Abstract
BACKGROUND AND PURPOSE:
Depression occurs frequently in PD; however the neural basis of depression in PD remains unclear. The aim of this study was to characterize possible depression-related white matter microstructural changes in the thalamus of patients with DPD compared with those with NDPD.
MATERIALS AND METHODS:
FA and MD maps from DTI were obtained in 14 patients with DPD and 18 patients with NDPD. Region-of-interest−guided VBA was conducted on the FA maps to detect possible microstructural differences in the thalamus between these 2 patient groups. Moreover, mean FA and MD in regions with a detected difference were compared between DPD and NDPD groups, and correlations between diffusion quantities and the severity of depression were analyzed.
RESULTS:
White matter microstructure differences were found between the patients with DPD and NDPD in the bilateral mediodorsal thalamic regions. In these regions, patients with DPD showed significantly decreased FA values (P < .005) compared with patients with NDPD, and the mean values of FA were negatively correlated with the scores of depression severity (P < .05) for patients with PD. No significant differences of MD were found in the mediodorsal thalamus between these 2 groups.
CONCLUSIONS:
Our results provide preliminary evidence that the mediodorsal thalamus may play an important role in depression in PD and suggest a relationship between FA in the mediodorsal thalamus and the presence of depressive symptoms in patients with DPD. These findings may be helpful for further understanding the potential mechanisms of depression in PD.
Depression occurs frequently in PD, in approximately 40% of patients,1–3 which is much higher than that in other equivalently disabled patients.3–6 Researchers have realized that depression may not just be a reaction to PD disabilities but rather results from neurodegenerative changes occurring in PD.7,8 However, the pathophysiology and neural basis of depression in PD remain unclear.3,5
The thalamus has reciprocal connections with the amygdala and dorsolateral prefrontal, orbitofrontal, cingulate, and insular cortices,9–11 and it plays an important role in the perception and regulation of emotion.12,13 Converging evidence suggests that there is a relationship between depression and morphologic and metabolic changes in the mediodorsal or “limbic” thalamus.5,11,14,15 For example, in a recent study, Young et al11 found an increased neuron count in individuals with MDD relative to the nonpsychiatric comparison subjects. Furthermore, it has been shown that depression and anxiety induced by the tyrosine hydroxylase inhibitor are associated with a marked reduction of glucose metabolism in the thalamus.14 The research by Cardoso et al5 showed a decreased activation in the left mediodorsal thalamus in patients with DPD compared with patients with NDPD in an fMRI study. In this same sample, a region-of-interest VBM study showed increased volume in the mediodorsal thalamus bilaterally. Research on the biochemical markers also suggests a role of the thalamus in depression in PD. Using [11C]RTI-32, a marker of dopaminergic and noradrenergic metabolism, Remy et al3 showed decreased binding in patients with DPD compared with patients with NDPD in a variety of regions, including the thalamus.
DTI16–19 has been increasingly used in the analysis of psychiatric disease.20–24 With DTI techniques, diffusion anisotropy characteristics can be fully extracted, characterized, and exploited, providing even more exquisite details on white matter microstructural integrity.18,25 Diffusion quantities, such as FA and MD, can be derived from DTI and are usually used for white matter integrity analysis.25,26 Previous DTI studies have also demonstrated a close relationship between depression and the thalamus.27,28 However, there are few studies characterizing white matter diffusion changes in patients with DPD. Matsui et al20 examined the FA value changes between patients with DPD and those with NDPD. In this study, 14 regions of interest were compared, and they found significant reductions in FA values in bilateral frontal regions of interest.20 However, the thalamic regions were not selected, and only FA values were analyzed in the selected regions.20
All the above findings led us study the possible white matter microstructural changes related to depression in the thalami of patients with DPD. Here we conducted a DTI study to analyze possible white matter microstructural differences in the whole thalamus between patients with DPD and those with NDPD and to investigate the relationship between the severity of depression and diffusion quantities in the thalamus of patients with PD.
