1/1 | 42/M | 4 | 3 | L AChoA, clip | Confusion, R drift | 6 | R ICA, 10 |
| L ICA, 10 | |||||||
| R VA, 10 | |||||||
| 1/2 | Confusion | 10 | R ICA, 10 | ||||
| L ICA, 10 | |||||||
| 2 | 42/M | 3 | 3 | L MCA, clip | Aphasia | 6 | L ICA, 10 |
| 3 | 43/F | 3 | 3 | R PcomA, clip | Confusion, agitation | 15 | R ICA, 10 |
| L ICA, 10 | |||||||
| R VA, 5 | |||||||
| 4 | 19/F | 2 | 3 | R AICA, coil | L weakness, L drift | 12 | R ICA, 10 |
| L ICA, 10 | |||||||
| L VA, 10 | |||||||
| 5 | 39/F | 4 | 3 | R AChoA, clip; R MCA, clip | L hemiparesis | 16 | R ICA, 20 |
| 6 | 49/F | 4 | 3 | R SHA, none | Bilateral lower extremity paresis | 13 | R ICA, 10 |
| L ICA, 10 | |||||||
| 7/1 | 34/F | 2 | 4 | None | Global aphasia | 6 | R ICA, 10 |
| L ICA, 10 | |||||||
| R VA, 10 | |||||||
| 7/2 | Global aphasia | 11 | R ICA, 10 | ||||
| L ICA, 10 | |||||||
| R VA, 10 | |||||||
| 8/1 | 57/F | 2 | 3 | L PcomA, clip | R hemiparesis, expressive aphasia | 5 | R ICA, 10 |
| L ICA, 20 | |||||||
| 8/2 | R hemiparesis, expressive aphasia | 8 | R ICA, 10 | ||||
| L ICA, 10 | |||||||
| 8/3 | L frontoparietal infarct | 11 | L ICA, 10 | ||||
| 9 | 59/F | 2 | 3 | AcomA, clip | Confusion | 1a | R ICA, 10 |
| 10 | 59/F | 2 | 3 | AcomA, coil | Bilateral ACA infarcts | 9 | R ICA, 10 |
| L ICA, 10 | |||||||
| 11 | 48/F | 3 | 3 | AcomA, clip | L neglect, L drift | 7 | R ICA, 10 |
| L ICA, 10 | |||||||
| 12 | 45/F | 4 | 3 | R MCA, clip | L hemiparesis | 4 | R ICA, 10 |
| L ICA, 10 | |||||||
| L VA, 10 |
Headache, photophobia, nausea, and vomiting began 5 days prior to admission and diagnosis.
Hemodynamic parameters during each procedure were obtained from anesthesia records (Table 2). Goals for MAP were obtained from inpatient records. The only patient to experience bradycardia was patient 1 during procedure 1 when his heart rate decreased to 57 beats per minute after treatment. Despite mild tachycardia at the beginning of the procedure, the patient's heart rate had gradually decreased to the sixties before treatment.
Table 2.
Hemodynamic parameters during 16 procedures involving intra-arterial vasospasm treatment in 12 patients
| Pt/Procedure No. | Total Verapamil (mg) | HHH Therapy | HR (bpm) |
MAP (mm Hg) |
ICP (mm Hg) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Nadir | Initial | Nadir | Goal | Initial | Peak | |||
| 1/1 | 30 | No | 102 | 57 | 108 | 86 | 17 | 17 | |
| 1/2 | 20 | No | 85 | 79 | 104 | 96 | 4 | 4 | |
| 2 | 10 | Yesa | 68 | 60 | 117 | 97 | 120–130 | 7 | 8 |
| 3 | 25 | No | 96 | 96 | 83 | 76 | 10 | 12 | |
| 4 | 30 | No | 78 | 75 | 114 | 91 | |||
| 5 | 20 | Yesa | 125 | 100 | 85 | 71 | 120–130 | ||
| 6 | 20 | Yesa | 87 | 80 | 136 | 100 | 120–130 | ||
| 7/1 | 30 | Noa | 95 | 86 | 129 | 112 | 6 | 10 | |
| 7/2 | 30 | Yesa | 94 | 91 | 130 | 130 | 145–155 | ||
| 8/1 | 30 | No | 73 | 64 | 77 | 64 | |||
| 8/2 | 20 | Yesa | 95 | 84 | 134 | 111 | 125–140 | ||
| 8/3 | 10 | Yesa | 125 | 94 | 117 | 103 | 135–145 | ||
| 9 | 10 | No | 85 | 68 | 85 | 81 | |||
| 10 | 20 | No | 131 | 106 | 114 | 112 | 16 | 20 | |
| 11 | 20 | No | 65 | 65 | 103 | 98 | 7 | 8 | |
| 12 | 30 | No | 90 | 86 | 105 | 95 | 15 | 15 | |
Refer to text for therapy changes during the procedure.
