Abstract
BACKGROUND AND PURPOSE:
The extended time window and theoretic reduction in hemorrhage make mechanical strategies an attractive approach in the treatment of patients with ischemic stroke. My purpose was to evaluate the recanalization efficacy and safety of the Solitaire FR Revascularization Device in a swine vessel occlusion model.
MATERIALS AND METHODS:
For recanalization efficacy, radio-opaque clots were used in 6 ascending pharyngeal arteries of the swine. Safety was assessed in 8 renal arteries with 3 passes of the device. Four vessels were harvested at 30 days, and 4, at 90 days, with microscopic examination to assess vessel damage.
RESULTS:
On deployment, immediate flow was seen in 3/6 vessels; and on retrieval, TIMI-3 recanalization was seen in 6/6. Vasospasm, which resolved on follow-up angiography with no distal emboli or vessel damage, was noted. In the renal vessels, safety evaluation findings were unremarkable, with the exception of 2 vessels harvested from a kidney at 90 days. These 2 vessels appeared without damage on microscopy; however, angiography showed shrinkage of the entire kidney, which was attributed to damage and stenosis of the main renal artery at the site of the guide-catheter insertion, likely leading to a chronic low-flow state and resultant atrophy of the entire kidney.
CONCLUSIONS:
The Solitaire Device showed good recanalization efficacy with acceptable safety. The unique stent-like design allows immediate flow restoration, a distinct advantage of the device. On the basis of this study, the device has good potential as a tool in the treatment of patients with ischemic stroke.
Limitations of thrombolysis, including low recanalization rate, limited time window for application, and increased risk of intracranial hemorrhage, attest to the need for further improvement in acute stroke treatment. The application of mechanical strategies for acute stroke treatment is now increasing with FDA approval of 2 devices, the Merci retriever (Concentric Medical, Mountain View, California) and the Penumbra system (Penumbra, Alameda, California).1–4 Regardless of the strategy used, early recanalization appears essential.5 Despite reporting high recanalization rates, current mechanical strategies do not allow immediate flow restoration, with times from ictus to treatment in excess of 4 hours and procedural times of ≤2 hours.1,3 A mechanical device that allows immediate flow restoration would be desirable to minimize occlusion times and reduce neuronal injury. In this study, we evaluated a new thrombectomy device, the Solitaire FR Revascularization Device (ev3, Irvine, California), with a unique stent-like design that allows immediate flow restoration followed by clot retrieval. The device was evaluated in a swine vessel occlusion model with regard to recanalization efficacy, clot-device interaction, safety, and potential device-related complications.
Materials and Methods
Animal experimentation was performed according to policies set by the National Institutes of Health and the Animal Research Committee of the local institution. Nine Yucatan swine of mixed sex, 3–4 months of age, weighing 20–40 kg, were used in this study. Following an overnight fast, the swine were premedicated with 20 mg/kg ketamine, intubated, and general anesthesia was maintained with mechanical ventilation and inhalation of 1%–2% halothane. For assessment of recanalization efficacy (6 animals, 6 vessels), clots were placed in the APAs of the swine as described below (see “Clot Production”). For assessment of safety (3 animals, 8 vessels), 3 deployments and recoveries of the device were made in the lobar and interlobar renal arteries of the swine, and the vessels were harvested for histologic evaluation as described below (see “Safety Study”).
Clot Production
Radio-opaque clots were prepared in vitro as previously described,6 with the following modifications: Soft and firm clots were prepared by addition of porcine fibrinogen (Sigma Aldrich, St. Louis, Missouri). Barium sulfate was used for radio-opacity.6 The clot was then introduced into the APA as described below with 3 swine receiving soft clots and 3 animals receiving firm clots (Table 1).