Materials and Methods
Subjects and Clinical Assessment
Thirty-six individuals with idiopathic PD with or without depression were enrolled in the present study. Patients had already been excluded if they had any other organic or central nervous system diseases. All patients fulfilled the UK Parkinson's Disease Society Brain Bank criteria for idiopathic PD.29 Motor and mental states were evaluated by the UPDRS III,30 the H-Y,30 and the MMSE.31 Patients with MMSE scores lower than the corresponding education level were also excluded. The included patients with PD were then divided into DPD and NDPD groups by the DSM-IV criteria.32 The HAMD33 was implemented to evaluate the severity of depression. Higher HAMD scores reflect greater severity of depressive symptoms. For every patient, all the psychometric and neurologic evaluations were conducted during a practically defined “off” state. The LEDD was also calculated on the basis of every patient's daily dosage as per Matsui et al.20 Between DPD and NDPD groups, unpaired 2-tailed t tests were used for parametric comparison of data for age, PD duration, MMSE, UPDRS, H-Y, HAMD, and LEDD; the Mann-Whitney U test was used for the nonparametric comparison for sex.
All patients were recruited from Xuanwu Hospital-Capital Medical University (Beijing, China), and written informed consent was obtained. The study was approved by Medical Research Ethical Committee of Xuanwu Hospital.
DTI
All the patients had taken their habitual anti-Parkinson dosage 30 minutes before MR imaging, and they were in the “on” state before and during MR imaging. DTI was performed with a 3T MR imaging scanner (Magnetom Trio; Siemens, Erlangen, Germany). The images were obtained by using echo-planar imaging sequences with 20 different motion-probing gradient directions (TR/TE, 6000/93 ms; matrix, 128 × 128; FOV, 256 × 256 mm; section thickness, 4 mm; b-value, 1000 s/mm2). In addition, 1 reference image without diffusion weighting (b = 0 image with a b-value of 0 s/mm2) was acquired. For each subject, DTI scans were acquired twice for subsequent averaging to improve the signal intensity–to-noise ratio.
Image Preprocessing and Diffusion Quantity Calculation
All of the following image preprocessing, diffusion quantity calculation, and data analysis were implemented by using the FSL 4.1 tools (FMRIB, Oxford, UK); Statistical Package for the Social Sciences, Version 17.0 (SPSS, Chicago, Illinois); and in-house software based on Matlab (MathWorks, Natick, Massachusetts).
Before calculating the diffusion tensors from DTI images, volume data were first re-sampled to a spatial isotropic dataset (voxel size, 1 × 1 × 1 mm) and then were preprocessed to remove skull data and correct the effects of head movement and eddy currents by using FSL.34,35 After the above image preprocessing, mean DTI images were calculated from the 2 preprocessed DTI acquisitions for every subject. Diffusion quantities of FA and MD were calculated subsequently on the mean DTI images. These diffusion quantities provide in vivo information about the organization of white matter.18,36
Region-of-Interest−Guided VBA in Bilateral Thalamic Regions
A region-of-interest−guided VBA method was used to detect significant white matter microstructure changes in the bilateral thalami between patients with DPD and those with NDPD. First, all FA images of every subject were aligned to a 1 × 1 × 1 mm MNI 152 standard space by using a nonlinear registration method provided by TBSS script in FSL tools,37 and all MD images were also registered to MNI 152 space by using the same calculated conversion matrixes. Subsequently, a neuroradiologist (J.L.) manually drew a 3D region-of-interest mask to cover the whole thalamic region on the standard MNI 152 T1 brain images. Finally, region-of-interest−guided VBA was performed on FA maps within the defined mask to detect the significantly different regions in the bilateral thalami between patients with DPD and those with NDPD. Statistical analysis was performed by using a voxelwise unpaired 2-tailed t test. Significance was indicated with P < .05.
Group Comparison
For every subject, the mean value of the diffusion quantities, FA and MD, would be calculated for regions in which there were significant differences detected by the previous region-of-interest−guided VBA method. On the basis of individual data, group comparisons were conducted between patients with DPD and NDPD.
Correlation Analysis
The correlations between the clinical score for the HAMD and diffusion quantities (mean FA or mean MD) in the confirmed significant regions were assessed respectively for all patients with PD by using a Pearson correlation coefficient with statistical significance evaluated by using nonparametric methods (P < .05) by SPSS.