Many patients required adjustment of their HHH therapy. Patient 2 was started on HHH therapy the morning of the procedure. During the procedure, norepinephrine was increased from 45 mcg/min to 55 mcg/min. For patient 5, the MAP goal was increased from 100–110 mm Hg to 120–130 mm Hg the morning of the procedure. This goal was not achieved before the procedure due to severe pulmonary edema and hemodynamic instability. During the procedure, phenylephrine was increased from 225 mcg/min to 300 mcg/min and norepinephrine was continued at 10 mcg/min. For patient 6, phenylephrine was titrated from 800 mcg/min to 740 mcg/min and then to 820 mcg/min during the procedure. During the first procedure for patient 7, a 5-mg bolus of labetolol was administered intravenously before intra-arterial verapamil infusion due to high MAP. During the second procedure for patient 7, phenylephrine was administered at 75 mcg/min at the beginning of the procedure and was titrated to 65 mcg/min and then to 160 mcg/min by the end of the procedure. During the second procedure for patient 8, phenylephrine was initially administered at 70 mcg/min and was titrated to 90 mcg/min after intra-arterial verapamil infusion. During the third procedure for patient 8, norepinephrine and phenylephrine were held constant at 30 mcg/min and 420 mcg/min, respectively. An intravenous bolus of 250-mL normal saline was administered for low MAP.
The nadir in MAP experienced by the patients averaged 14 ± 10 mm Hg lower than the MAP at the beginning of the procedures (P = .00002, Student t test). Decreases in MAP tended to persist for 10–30 minutes, depending on dosing schedule and number of separate arterial distributions treated. At the conclusion of each procedure, which was typically 1 hour after the first verapamil infusion, heart rate, MAP, and intracranial pressure changed to −2 ± 16 beats per minute (mean), −0.7 ± 11 mm Hg, and −1 ± 4 mm Hg, respectively, compared with the first measurement during each procedure. These changes were not significant (P = .41, P = .31, and P = .23, respectively).
Three patients underwent angioplasty and intra-arterial verapamil treatment for cerebral vasospasm at some point before a treatment included in this study. Patient 3 underwent angioplasty of the bilateral M1 MCA segments and bilateral supraclinoid ICA segments and infusion of 10-mg verapamil into each cervical ICA on posthemorrhage day 11. Patient 7 underwent angioplasty of the left M1 MCA segment, right P1 PCA segment, basilar artery, and distal right VA on posthemorrhage day 8. During the same procedure, 20-mg verapamil was infused into the right ICA, 5-mg verapamil was infused into the left ICA, and 10-mg verapamil was infused into the right VA. Patient 8 underwent angioplasty of the left supraclinoid ICA and left M1 MCA segment on posthemorrhage day 5 after the first of 3 procedures involving treatment with intra-arterial verapamil alone (Table 1).