Table 1:
Angiographic results of clot retrieval
| Vessel | Animal Number |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
|
| R APA | L APA | R APA | L APA | R APA | L APA | |
| Vessel diameter | 2 | 2.5 | 2 | 2.1 | 2.1 | 2.3 |
| Clot type | Soft | Soft | Soft | Firm | Firm | Firm |
| TIMI postdevice deploymenta | 0 | 2 | N/Ab | 0 | 1 | 2 |
| Time to immediate flow (min)c | N/A | 3 | N/Ab | N/A | 3 | 5 |
| Clot retrieved | Yes | Yes | Yes | Yes | Yes | Yes |
| No. passes with device | 1 | 1 | 1 | 2 | 1 | 1 |
| Final TIMI post-clot retrieval | 3 | 3 | 3 | 3 | 3 | 3 |
| Time to successful recanalization (min)d | 7 | 6 | 9 | 35 | 4 | 8 |
| Distal embolization | No | No | No | No | No | No |
| Vessel damage | No | No | No | No | No | No |
| Vasospasm | Present | Present | Present | Present | Present | Present |
| Thrombus | No | No | No | No | No | No |
TIMI flow immediately postdeployment of the device into the clot.
Angiogram not obtained after deployment of device.
Time from insertion of microcatheter into the guide catheter to immediate flow restoration.
Time from insertion of microcatheter to retrieval of clot into the guide catheter.
Clot Placement
Biplane angiography was performed via a right transfemoral approach. An angled guide catheter (Cordis, Miami Lakes, Florida) was used to catheterize the right or left common carotid artery for imaging of the ipsilateral rete mirabile. For soft clots 6F (Envoy; Cordis) and for firm clots 7F guide catheters (Envoy; Cordis) were used. The tip of the catheter was placed in the origin of the APA. The clot was then manually introduced into the guide catheter and gently injected into the APA. In most instances, the clot migrated to the mid-to-distal APA to achieve occlusion (Figs 1 and 2). Angiography was performed to document occlusion followed by removal of the guide catheter. The occlusion was maintained for 3 hours (±30 minutes) before attempted thromboembolectomy.
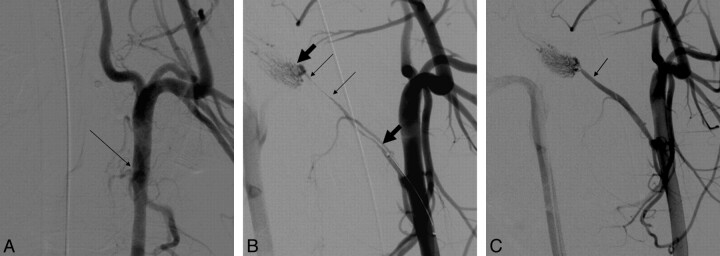
Fig 1.
A, Anteroposterior angiogram of the left common carotid artery in a swine. The arrow points to the origin of the APA, which is occluded here due to a clot in the vessel. A radio-opaque clot is not seen in this subtracted angiogram. B, Right anterior oblique angiogram of the left common carotid artery following deployment of the device shows immediate TIMI 2 flow restoration, with patency of the APA. Thin arrows point to the length of the clot, while the thick arrows show the length of device deployed. C, Right anterior oblique angiogram of the left common carotid artery postretrieval of the clot shows TIMI 3 complete recanalization. Arrow points to the original site of occlusion in the vessel.
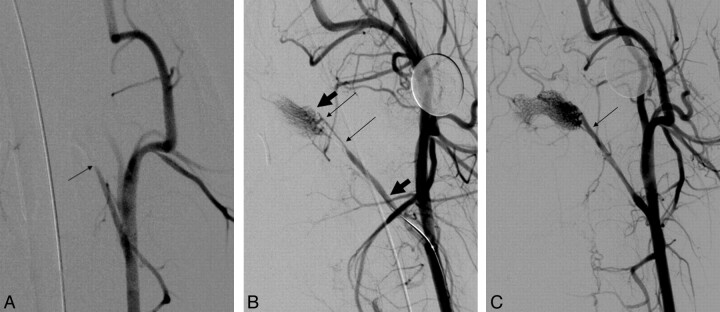
Fig 2.