Results
Clinical Results
Of the 36 recruited candidates, 4 patients were excluded from this study (2 due to claustrophobia and 2 due to motion artifacts). On the basis of the DSM-IV criteria, the 32 included patients were divided into 2 groups: 14 patients with DPD and 18 patients with NDPD. The clinical and demographic characteristics of the patients are given in Table 1. There was no significant difference between the DPD and NDPD patient groups as to age, sex, PD duration time, MMSE, UPDRS, H-Y, or LEDD. The 2 groups showed statistically significant differences in the HAMD score (P < .001).
Table 1:
Clinical and demographic characteristics of patients
| Groups | DPD (n = 14) | NDPD (n = 18) | P Valuea |
|---|---|---|---|
| Sex (M/F) | 4/10 | 10/8 | NS |
| Age (yr) | 65.28 ± 8.89 | 61.05 ± 10.17 | NS |
| PD duration | 6.29 ± 5.51 | 5.67 ± 2.57 | NS |
| MMSE | 29.5 ± 0.7 | 29.2 ± 1.0 | NS |
| UPDRS | 39.04 ± 22.28 | 33.83 ± 15.09 | NS |
| H-Y | 1.96 ± 0.99 | 1.83 ± 0.75 | NS |
| HAMD | 15.64 ± 4.21 | 4.44 ± 2.14 | <.001 |
| LEDD (mg/day) | 315 ± 201 | 363 ± 197 | NS |
Unpaired 2-tailed t tests were used for parametric comparison of data between the 2 groups for age, PD duration, MMSE, UPDRS, H-Y, HAMD, and LEDD; The Mann-Whitney U test was used for nonparametric comparison of sex between the 2 groups. Significance was set at P <. 05.
VBA in the Whole Thalamus
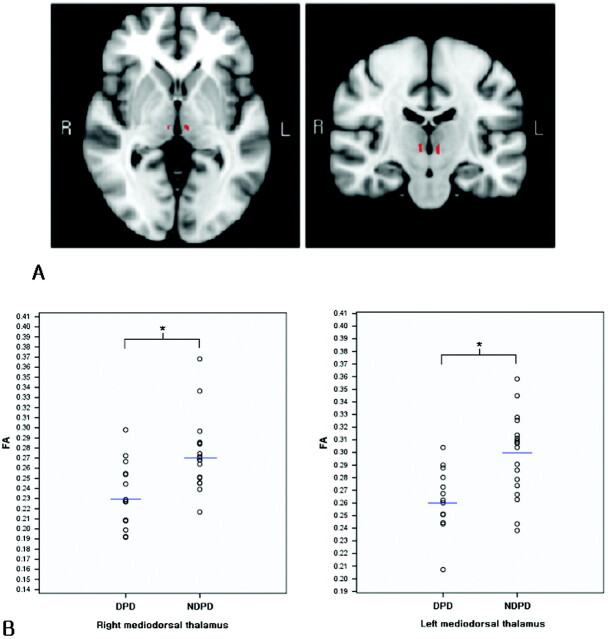
By region-of-interest−guided VBA analysis, a decreased FA value was found in the thalamus bilaterally in patients with DPD compared with patients with NDPD (P < .05). As shown in red in Fig 1A, the 2 regions differed significantly. These regions were in the right and left mediodorsal thalami, respectively. Compared with those in patients with NDPD, no regions in the thalamus of patients with DPD showed significantly increased FA values.
Fig 1.
Illustration of regions of difference in patients with DPD and NDPD in the bilateral thalami. A, Standard MNI 152 T1 brain images (in axial and coronal directions) are overlaid with the statistically significant differing regions (in red). FA in the mediodorsal thalamus differs in patients with DPD compared with patients with NDPD. B, Scatterplots show the mean FA values in the detected significant regions for every subject in the DPD and NDPD groups. Blue lines denote the mean values for each group. Asterisks indicate significant differences between these 2 groups with P < .005.