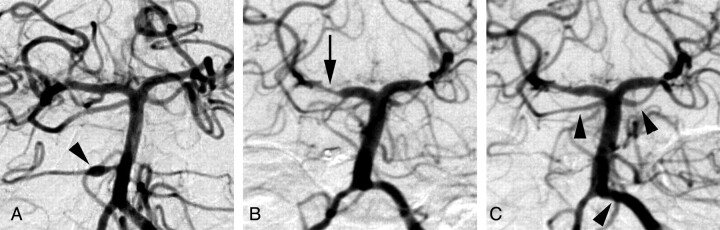
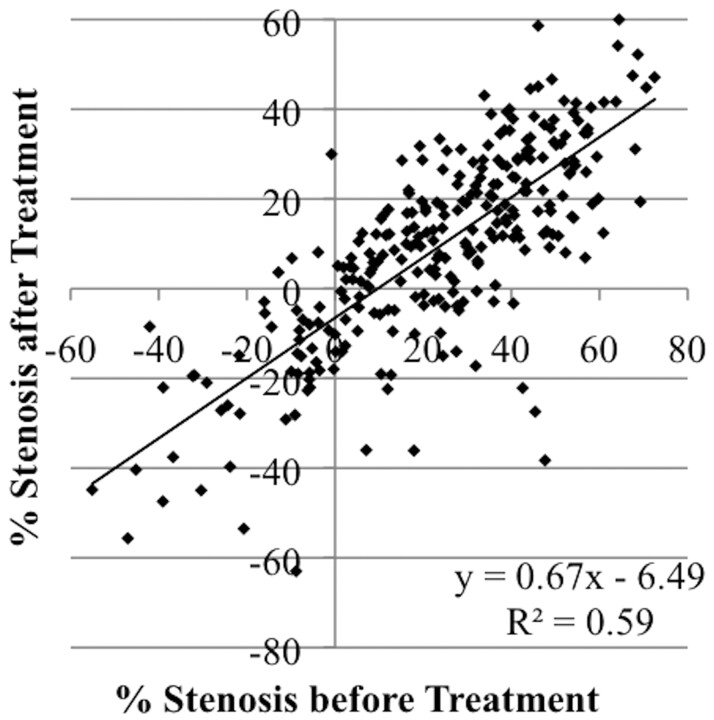
Figure 1 demonstrates an example of improvement in angiographic vasospasm in the posterior circulation after intra-arterial infusion of 10-mg verapamil into the left vertebral artery. Figure 2 plots data describing the changes in the diameters of all 275 arterial segments measured in all 34 arterial distributions included in this study. Note that several data points demonstrate a negative degree of stenosis before treatment (ie, increased diameter at the time of vasospasm treatment relative to initial presentation). These data suggest that some of the arteries demonstrated angiographic vasospasm at presentation. Note also that the linear regression line does not pass through the origin. This finding may be secondary to a combination of vasospasm in some arteries at presentation and enlargement of some arteries beyond their normal baseline diameters after treatment. For the 34 arterial distributions treated, the segments with the worst angiographic vasospasm from each arterial distribution averaged 51 ± 13% stenosis, which improved to 29 ± 18% stenosis (P < .001).
Fig 1.
Anteroposterior views of left vertebral angiograms from patient 4 obtained after presentation with SAH (A) and 12 days later before (B) and after (C) treatment with 10-mg intra-arterial verapamil. The arrowhead in A indicates a dissecting aneurysm of the right AICA, which was subsequently coil-embolized. The arrow in B indicates the right P2 PCA segment, which improved from 50% stenosis to 38% stenosis with treatment. The arrowheads in C indicate improvement in angiographic vasospasm in the superior cerebellar arteries and distal left VA, analysis of which was beyond the scope of this study.
Fig 2.
Scatterplot demonstrating improvement in angiographic cerebral vasospasm after treatment with intra-arterial verapamil. During 16 procedures in 12 patients, 34 arterial distributions were selectively treated. Cerebral angiograms were obtained after patient presentation with SAH and days later before and after intra-arterial verapamil treatment of cerebral vasospasm. Artery diameters were measured in a blinded fashion at multiple predetermined sites (whether or not vasospasm was present) in each arterial distribution from each of the 3 angiograms, and the resulting 275 data points were plotted.