A, Anteroposterior angiogram of the left common carotid artery in swine. Occlusion of the APA is seen (arrow). B, Right anterior oblique angiogram of the left common carotid artery with the device deployed shows immediate flow restoration. The thin arrows indicate the proximal and distal aspects of the clot, while the thick arrows point to the proximal and distal ends of the device. C, Right anterior oblique angiogram of the left common carotid artery postretrieval of the clot shows complete restoration of flow. Arrow shows the original site of occlusion.
Device Description
The ev3 Solitaire FR Revascularization Device is a stent-like device, which can be fully deployed, fully resheathed, and recovered (Fig 3). Its stent-like design allows immediate blood flow restoration and clot retrieval. The device is designed for use in the neurovasculature, such as the internal carotid artery, M1 and M2 segments of the MCA, anterior cerebral artery, and the basilar and vertebral arteries. It is available in 2 sizes: 4 and 6 mm in diameter. The 4-mm device is available in 15- and 20-mm lengths, and the 6-mm device, in 20- and 30-mm lengths. The 4-mm device is designed to be delivered through a microcatheter with a minimal luminal diameter of 0.021 inches, and the 6-mm device, through an inner lumen of 0.027 inches. The 4-mm device (20 mm in length) was used in the experiments described herein.
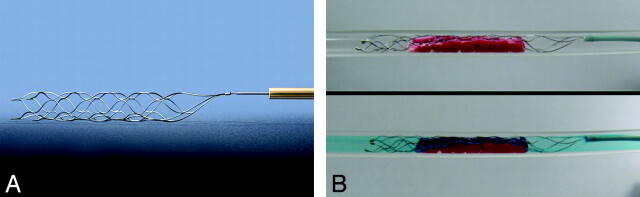
Fig 3.
A, The Solitaire FR Revascularization Device. Note the stent-like design with attached delivery wire allowing deployment and retrieval of the device. B, The upper panel shows an in vitro image of the device deployed within a clot in a silicone tube. Note compression of the clot by the device. The lower panel shows colored saline flowing through the open channel created by the device, simulating immediate flow restoration as seen in Figs 1B and 2B.
Thrombectomy Procedure
An 8F balloon guide catheter (Concentric Medical) was placed in the common carotid artery. Coaxially through the guide catheter, a Rebar-18 microcatheter (ev3) and a SilverSpeed 14 microguidewire (ev3) were used to catheterize the APA with the tip of the microcatheter placed just beyond the clot. The 4 × 20 mm Solitaire FR Revascularization Device was then introduced into the microcatheter via a Y-connector and advanced to the distal tip of the microcatheter. The device was then fixed in place while the microcatheter was withdrawn to unsheathe the Solitaire FR Revascularization Device until the tip of the microcatheter was just proximal to the proximal marker on the device. Following deployment, angiography was performed to document immediate flow restoration. The device and microcatheter were then withdrawn to retrieve the clot. During the retrieval, the balloon at the tip of the guide catheter was inflated to arrest antegrade flow. Once the clot was in the common carotid artery, aspiration was performed through the guide catheter to capture the clot and prevent dislodgement of the clot from the device. A postretrieval angiogram was obtained to document recanalization and any vessel damage, including vasospasm, dissection, thrombus formation, perforation, or distal embolization. The animals were euthanized postprocedure. The TIMI scale7 was used to assess recanalization as follows: TIMI 0, no recanalization; TIMI 1, minimal recanalization, flow past the clot but no opacification of the distal territory; TIMI 2, partial recanalization, flow past the clot with opacification of the distal territory; TIMI 3, complete recanalization, no clot present with complete opacification of the distal territory.