Group Comparisons
These 2 detected regions were taken as regions of interest for a further comparison of patients with DPD and NDPD. Figure 1B shows the scattergraphs of the mean FA values in the detected bilateral mediodorsal thalami for every subject. Table 2 shows the mean values and SDs of FA and MD in the detected regions of interest of DPD and NDPD groups respectively and the comparison results of mean FA and mean MD between these 2 groups. Patients with DPD show significantly decreased mean FA values in the bilateral mediodorsal thalami compared with patients with NDPD (Fig 1A, right region of interest, P = .003; left region of interest, P = .002). There were no significant differences in MD between patients with DPD and NDPD in the same regions (P > .05).
Table 2:
Diffusion quantity comparison between patients with DPD and those with NDPD
| Mediodorsal Thalamus | mFA |
t Valuea | P Value | mMD (×10−4 mm2/s) |
t Valuea | P Value | ||
|---|---|---|---|---|---|---|---|---|
| DPD | NDPD | DPD | NDPD | |||||
| Right | 0.23 ± 0.03 | 0.27 ± 0.04 | −3.300 | .003b | 8.92 ± 2.06 | 8.26 ± 1.22 | 1.061 | .301 |
| Left | 0.26 ± 0.02 | 0.30 ± 0.03 | −3.463 | .002b | 8.27 ± 1.03 | 7.97 ± 0.71 | 0.924 | .365 |
Indicates unpaired 2-tailed t test.
Indicates statistical significance with P < .005.
Correlation Analysis
Mean FA and MD in the detected mediodorsal thalamic regions were used for correlation analysis with HAMD. Higher HAMD scores reflect greater severity of depressive symptoms. Table 3 shows the results of the Pearson correlation between HAMD and the diffusion quantities, mean FA and mean MD, for all patients with PD. Parameter r represents the Pearson correlation coefficient. Figure 2shows the scatterplots with trend lines indicating the correlation relationship of mean FA and MD with HAMD for all subjects with PD. From Table 3 and Fig 2, one can see that HAMD was significantly negatively correlated with mean FA values in the bilateral mediodorsal thalami for all subjects with PD (P < .05). The mean FA did not show a correlation with HAMD for patients with DPD or NDPD separately. The mean MD did not show a correlation with HAMD for all patients with PD or for those with DPD or NDPD. Results showed that there was a negative correlation between the severity of depression (HAMD scores) and FA values in the detected right and left mediodorsal thalami for all subjects with PD.
Table 3:
Pearson correlation between HAMD and diffusion quantities for PDa
| Regions | mFA |
mMD |
||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| Right mediodorsal thalamus | −0.362 | .041b | 0.134 | .466 |
| Left mediodorsal thalamus | −0.404 | .022b | 0.063 | .731 |
SPSS, Version 17.0, was used for correlation analysis. For correlation coefficient calculation, the Pearson test was selected; for the significance test of r, a 2-tailed t test was selected.
Indicates statistical significance with P < .05.
Fig 2.