Figure 2 demonstrates that the regression coefficient (slope) calculated from a plot of the degree of stenosis before treatment versus the degree of stenosis after treatment was 0.67 ± 0.03 with a y-intercept of −6.5 ± 1.2. Subgroup analysis was performed to compare the effects of intra-arterial verapamil on distal vessels with the effects on proximal vessels. Proximal segments defined as the basilar artery, supraclinoid ICA, A1 ACA segment, M1 MCA segment, and P1 PCA segment showed a regression coefficient that was not statistically significantly different from that of distal segments defined as A2 and A3 ACA segments; M2, M3, and M4 MCA segments; PcomA; and P2 and P3 segments (P > .05, analysis of variance). Subgroup analysis was also performed to compare the effects of intra-arterial verapamil on the anterior circulation with those on the posterior circulation. Again, the regression coefficients did not demonstrate a statistically significant difference (P > .05). Note that data in Fig 2 represent averaged observations from 2 independent blinded reviewers. As a measure of inter-rater reliability, regression coefficients were calculated from data from each reviewer independently. The regression coefficients did not demonstrate a statistically significant difference (P > .05).
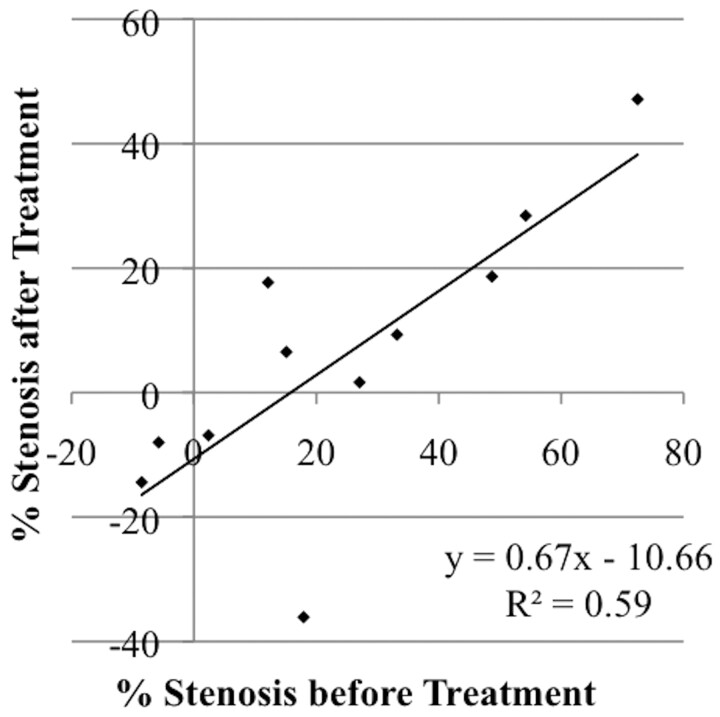
Four treatments included in the data plotted in Fig 2 involved patients treated with angioplasty for vasospasm at some point in the days before intra-arterial verapamil administration. Subgroup analysis of arterial segments previously treated with angioplasty was performed to assess the responsiveness of those arterial segments to subsequent treatment with intra-arterial verapamil. Figure 3 demonstrates that the regression coefficient calculated from a plot of degree stenosis before treatment versus degree stenosis after treatment was 0.67 ± 0.19 with a y-intercept of −10.7 ± 6.4. Arterial segments previously treated with angioplasty demonstrated responsiveness to intra-arterial verapamil that was not different from that of arterial segments that had not been previously treated with angioplasty (P > .05).
Fig 3.
Subgroup analysis of all arterial segments with history of angioplasty treatment demonstrating improvement in recurrent angiographic cerebral vasospasm after treatment with intra-arterial verapamil. Data from 4 procedures in 3 patients documenting 11 treatment changes in 9 arterial segments were plotted. Treatment response was not different in arterial segments with a history of recent angioplasty treatment compared with other arterial segments (P > .05).