Safety Study
Three animals were used for this portion of the study. For evaluation of device safety, the lobar and interlobar renal arteries (4 vessels in 1 animal with a 30-day follow-up and 2 vessels each in the other 2 animals with a 90-day follow-up for a total of 8 vessels) were chosen with 3 deployments and recoveries of the device followed by harvesting of the kidneys for histologic evaluation. Initial angiography was performed using a 6F Envoy angled guide catheter (Cordis) placed in the main trunk of the right or left renal artery. Lobar or interlobar renal arteries of approximately 2 mm in size (±0.5 mm) were chosen similar in size to the M1 and M2 segment of the MCA. Coaxially through the guide catheter, a Rebar-18 microcatheter and a SilverSpeed 14 microguidewire were used to catheterize the target vessel. The microguidewire was then removed, and the 4 × 20 mm Solitaire Device was introduced through a Y-connector and advanced until the distal markers were at the distal tip of the microcatheter. As described above, the device was then held in position while the microcatheter was withdrawn to expand the device into the target vessel. The device and microcatheter were then withdrawn as a unit to simulate clot retrieval. Postangiography was then performed to evaluate the vessel for damage, including dissection, vasospasm, thrombus formation, or vessel rupture. Three such deployments were made in each of 8 vessels selected.
At the target 30-day (4 vessels) and at 90-day (4 vessels) follow-up period, angiograms were obtained to evaluate the vessel for damage, including dissection, stenosis, thrombus formation, or vessel rupture with a 6F angled guide catheter (Cordis) placed in the main trunk of the right or left renal artery. Detachable platinum coils were then placed in the treated vessels distal to the deployment site for identification of the vessel by the pathologist. The vessels were catheterized with the Echelon 10 microcatheter (ev3) and a SilverSpeed 14 microguidewire, with the microcatheter placed distal to the site of deployment of the Solitaire Device. One Axium coil (ev3), ranging from 2 to 3 mm in diameter by 2–8 cm in length was placed in each of the vessels. Following completion of the study, the animal was euthanized, and the kidneys were harvested for histologic evaluation as described in the next section.
Histopathologic Examination
The fixed kidneys were examined in conjunction with copies of digital images to aid in the location of the treated vessels. Target vessels were located, dissected away from surrounding tissue, and cut transversely into 8 individual pieces numbered proximal to distal with regard to arterial blood flow. The vessel sections from each of the kidneys were processed and embedded in paraffin according to the laboratory standard operating procedures. They were subsequently sectioned at 5–7 μm and stained with hematoxylin-eosin.
The sections were examined by a board-certified veterinary pathologist. Microscopic examination included evaluating intimal thickening, medial/adventitial proliferation, inflammation (chronic or granulomatous), and stenosis. Lesions seen were recorded, and the severity was graded on a 4-point scale of 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked. If the severity varied among artery sections, then the severity grade diagnosed was that of the greatest degree of severity seen among the sections. In addition, when stenosis of the artery was present, calculation of the percentage of stenosis was made by comparing the size of the lumen with the original size of the lumen estimated as the area within the internal elastic lamina. If the degree of stenosis differed among artery sections, the maximum degree of stenosis was reported.
Results
Recanalization Success
TIMI 3 recanalization was obtained in all animals following clot retrieval, with only 1 pass required to achieve this in 5 animals and 2 passes, in 1 animal (Table 1). The time to achieve recanalization calculated from the time the microcatheter was deployed to clot retrieved into the guide catheter was a mean of 11.4 ± 11.8 minutes (range, 4.1–35.2 minutes).
After initial deployment and expansion of the device across the clot, immediate flow restoration was achieved in 3 of 5 animals (Figs 1B and 2B) with TIMI 1 flow in 1 animal and TIMI 2 flow in the other 2 animals (Table 1). In the 6th animal, angiography was not performed after deployment and before retrieval. The time from insertion of the microcatheter into the guide catheter to immediate flow restoration was 3, 3, and 5 minutes in animals 2, 5, and 6, respectively (Table 1). In 2 of 5 animals, immediate flow restoration was TIMI 0. In these, vasospasm likely contributed significantly to lack of immediate flow restoration.
Radio-opaque clots allowed observation of clot-device interaction. There was no fragmentation of the clot noted during deployment of the device or during pull-back. It was, however, noted that there can be fragmentation of the clot at the tip of the balloon catheter during retrieval. In all cases, the clot could be retrieved with aspiration through the balloon guide catheter lumen.