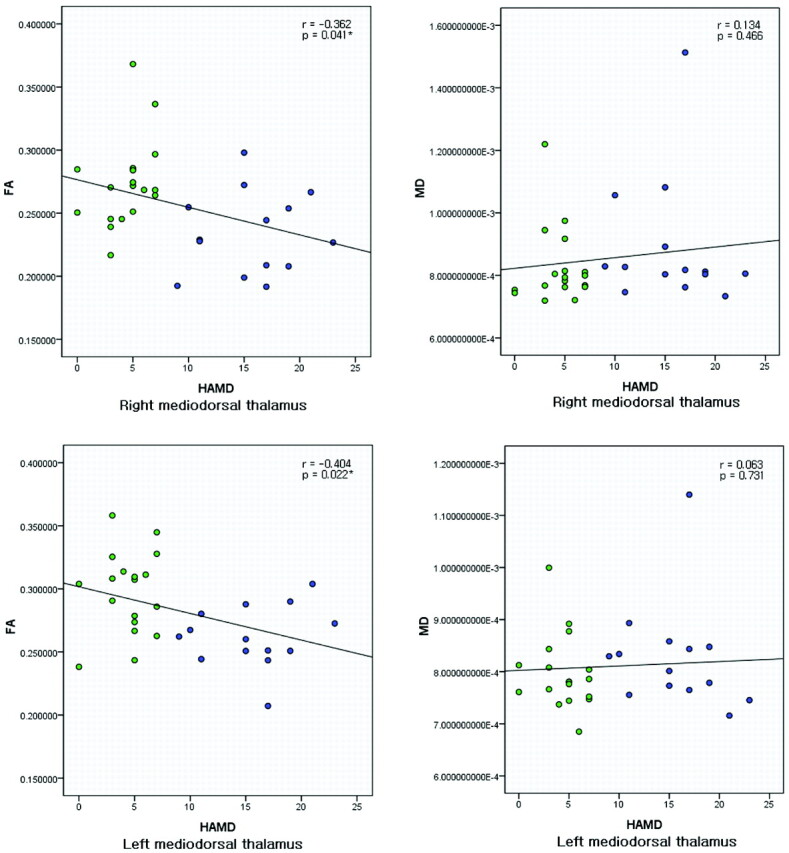
Scatterplots with trend lines showing the correlations of diffusion quantities (FA and MD) with HAMD for 32 PD subjects in the right and left mediodorsal thalami. Green represents patients with NDPD; blue represents patients with DPD. The asterisks indicate that a significant negative correlation was found between FA and HAMD.
Discussion
To the best of our knowledge, this is the first study of FA and MD differences in the thalami of patients with DPD compared with patients with NDPD by using DTI. On the basis of the VBA of FA maps in the whole thalamus, a significant difference was detected between patients with DPD and NDPD in the bilateral mediodorsal thalami (Fig 1A). In the detected regions showing a difference, patients with DPD had decreased mean FA values in the right (P = .003) and left (P = .002) mediodorsal thalami, compared with patients with NDPD (Fig 1B and Table 2). Our findings are consistent with a recent study of Cardoso et al,5 who found a decreased activation in the mediodorsal thalamus between their patients with DPD and those with NDPD by using fMRI. Decreased FA in the thalamus of patients with DPD indicates a change in neuron cell integrity or a possible fiber degeneration in the bilateral mediodorsal thalami relative to patients with NDPD. This finding may be the cause or result of a decrease in either incoming or projecting white matter pathways associated with the mediodorsal thalamus. The mediodorsal thalamus receives strong dopaminergic projections, which are important for modulation of thalamic activity,5,13 and loss of dopamine was found in the limbic system in patients with DPD compared with patients with NDPD, including the mediodorsal thalamus.3 An increased neuronal count was reported in the mediodorsal thalamus of patients with MDD in previous studies,11 indicating that there may also be a change in the diffusion style and anisotropy of free water inside the brain. Our results of decreased FA in the mediodorsal thalamus of patients with DPD, together with other reports,5,11 highlight the importance of the limbic thalamus in depression in PD.
While we show a decreased FA in the mediodorsal thalamus, no significant differences in MD were found in the same mediodorsal thalamic regions between these 2 groups. MD characterizes the average magnitude of overall molecular displacement by diffusion17,18 and represents the integrity of the cellular matrix.24 FA describes how much molecular displacement varies in space17,18 and can reflect the degeneration of axons in the major fiber bundles.24 Therefore, our findings suggest that the arrangement and organization of white matter in the mediodorsal thalamus of patients with DPD were different from such organization in patients with NDPD, while the integrity of the cellular matrix did not change obviously in the same regions between these 2 groups in our study.
Our study also showed a significantly negative correlation between the severity of depression (HAMD scores) and the mean FA values in bilateral mediodorsal thalamic regions (P < .05) for patients with PD (Fig 2 and Table 3). The lower FA values in this region correlated with the increased depressive symptoms in patients with PD. This finding provides new information for further understanding the potential mechanisms of depression in patients with PD.