Discussion
This study included all patients treated with intra-arterial verapamil, but not concurrent angioplasty, for cerebral vasospasm between January 1, 2006, and December 31, 2008, at our medical center, where follow-up angiography was performed ≥20 minutes after treatment. Clinical information for this patient cohort is detailed in Table 1. This study population had a selection bias on the part of the intensivists, neurosurgeons, and neurointerventional radiologists caring for the patients, but this population does share characteristics of a random population of patients with nontraumatic SAH. Most cases of nontraumatic SAH are caused by rupture of an intracranial aneurysm, which accounts for approximately 85% of cases.2 Ruptured aneurysm was diagnosed as the cause of SAH in 10 of the 12 cases presented here. The incidence of SAH increases with age and is higher in women than in men. The average age here was 45 ± 11 years. Women accounted for 10 of the 12 patients treated.
The optimum dosage and infusion rate of intra-arterial verapamil are not known. The average dosage in this study was 10 ± 3 mg per vessel for a total of 22 ± 8 mg per procedure (Table 1). These dosages were selected on the basis of anecdotal experience and, in some cases, were the result of titration to a desired effect. Mazumdar et al9 used an average of 7.4 mg with a range from 2.5 to 10 mg but did not specify if the dose was per vessel or per procedure. In that study, the diameters of vasospastic arteries were not significantly different after treatment. Other groups have reported improvement in angiographic vasospasm after treatment. Feng et al7 used an average of 2 mg per vessel for a total of 3.1 ± 0.3 mg per procedure. The highest dose used in a single vessel was 8 mg. Keuskamp et al8 used much higher doses at 41 ± 29 mg per procedure. The duration of action of a pulsed dose of locally high-concentration intra-arterial verapamil is likely in the range of several hours, and repeat treatment may sometimes be required for ongoing optimal clinical effect.12,13
Although most patients in this study received only 1 treatment, some patients were treated 2 or 3 times during the course of several days. Albanese et al10 used in-dwelling microcatheters for prolonged infusions of verapamil at rates of 10–50 mg/h for infusion times from 1 to 20.5 hours for total dosages ≤720 mg. Treatment intervals have been typically based on clinical status rather than pharmacokinetics. Given differences in methods used to quantify results, direct comparison between these dosage schemes is not straightforward.
Administration of intravenous verapamil may produce undesired systemic effects that decrease cerebral perfusion pressure. Table 2 details hemodynamic parameters for each treated patient during each procedure. After initiation of treatment, patients experienced transient decreases in MAP, typically for 10–30 minutes. This time span is likely a function of the number and spacing of treatment boluses to multiple arterial distributions. Because arterial distributions were treated sometimes only a few minutes apart, the effect of individual boluses is not clear. These changes resolved by the end of the procedures. In a study using lower doses of verapamil, Feng et al7 reported statistically significant decreases in MAP of 3.8 ± 1.0% at 10 minutes after treatment and 1.7 ± 1.1% at 20 minutes after treatment. In a study using higher doses of verapamil than those in the current study, Keuskamp et al8 reported qualitative transient decreases in heart rate and/or blood pressure in some of their patients lasting, at most, 5 minutes with no episodes of hypotension or new bradycardia. Some of the decreases in MAP were greater than 10 mm Hg, but specific measurements were not given. They measured no significant change in hemodynamic parameters when comparing the beginning and end of the procedures.
Results shown in Fig 2 plot the degree of stenosis before and after treatment in a series of patients with vasospasm. The arterial segments most negatively affected by angiographic vasospasm were the segments that demonstrated the greatest improvement with treatment, a result also reported by Feng et al.7 This dependence of the degree of improvement on the degree of angiographic vasospasm is important in evaluation of other studies on this topic. A common convention has been to report treatment effect as a percentage increase in arterial diameter of vasospastic arteries after treatment relative to before treatment.7,9 Unfortunately, when the degree of vasospasm before treatment is not reported, the absolute change in artery diameter is unknown, and results can be difficult to compare between studies. In the case series by Mazumdar et al,9 this confounding variable may have contributed to the lack of statistical significance of those results because data from arterial segments that were not affected or only mildly affected by vasospasm may have obscured the true treatment effect on more severely vasospastic arteries.
