Abstract
BACKGROUND AND PURPOSE:
Aneurysms are rarely associated with symptomatic intracranial stenosis. We report the results of recanalization by stent placement in patients with symptomatic severe intracranial stenosis associated with adjacent aneurysms.
MATERIALS AND METHODS:
Of 139 patients who underwent intracranial stent placement during a 5-year period, 10 (7%) had symptomatic severe intracranial stenosis associated with adjacent aneurysms. Five were in the VA, 3 in the BA, and 2 in M1. The types of aneurysm were atherosclerotic fusiform (n = 5), ulcerative (n = 4), and saccular (n = 1). We analyzed angiographic findings based on biplane and 3D angiograms and assessed patient outcomes and complications after stent placement. The results were compared with those of a control group without aneurysms who underwent stent placement during the same study period.
RESULTS:
Aneurysm locations were post- (n = 6), in- (n = 2), and pre-stenotic (n = 2). After angioplasty with stent placement and/or aneurysm embolization, there were no lesion-related strokes or deaths during a median follow-up period of 25 months (range, 11–43 months). One patient had asymptomatic restenosis. The final mRS score was good (≤2) in all patients. There were no statistically significant differences in event or restenosis rates compared with the control group.
CONCLUSIONS:
Adjacent aneurysms were rarely associated with severe intracranial stenosis but were more common in the posterior circulation. Intracranial stent placement may be performed without additional stroke risk, regardless of the type and location of the aneurysm.
Intracranial atherosclerosis is a major cause of ischemic stroke, accounting for 8%–15% of all strokes.1,2 Intracranial angioplasty and stent placement have become therapeutic options for patients with symptomatic intracranial arterial stenoses unresponsive to medical therapy.3–6 The North American Symptomatic Carotid Endarterectomy Trial reported that 3.1% of patients with carotid stenosis had aneurysms.7 To the best of our knowledge, however, there have been no reports on intracranial stenoses associated with adjacent aneurysms in the same arterial segment. Recanalization by stent placement of intracranial stenoses in patients with adjacent aneurysms may be associated with a greater risk of complications than is present in patients without aneurysms. Stent-placement procedures in the former patient group can lead to difficulties because of the need to manage an aneurysm in the same anatomic segment. Although stent placement can protect the neck of the aneurysm, dilation of the stenotic vessel can increase the risk of aneurysm rupture.
To assess clinical outcomes in patients with intracranial aneurysms adjacent to stenoses, we analyzed the morphologic type and location of aneurysms. We compared outcomes in such patients with those of a control group of patients with intracranial stenoses but without aneurysms who underwent stent placement during the study period.
Materials and Methods
Review of the prospectively collected neurointerventional data registry of the Asan Medical Center showed that of the 139 consecutive patients who underwent intracranial stent placement between August 2003 and December 2008, 10 (7%) had aneurysms. All such patients had symptomatic severe intracranial (≥70%) stenoses associated with adjacent aneurysms in the same arterial anatomic segment.5,8 We excluded patients with acute or unstable stroke and those with aneurysms not adjacent to stenoses (n = 8). The study was approved by our institutional review board.
Patient age ranged from 54 to 73 years of age (mean, 62 years). There were 9 men and 1 woman. All patients were symptomatic (6 with stroke and 4 with TIA), and all underwent brain MR imaging before stent placement. DWI performed in 7 patients showed that 6 had new ischemic lesions. These were in the borderzone in 2 patients, scattered superficially in the cortex in 3, and in the brain stem in 1 patient. The single patient without any diffusion abnormality had an old lacunar infarction in the left basal ganglia and showed symptoms of TIA with severe stenosis of the mid-BA on MR angiography. Of the 3 patients who did not undergo DWI, 1 had a subacute infarction and 2 had no brain parenchymal lesions. In the latter patients, severe atherosclerotic stenosis in the vertebral system was associated with symptoms of TIA, such as severe intermittent dizziness or vertigo.