Postretrieval vasospasm was noted in all animals. This resolved in all cases at follow-up angiography after 1 hour of observation. There was no evidence of distal embolization, vessel damage, or thrombus formation.
Device Safety
Angiography.
A total of 8 renal arteries in 3 animals (4 vessels in 1 animal and 2 vessels each in 2 animals) were used for assessment of safety, with 3 deployments and retrievals of the device in the vessels (Table 2). Angiograms were obtained after each deployment and retrieval, and they showed no evidence of thrombus formation, dissection, or distal embolization. Follow-up angiograms were obtained up to 1 hour after the last retrieval. No angiographic evidence of vessel injury could be identified on the delayed angiograms. Of note, following the deployments, there was vasospasm in all 8 vessels examined. This always resolved on the follow-up angiograms.
Table 2:
Angiographic and histologic results of clot retrieval in the renal arteries
| Vessel | Animal 7 Renal A |
Animal 8 Renal A |
Animal 9 Renal A |
|||||
|---|---|---|---|---|---|---|---|---|
| Lower L | Upper L | Upper R | Lower R | Lower L | Upper L | Upper R | Lower R | |
| Vessel diameter | 1.8 | 2 | 2 | 1.8 | 1.8 | 2.3 | 2.0 | 2.0 |
| Chronic period (days) | 33 | 33 | 33 | 33 | 102 | 102 | 91 | 91 |
| TIMI post-chronic period | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 |
| Distal embolizationa | No | No | No | No | No | No | No | No |
| Vessel damagea | No | No | No | No | Nob | Nob | No | No |
| Vasospasma | Present only at time 0 | Present only at time 0 | Present only at time 0 | Present only at time 0 | Present only at time 0 | Present only at time 0 | Present only at time 0 | Present only at time 0 |
| Thrombusa | No | No | No | No | No | No | No | No |
| Proliferation, medial/adventitialc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thickening, intimalc | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 |
| Inflammation, chronicc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inflammation, granulomatousc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stenosis | 5% | 5% | 1% | 5% | 1% | 5% | 5% | 5% |
Distal embolization, vessel damage, vasospasm, and thrombus were evaluated at time 0 and at the chronic period. The rating is for both time periods, unless stated otherwise.
There were no angiographic observations of vessel damage, but the entire kidney was diminished in size.
Severity grade of lesions at histology: 1 = minimal, 2 = mild, 3 = moderate, 4 = marked.
The 8 vessels treated were re-examined at 30 days (4 vessels, 1 swine) and at 90 days (4 vessels, 2 swine). Angiography showed no evidence of vessel damage, including no evidence of dissection, thrombus formation, distal emboli, or vessel stenosis in 6 of the 8 vessels examined. These included 4 vessels (1 swine) that were 30 days posttreatment and 2 vessels (1 swine) that were 90 days posttreatment. The swine were sacrificed, and the kidneys were harvested for histopathologic examination as described below.
Follow-up angiography on the swine with the remaining 2 vessels that were 90 days posttreatment showed that there had been interval shrinkage of the entire kidney. The treated vessels angiographically showed no evidence of damage with no dissection, thrombus formation, or distal emboli. The appearance was consistent with a chronic low-flow state with resultant atrophy of the entire kidney. There was stenosis noted in the trunk of the main renal artery at the site of the previous guide-catheter insertion.
Histology.
Microscopic examination of the 30-day (4 vessels) and 90-day (4 vessels) specimens showed mild (grade 2) intimal thickening in the 30-day vessels and minimal (grade 1) to mild (grade 2) intimal thickening in the 90-day vessels (Table 2). This intimal thickening led to an approximately 5% luminal narrowing in 3 vessels and 1% luminal narrowing in 1 vessel in both groups (Table 2). There was no evidence of chronic or granulomatous inflammation or medial/adventitial proliferation.