There is lag time between vasodilator infusion and therapeutic response, a concept that becomes important when repeat DSA runs are performed to adjust vasodilator dosage or quantify overall treatment outcome. In an in vitro study of dog cerebral arteries, verapamil-induced changes in vessel walls developed gradually and stabilized within 15–30 minutes.14 In 14 of the 18 cases reported by Mazumdar et al,9 follow-up angiography was performed 5 minutes after the conclusion of verapamil infusion. This delay may have been insufficient and may have contributed to the statistically insignificant results. In contrast, Keuskamp et al8 performed follow-up angiography 1–2 minutes after each 5-mg intra-arterial verapamil bolus. Multiple doses were often used because lower doses were not effective. Even though the infusions tended to be performed during relatively long time periods, the lower doses may have been more effective if longer time periods had been observed before follow-up angiography. Feng et al7 demonstrated improvement in angiographic vasospasm when angiography was performed 10–15 minutes after treatment by using relatively low-dose treatments. In cases in which pretreatment DSA is compared with posttreatment DSA performed ≤ 35 minutes later as in these studies, it is reasonable to assume that any change in vessel diameter will be secondary to treatment, without necessarily including an untreated or sham-treated cohort. Albanese et al10 treated patients with continuous vasodilator infusions by indwelling microcatheters for ≤20.5 hours. The assumption that posttreatment artery diameter is completely a function of medication infusion may not be valid at such long time periods.
Angioplasty can be complementary to selective intra-arterial vasodilator infusion in the treatment of vasospasm. Due to mechanical considerations, angioplasty is limited to relatively large proximal arteries. It has been suggested by others that distal vessels are more affected by medication infusion than proximal vessels.7,10 In this study, the effects of intra-arterial verapamil were not different between proximal and distal arterial segments. As shown in Fig 1, intra-arterial vasodilator infusion has the benefit of simultaneous treatment of an entire arterial distribution, including distal vessels not accessible by angioplasty. As shown in Fig 3, arterial segments previously treated with angioplasty did not demonstrate a difference in responsiveness to intra-arterial verapamil compared with arterial segments not previously treated with angioplasty. In a study of canine carotid arteries, Megyesi et al15 reported that arterial segments previously treated with angioplasty in vivo were less responsive to several pharmacologic agents when studied in vitro after animal sacrifice. In that study, arteries were balloon-dilated to 150% of their normal diameter, a degree of dilation far beyond that used in clinical practice. In a similar study involving rabbit carotid arteries, Macdonald et al16 reported that vasospastic arteries dilated to 100% of their normal diameter demonstrated reduced responsiveness to multiple pharmacologic agents. The etiology of the disagreement between those results and results in this study is not clear.
Randomized controlled trials have not been performed to prove the efficacy of intra-arterial verapamil in the treatment of clinical vasospasm. Other studies have reported changes in gross neurologic status in treated patients.7–10,17 Those parameters were outside the scope of the current study. In the absence of a control patient population, those data may be of limited value. It is well established that complications may be under-reported and benefit may be exaggerated in studies that do not include a control patient population. Randomized controlled trials have also not been performed to compare angioplasty with intra-arterial vasodilator infusion or to compare the different available vasodilators.
Conclusions
Intra-arterially administered verapamil improves angiographic vasospasm after SAH when administered at 10 ± 3 mg per arterial distribution. If repeat angiography is performed to adjust vasodilator dosage or assess treatment response, a delay of 15–30 minutes may be required. Optimal dose, infusion rate, and retreatment interval remain to be determined. Randomized controlled trials are needed to prove efficacy in the treatment of clinical vasospasm.