We assessed the type, shape, and location of each aneurysm adjacent to a stenosis, with “adjacent” being defined as the presence of both the stenosis and the aneurysm in the same arterial anatomic segment.5,9 An “aneurysm” was defined as an artery diameter >1.5-fold that of the adjacent normal lumen. To the best of our knowledge, aneurysm configurations related to severe intracranial atherosclerotic stenoses have not been previously described. We defined such configurations as “atherosclerotic fusiform” or “atherosclerotic ulcerative” and “saccular” (Table). An “atherosclerotic fusiform” aneurysm (n = 5) was defined as an aneurysm consisting of a dilated arterial segment without definite neck formation; this was differentiated from a poststenotic focal dilation by the location, degree, and length of dilation. We distinguished fusiform aneurysms by size criteria because it may be difficult to differentiate post-stenotic dilations from fusiform aneurysms.10–12 An “atherosclerotic ulcerative” aneurysm (n = 4) was defined as an aneurysm with a sac-like undermining ulcer, an aneurysmal neck, and an irregular adjacent arterial wall and was differentiated from a dissecting aneurysm by the clinical course and angiographic features, defined as focal involvement of the ulceration within the atherosclerotic stenotic segment. As defined here, an ulcerative aneurysm may correspond to a penetrating atherosclerotic ulcer or a saccular pseudoaneurysm in the aorta.13,14 A “saccular” aneurysm (n = 1) was defined as an aneurysm with a smooth berry-shaped body and a neck that had enlarged by the 2-year follow-up in the single affected patient (patient 9).
Summary of the patient data
| No. | Sex/Age (yr) | Symptom | Initial NIHSS Score | Stenosis Location | Aneurysm |
FU (mo) | Final mRS Score | |
|---|---|---|---|---|---|---|---|---|
| Shape | Location | |||||||
| 1 | M/61 | Stroke | 3 | M1 | Fusiform | Pre- | 23 | 1 |
| 2 | M/57 | Stroke | 3 | BA | Fusiform | Post- | 35 | 1 |
| 3 | F/60 | Stroke | 4 | M1 | Fusiform | Post- | 26 | 0 |
| 4 | M/73 | Stroke | 6 | BA | Fusiform | Post- | 25 | 1 |
| 5 | M/54 | Stroke | 6 | VA | Ulcerative | In- | 16 | 1 |
| 6 | M/58 | TIA | 0 | VA | Fusiform | Post- | 29 | 0 |
| 7 | M/65 | TIA | 3 | VA | Ulcerative | In- | 18 | 0 |
| 8 | M/69 | TIA | 0 | VA | Ulcerative | Post- | 43 | 0 |
| 9 | M/56 | TIA | 0 | BA | Saccular | Pre- | 25 | 0 |
| 10 | M/62 | Stroke | 3 | VA | Ulcerative | Post- | 11 | 1 |
The location of each aneurysm was classified as post-, in-, or prestenotic. Lumen size or degree of stenosis and dilation were measured by using Quantitative Vascular Analysis software (Pie Medical Imaging, Maastricht, the Netherlands) based on the Warfarin-Aspirin Symptomatic Intracranial Disease trial methods.15
Stent placement was performed after balloon angioplasty due to recoiling in the M1 portion of the middle cerebral artery in 2 patients, in the intradural VA in 5, and in the BA in 3 (Fig 1). Seven patients received 1 stent each, whereas 3 each received 2 stents. We used balloon-expandable stents, including RX Driver (Medtronic, Minneapolis, Minnesota) in 8 vessels, Vision stents (Guidant, Santa Clara, California) in 2, Cypher select stents (Cordis, Miami Lakes, Florida) in 1, and Neuroform stents (Boston Scientific/Target, Fremont, California) in 2. In addition, 3 patients who required additional bailout stent placement because of a growing intraluminal filling defect at the margin of the first stent, suggesting dissection of the intimal flap, received 1 stent each, 2 of which were Neuroform and Vision. No patient underwent MR imaging after stent placement unless a newly developed neurologic deficit was evident.
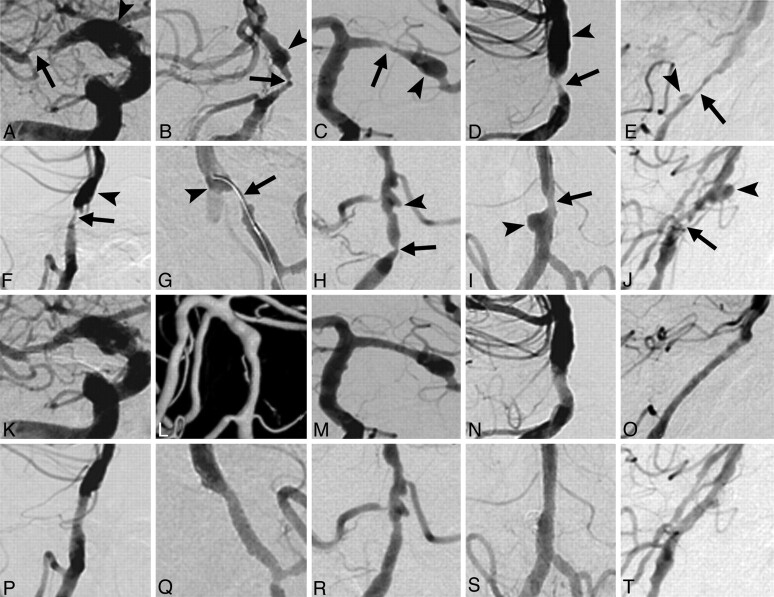
Fig 1.