DTI provides information about white matter microstructure integrity. This unique imaging technique enables us to quantify the thermal random motion of water molecules.18,38,39 As mentioned above, the literature on DPD research using DTI is quite limited. Matsui et al20 examined FA changes between NDPD and DPD groups by using a region-of-interest–analysis method, but the authors did not evaluate thalamic regions. Their method depended on a priori assumptions of the location, size, and shape of the regions.20 Moreover, their method was labor-intensive, and their results were operator-dependent. In our study, a region-of-interest−guided VBA method was adopted. Compared with the region-of-interest–analysis method, the region-of-interest−guided VBA method can reliably locate the regions with diffusion quantity differences, providing a feasible approach to obtaining more accurate clinical results.
Although the region-of-interest−guided VBA method has the above advantages, it still has some disadvantages. The VBA method is sensitive to various image preprocessing steps, such as image registration. Moreover, the region-of-interest−guided VBA method requires conducting a VBA within the defined region of interest. In our study, the thalamus was studied as a specific region of interest, but the thalamus may be involved in a complex system with other related regions, which together modulate the depressive symptoms of patients with PD.
There are several limitations of the present study, which future studies can address. First, because we conducted a preliminary analysis, the relatively small sample size decreased the power of our results. Although we obtained significant difference in FA between patients with DPD and NDPD, more subjects are now being recruited for subsequent research to obtain a larger sample size. Second, subjects with more consistent demographic characteristics will be considered. In the current study, though there were no significant differences between DPD and NDPD groups with regard to age, sex, PD duration, MMSE, UPDRS, H-Y, and LEDD, the distribution of sex for these 2 groups was not exactly equal. Subjects in our future studies will be recruited to obtain a sample that is better matched for sex. Third, in further studies, DTI data will be collected with a higher spatial resolution. In this study, the section thickness of DTI was 4 mm. To get more accurate analysis results and for further fiber tractography−based analysis, we will acquire images with higher spatial resolution. Finally, although our current study suggested the mechanistic importance of the thalamus in depression in patients with PD by comparing patients with DPD with patients with NDPD, it is still a preliminary study. Healthy control subjects, together with individuals with de novo depression, will be recruited in the future. Through comparison among DPD, NDPD, de novo depression, and heathy controls, we should have a clearer view of white matter microstructure evolution in the thalamus associated with depression in patients with PD.
Conclusions
It is striking that decreased FA was found in the bilateral mediodorsal thalamic regions of patients with DPD compared with patients with NDPD, and the mean value of FA was negatively correlated with depression severity for patients with PD. Our preliminary results provided evidence that the mediodorsal thalamus may play an important role in depression in PD, and there is a relationship between FA in the mediodorsal thalamus and the presence of depressive symptoms in patients with DPD. These findings may be helpful for further understanding potential neural mechanisms of depression in PD.
Abbreviations
- DPD
depressed PD
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- fMRI
functional MR imaging
- FMRIB
Functional MR Imaging of the Brain
- FSL
FMRIB Software Library
- HAMD
Hamilton Depression Scale
- H-Y
Hoehn–Yahr staging
- LEDD
L-dopa equivalent daily dosage
- MD
mean diffusivity
- MDD
major depressive disorder
- mFA
mean value of FA
- mMD
mean value of MD
- MMSE
Mini-Mental State Examination
- MNI
Montreal Neurological Institute
- NDPD
nondepressed PD
- NS
not significant
- PD
Parkinson disease
- UPDRS
Unified Parkinson Disease Rating Scale
- VBA
voxel-based analysis
- VBM
voxel-based morphometry
Footnotes
This work was partly supported by the National Natural Science Foundation of China grants 30970769, 60532050, 60910006, 30770620, 30970771; and CAS Hundred Talents Program grant YZ200766.