Discussion
My study showed that the Solitaire FR Revascularization Device is efficacious at clot removal. In 5 of 6 APAs, the clot was removed with 1 deployment, with time to recanalization from insertion of the microcatheter to withdrawal of the clot into the guide catheter varying from approximately 4 to 9 minutes. In the 6th vessel, the clot was removed in 2 deployments within 35 minutes. There is a theoretic risk of clot fragmentation and distal embolization with any thrombectomy device; however, this was not observed in our experiments. The strut attenuation allows greater device-clot interaction, with many of the metal struts embedding into the clot to prevent slippage from the device. With the clot engaged with the struts, the chance of fragmentation and distal embolization may be less. It was observed however, that in many cases, the clot would shear off the device during retrieval into the tip of the balloon-guide catheter. In every case, it was possible to aspirate the clot into the guide catheter and, therefore, prevent distal embolization. As noted by other investigators, this makes the use of the balloon-guide catheter essential.8
Because the clots used in these experiments were radio-opaque, it was possible to closely observe device-clot interaction during deployment of the device and retrieval. As the device expands in the vessel, the clot can become compressed against the vessel wall, potentially establishing immediate blood flow. In 3 of 6 vessels treated, immediate flow restoration was observed, with TIMI 2 flow in 2 vessels and TIMI 1 flow in 1 vessel (Table 1). This is a unique aspect of the device in that some degree of antegrade flow is present during the retrieval procedure. None of the devices currently FDA-approved for clot removal in patients with ischemic stroke can establish immediate flow,1,3,4 and time to restoration of blood flow can be an important determinant of clinical outcome in acute stroke treatment.5 In the 3 cases in which immediate flow was not restored, vasospasm was a significant contributor in 2 cases, and in 1 case, we simply did not document angiographically whether flow was restored on deployment.
The success in establishing immediate flow may depend partly on clot composition. We noted that with firm clots, the device was better able to compress the clot and re-establish some degree of antegrade flow. As noted in Table 1, in 2 of 3 firm clots, antegrade flow was established immediately. With soft clots, it was noted that though there was some compression of the clot, there was protrusion of the clot through the struts, thus occluding the vessel lumen and not allowing immediate flow restoration. This finding is noted in Table 1, because 1 of 3 soft clots had antegrade flow documented.
As mentioned above, in 5 of 6 vessels, the clot was retrieved with only 1 deployment. The Solitaire FR Revascularization Device, by virtue of its stent-like design, compresses the clot and dislodges it from the vessel wall on deployment. The retrieval then is made less difficult because the clot, disengaged from the vessel wall, is easier to retrieve. The greatest difficulty in retrieving clots in clinical cases with the Merci device has been to dislodge the clot because being adherent to the vessel, the clot is very difficult to pull back, often leading to stretching of the Merci device. In the experiments, the ease of retrieval of the clot was noteworthy compared with my clinical experience with the Merci device, and this I attribute to the above-described reason.
With any thrombectomy device, safety is of paramount importance. The effect of the Solitaire FR Revascularization Device on the vasculature was tested in the renal arteries of swine. The swine was chosen because not only did the renal arteries possess proper-sized vessels similar to the MCA in humans of approximately 2 mm in diameter but also the kidneys are easy to harvest for histologic examination. Angiography during repeated deployment and retrievals was only remarkable for vasospasm. This resolved in every case without sequelae. This high rate of vasospasm might be explained by the model, with the swine renal vessels particularly prone to spasm as other investigators have also noted.9 There was no evidence of vessel damage (ie, dissection, perforation, clot formation) related to device deployment. Thirty- and 90-day follow-up angiographic findings were unremarkable, with the exception of 1 swine with 90-day follow-up showing shrinkage of the entire kidney (Table 2, animal 8). On further review, it was noted that the main renal artery had an area of narrowing where the guide catheter had been placed. It was surmised that the likely scenario here was guide catheter damage to the main trunk of the renal artery (ie, dissection or clot formation or both), followed by reduction in the flow to the kidney during a prolonged period, leading to atrophy of the entire kidney. The 2 vessels into which the device had been deployed showed no evidence of narrowing, dissection, distal occlusions, or pseudoaneurysm formation.