Prestenting (A–J) and poststenting (K−T) angiograms of the 10 patients, matched to patients 1–10 in the Table. Severe stenoses (arrows) with adjacent aneurysms (arrowheads) were recanalized by stent placement. E and O, After stent placement, a branch of the anteroinferior cerebellar artery was retrogradely opacified on delayed imaging (not shown). G and Q, A guidewire was repeatedly rolled within each ulcerative aneurysm, which disappeared after stent placement. I, J, S, and T, Stent-assisted coil embolization was also performed for the aneurysms of patients 9 and 10.
Patients were followed clinically for 11-43 months (median, 25 months). Follow-up imaging to detect possible restenosis was performed in 9 patients in the 7- to 35-month interval (median, 16 months) by using digital subtraction angiography (n = 3), TCD (n = 1), or CTA (n = 5). Restenosis (>50%) was defined by cerebral angiography and increased flow velocity relative to the prestenting value on follow-up TCD.15 Restenosis in 5 patients, followed by CTA, was determined by binary estimation (>50%) after delineation of traced-stented vessel segments by using Advanced Vessel Analysis (Siemens Medical Systems, Erlangen, Germany) or visual inspection of luminal patency along the stented vessel in conjunction with TCD.
The percentage diameter of stenosis, the minimal lumen diameter, and the reference diameter before and after stent placement were measured. The preprocedure as well as the procedure per se were identical to the intracranial stent placement method previously described.4 On completion of angioplasty and stent placement, each patient received clopidogrel, 75-mg by mouth once a day for at least 6 months, and aspirin 100 mg by mouth once a day for life. Patients having a long lesion or a stent <2.5 mm in lumen diameter were also prescribed cilostazol, 50–100 mg twice a day for 1–6 months.
Bivariate analysis of event and restenosis rates was performed by using the χ2 test and the Fisher exact test. All reported probability values are 2-sided, and a P value < .05 was considered statistically significant. All statistical analyses were performed by using SAS, Version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
Successful recanalization by stent placement was achieved in all 10 patients (Fig 1). Of the 8 patients who underwent angioplasty and stent placement, 3 had in-stenotic ulcerative aneurysms, which disappeared immediately after stent placement; and these patients did not require further treatment. Two patients, 1 with a saccular sidewall broad-necked aneurysm in the proximal portion of the stenosis of the mid-BA and the other with a saccular aneurysm in the distal portion of the stenosis of the right intradural vertebral artery, underwent stent-assisted embolization of the aneurysm followed by stent placement of the stenosis. After ensuring that the broad aneurysmal neck was protected with a self-expandable stent, we embolized the aneurysm with a Guglielmi detachable coil, and an additional balloon-expandable stent was inserted to relieve the stenosis.
There were no lesion-related strokes or deaths during the 11- to 43-month follow-up period (median, 25 months). The final mRS score was good (≤2) in all patients, with restenosis required in only 1 of 9 patients during the 7- to 35-month follow-up period (median, 16 months).
The control group, consisting of 129 patients without aneurysms who underwent stent placement during the same period, had 12 events 1–45 months (median, 15 months) after stent placement. Restenosis or occlusion or both were observed in 7 of 87 patients after 1–36 months (median, 12 months). No significant differences in event frequency (P = .601) or restenosis (P = .556) rates were observed between the 2 groups.
Discussion
Although we found that aneurysms were rarely associated with symptomatic severe intracranial stenosis (7%) and were more common in the posterior circulation (80%), the aneurysms were of various types, including fusiform, ulcerative, and saccular. Because bypass surgery for intracranial stenosis or surgery to treat the aneurysm could not be considered in such patients, stent placement may be a good therapeutic option to improve perfusion status regardless of aneurysm type. Therefore, each type of aneurysm required a different stent-placement strategy both to correct the stenosis and to protect the aneurysm.