References
- 1. Cummings JL, Masterman DL. Depression in patients with Parkinson's disease. Int J Geriatr Psychiatry 1999;14:711–18 [PubMed] [Google Scholar]
- 2. Shulman LM, Taback RL, Bean J, et al. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–10 [DOI] [PubMed] [Google Scholar]
- 3. Remy P, Doder M, Lees A, et al. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005;128:1314–22 [DOI] [PubMed] [Google Scholar]
- 4. Rodin G, Voshart K. Depression in the medically ill: an overview. Am J Psychiatry 1986;165:333–39 [DOI] [PubMed] [Google Scholar]
- 5. Cardoso EF, Maia FM, Fregni F, et al. Depression in Parkinson's disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage 2009;47:467–72 [DOI] [PubMed] [Google Scholar]
- 6. Cole SA, Woodard JL, Juncos JL, et al. Depression and disability in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1996;8:20–25 [DOI] [PubMed] [Google Scholar]
- 7. Burn DJ. Depression in Parkinson's disease. Eur J Neurol 2002;9:44–54 [DOI] [PubMed] [Google Scholar]
- 8. McDonald WM, Richard IH, Delong MR. Prevalence, etiology, and treatment of depression in Parkinson's disease. Biol Psychiatry 2003;54:363–75 [DOI] [PubMed] [Google Scholar]
- 9. Price JL. Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci 1999;877:383–96 [DOI] [PubMed] [Google Scholar]
- 10. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000;23:155–84 [DOI] [PubMed] [Google Scholar]
- 11. Young KA, Holcomb LA, Yazdani U, et al. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry 2004;161:1270–77 [DOI] [PubMed] [Google Scholar]
- 12. Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry 2003;54:504–14 [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, et al. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 2007;34:965–84 [DOI] [PubMed] [Google Scholar]
- 14. Bremner JD, Vythilingam M, Ng CK, et al. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA 2003;289:3125–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carlson PJ, Singh JB, Zarate JCA, et al. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx 2006;3:22–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 18. LeBihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46 [DOI] [PubMed] [Google Scholar]
- 19. Voss HU, Schiff ND. MRI of neuronal network structure, function, and plasticity. Prog Brain Res 2009;175:483–96 [DOI] [PubMed] [Google Scholar]
- 20. Matsui H, Nishinaka K, Oda M, et al. Depression in Parkinson's disease: diffusion tensor imaging study. J Neurol 2007;254:1170–73 [DOI] [PubMed] [Google Scholar]
- 21. Menke RA, Scholz J, Miller KL, et al. MRI characteristics of the substantia nigra in Parkinson's disease: a combined quantitative T1 and DTI study. Neuroimage 2009;47:435–41. Epub 2009 May 15 [DOI] [PubMed] [Google Scholar]
- 22. Lee DY, Fletcher E, Martinez O, et al. Regional pattern of white matter microstructural changes in normal aging: MCI and AD. Neurology 2009;73:1722–28. Epub 2009 Oct 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mielke MM, Kozauer NA, Chan KC, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neuroimage 2009;46:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol 2009;30:1222–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 27. Alexopoulos GS, Kelly RE, Jr. Research advances in geriatric depression. World Psychiatry 2009;8:140–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord 2009;119:132–41. Epub 2009 Apr 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scanlon BK, Katzen HL, Levin BE, et al. A formula for the conversion of UPDRS-III scores to Hoehn and Yahr stage. Parkinsonism Relat Disord 2008;14:379–80. Epub 2007 Nov 19 [DOI] [PubMed] [Google Scholar]
- 31. Kohn R, Vicente B, Rioseco P, et al. The Mini-Mental State Examination: age and education distribution for a Latin American population. Aging Ment Health 2008;12:66–71 [DOI] [PubMed] [Google Scholar]
- 32. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov Disord 2007;22:1061–68 [DOI] [PubMed] [Google Scholar]
- 33. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol 2007;2:1332–42 [DOI] [PubMed] [Google Scholar]
- 34. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:208–19 [DOI] [PubMed] [Google Scholar]
- 36. Westin CF, Maier SE, Mamata H. Processing and visualization for diffusion tensor MRI. Med Image Anal 2002;6:93–108 [DOI] [PubMed] [Google Scholar]
- 37. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505 [DOI] [PubMed] [Google Scholar]
- 38. Karagulle Kendi AT, Lehericy S, Luciana M, et al. Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol 2008;29:501–05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scherfler C, Schocke MF, Seppi K, et al. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson's disease. Brain 2006;129:538–42 [DOI] [PubMed] [Google Scholar]