Abbreviations
- ACA
anterior cerebral artery
- AChoA
anterior choroidal artery
- AcomA
anterior communicating artery
- AICA
anterior inferior cerebellar artery
- DSA
digital subtraction angiography
- HH
Hunt and Hess
- HHH
hypertension, hypervolemia, and hemodilution
- HR
heart rate
- ICA
internal carotid artery
- ICP
intracranial pressure
- L
left
- MAP
mean arterial blood pressure
- MCA
middle cerebral artery
- PCA
posterior cerebral artery
- PcomA
posterior communicating artery
- PHD
posthemorrhage day when intra-arterial verapamil was administered
- Pt
patient
- R
right
- SAH
subarachnoid hemorrhage
- SHA
superior hypophyseal artery
- VA
vertebral artery
References
- 1. de Rooij NK, Linn FHH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124(pt 2):249–78 [DOI] [PubMed] [Google Scholar]
- 3. Macdonald RL. Management of cerebral vasospasm. Neurosurg Rev 2006;29:179–93 [DOI] [PubMed] [Google Scholar]
- 4. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med 2006;354:387–96 [DOI] [PubMed] [Google Scholar]
- 5. Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurg Focus 2006;21:E8. [DOI] [PubMed] [Google Scholar]
- 6. Eddleman CS, Hurley MC, Naidech AM, et al. Endovascular options in the treatment of delayed ischemic neurological deficits due to cerebral vasospasm. Neurosurg Focus 2009;26:E6. [DOI] [PubMed] [Google Scholar]
- 7. Feng L, Fitzsimmons B, Young WL, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol 2002;23:1284–90 [PMC free article] [PubMed] [Google Scholar]
- 8. Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurismal subarachnoid hemorrhage. J Neurosurg 2008;108:458–63 [DOI] [PubMed] [Google Scholar]
- 9. Mazumdar A, Rivet DJ, Derdeyn CP, et al. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus 2006;21:E15. [DOI] [PubMed] [Google Scholar]
- 10. Albanese E, Russo A, Quiroga M, et al. Ultrahigh-dose intraarterial infusion of verapamil through an indwelling microcatheter for medically refractory severe vasospasm: initial experience. J Neurosurg 2010;113:913–22 [DOI] [PubMed] [Google Scholar]
- 11. Eskesen V, Karle A, Kruse A, et al. Observer variability in assessment of angiographic vasospasm after aneurismal subarachnoid hemorrhage. Acta Neurochir (Wien) 1987;87:54–57 [DOI] [PubMed] [Google Scholar]
- 12. Mussa S, Prior T, Alp N, et al. Duration of action of antispasmodic agents: novel use of a mouse model as an in vivo pharmacological assay. Eur J Cardiothorac Surg 2004;26:988–94 [DOI] [PubMed] [Google Scholar]
- 13. Scholz H. Pharmacological aspects of calcium channel blockers. Cardiovasc Drugs Ther 1997;10(suppl 3):869–72 [DOI] [PubMed] [Google Scholar]
- 14. Shimizu K, Ohta T, Toda N. Evidence for greater susceptibility of isolated dog cerebral arteries to Ca antagonists than peripheral arteries. Stroke 1980;11:261–66 [DOI] [PubMed] [Google Scholar]
- 15. Megyesi JF, Findlay JM, Vollrath B, et al. In vivo angioplasty prevents the development of vasospasm in canine carotid arteries. Stroke 1997;28:1216–24 [DOI] [PubMed] [Google Scholar]
- 16. Macdonald RL, Zhang J, Han H. Angioplasty reduces pharmacologically mediated vasoconstriction in rabbit carotid arteries with and without vasospasm. Stroke 1995;26:1053–60 [DOI] [PubMed] [Google Scholar]
- 17. Westhout FD, Nwagwu CI. Intra-arterial verapamil-induced seizures: case report and review of the literature. Surg Neurol 2007;67:483–86 [DOI] [PubMed] [Google Scholar]