Pre- and post-stenotic fusiform aneurysms did not require specific management if the stent size and the length of the stenotic segment could be carefully determined on the basis of the proximal or distal arterial segment or of the normal opposite arterial segment. Stent placement of the stenotic segment, including ulcerative aneurysms, led to good luminal patency if the stent placement covered the ulcerative aneurysmal segment. In such patients, the aneurysmal lumen finally collapsed and disappeared after stent placement.
Although none of our 10 patients had a stroke during the median 25-month follow-up period, indicating that the outcome of concomitant treatment of the adjacent aneurysm associated with intracranial stenosis was relatively good, longer follow-up and/or a larger number of patients will be required to confirm the efficacy and safety of this approach. Although we did not routinely perform DWI after the procedure, stent placement may be associated with subclinical neurologic ischemic events that can only be detected by DWI. In addition, knowledge of the exact pathology of the aneurysm may further clarify the pathologic features of such disease entities.
Conclusions
Adjacent aneurysms were rarely associated with severe intracranial stenosis and were more common in the posterior circulation. Our findings suggest that stent placement in an intracranial stenosis in the presence of an adjacent aneurysm does not increase either event or restenosis rates. Stent placement can, therefore, be performed without increasing the risk of stroke, regardless of the type and location of the aneurysm.
Acknowledgments
We acknowledge the assistance of Sun Moon Whang, BS, and Eun Hye Kim, RN, in patient data collection, as well as that of Yun Gyeong Jeong in preparing the manuscript. We thank Bonnie Hami, MA (United States), for her English editorial assistance.
Abbreviations
- BA
basilar artery
- CTA
CT angiography
- DWI
diffusion-weighted imaging
- FU
follow-up
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- Pre-
pre-stenotic
- In-
in-stenotic
- Post-
post-stenotic
- TCD
transcranial Doppler sonography
- TIA
transient ischemic attack
- VA
vertebral artery
Footnotes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A080201).
References
- 1. Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: The Northern Manhattan Stroke Study. Stroke 1995;26:14–20 [DOI] [PubMed] [Google Scholar]
- 2. Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996;27:1974–80 [DOI] [PubMed] [Google Scholar]
- 3. Lylyk P, Cohen JE, Ceratto R, et al. Angioplasty and stent placement in intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol 2002;23:430–36 [PMC free article] [PubMed] [Google Scholar]
- 4. Suh DC, Kim JK, Choi JW, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol 2008;29:781–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pyun HW, Suh DC, Kim JK, et al. Concomitant multiple revascularizations in supra-aortic arteries: short-term results in 50 patients. AJNR Am J Neuroradiol 2007;28:1895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi JW, Kim JK, Choi BS, et al. Angiographic pattern of symptomatic severe M1 stenosis: comparison with presenting symptoms, infarct patterns, perfusion status, and outcome after recanalization. Cerebrovasc Dis 2010;29:297–303 [DOI] [PubMed] [Google Scholar]
- 7. Kappelle LJ, Eliasziw M, Fox AJ, et al. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis: North American Symptomatic Carotid Endarterectomy Trial Group. Neurology 2000;55:307–09 [DOI] [PubMed] [Google Scholar]
- 8. Suh DC, Kim EH. The therapeutic time window related to the presenting symptom pattern: that is, stable versus unstable patients, can affect the adverse event rate of intracranial stenting. Stroke 2009;40:e588–89, author reply e90. Epub 2009 Sep 10 [DOI] [PubMed] [Google Scholar]
- 9. Jang Y-G, Ryu CW, Kim JS, et al. Dissecting aneurysm of the basilar arterial trunk presenting with pontine infarction: coil obliteration of the dissecting aneurysm including the diseased basilar arterial segment. Interv Neuroradiol 2007;13:381–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stehbens WE. Evaluation of aneurysm models, particularly of the aorta and cerebral arteries. Exp Mol Pathol 1999;67:1–14 [DOI] [PubMed] [Google Scholar]
- 11. Dobrin PB. Poststenotic dilatation. Surg Gynecol Obstet 1991;172:503–08 [PubMed] [Google Scholar]
- 12. Rainer WG. Coronary arterial aneurysm vs poststenotic dilatation. Chest 1976;70:688–89 [DOI] [PubMed] [Google Scholar]
- 13. Macura KJ, Corl FM, Fishman EK, et al. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol 2003;181:309–16 [DOI] [PubMed] [Google Scholar]
- 14. Yoo SM, Lee HY, White CS. MDCT evaluation of acute aortic syndrome. Radiol Clin North Am 2010;48:67–83 [DOI] [PubMed] [Google Scholar]
- 15. Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–46 [PMC free article] [PubMed] [Google Scholar]