Microscopic examination of the vessels was only remarkable for intimal thickening, which was graded as mild to minimal, leading to an approximately 1%–5% narrowing. This intimal thickening and stenosis were not visible on angiography, were only noted on microscopic examination, and in the clinical setting would likely be without sequelae. Of note, there was no microscopic evidence of dissection, pseudoaneurysm formation, or inflammation in the vessel wall. Particular attention was directed to the swine that showed shrinkage of the entire kidney (Table 2, animal 8). The vessels in this swine did not show any evidence of injury or inflammation. Overall, the microscopic evidence suggests that the device is safe, on the basis of the 3 deployments and retrievals performed in these experiments.
Although animal studies can provide an insight into the efficacy and safety of a device, the experience in the clinical setting is complex and differs from 1 stroke patient to the next. It is, therefore, difficult to predict device performance in humans solely on the basis of animal studies. The swine vessels are less tortuous without atherosclerosis and are prone to vasospasm compared with cerebral vessels in humans. Furthermore, in our experiments, thrombi were made to be visible under fluoroscopy to allow assessment of device-clot interactions. Clinical experience in humans would provide a better assessment of the device; however, to date, there has been limited experience with the device in the setting of acute ischemic stroke.10,11 Although the initial clinical experience with the Solitaire device appears promising, questions regarding safety and efficacy can only be answered in the setting of a clinical trial. Currently ev3 is sponsoring an FDA-approved study of the use of the Solitaire device in the setting of acute ischemic stroke. The study entitled Solitaire With the Intention For Thrombectomy is approved to enroll 200 patients and started enrolling in the first quarter of 2010.
Conclusions
Our animal data suggest that the Solitaire FR Revascularization Device is efficacious at clot removal with acceptable safety. Further evaluation in the clinical setting would be required for it to be considered as a thrombectomy device in acute ischemic stroke.
Abbreviations
- A
artery
- APA
ascending pharyngeal artery
- FDA
US Food and Drug Administration
- FR
flow restoration
- L
left
- MCA
middle cerebral artery
- N/A
not applicable
- R
right
- Renal A
renal artery
- TIMI
Thrombolysis In Myocardial Infarction
- UCLA
University of California, Los Angeles
References
- 1. Penumbra Pivotal Trial Stroke Investigators. The Penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–68. Epub 2009 Jul 9 [DOI] [PubMed] [Google Scholar]
- 2. Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: results of the Multi Mechanical Embolus Removal In Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006;27:1177–82 [PMC free article] [PubMed] [Google Scholar]
- 3. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 4. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 5. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–74 [DOI] [PubMed] [Google Scholar]
- 6. Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006;27:1357–61 [PMC free article] [PubMed] [Google Scholar]
- 7. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings—TIMI Study Group . N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 8. Gralla J, Schroth G, Remonda L, et al. Mechanical thrombectomy for acute ischemic stroke: thrombus-device interaction, efficiency, and complications in vivo. Stroke 2006;37:3019–24 [DOI] [PubMed] [Google Scholar]
- 9. Brekenfeld C, Schroth G, El-Koussy M, et al. Mechanical thromboembolectomy for acute ischemic stroke: comparison of the catch thrombectomy device and the Merci retriever in vivo. Stroke 2008;39:1213– 19. Epub 2008 Feb 28 [DOI] [PubMed] [Google Scholar]
- 10. Castano C, Serena J, Davalos A. Use of the new Solitaire AB device for mechanical thrombectomy when Merci clot retriever has failed to remove the clot: a case report. Interv Neuroradiol 2009;15:209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henkes H, Liebig T, Miloslavski E, et al. Endovascular acute ischemic stroke treatment using the self-expanding and fully retrievable Solitaire stent. Stroke 2009;40:e247–48 [Google Scholar]