Abstract
BACKGROUND AND PURPOSE:
Intracranial fusiform aneurysms, which incorporate the branch vessel and require salvaging of the parent vessel, are difficult to manage. The goal of this study was to evaluate the efficacy of reconstructive endovascular treatment of intracranial fusiform aneurysms by using a 1-stage procedure with a stent and balloon.
MATERIALS AND METHODS:
During a 3-year period, 20 patients with 20 intracranial fusiform aneurysms were treated by using a 1-stage procedure involving a balloon and stent. Subarachnoid hemorrhage was present in 15 patients. Five aneurysms were located in the anterior circulation and 15, in the posterior circulation. Clinical outcomes and periprocedural complications were evaluated in all patients. The extent of coil packing was evaluated by control angiography after embolization and classified as either complete occlusion or partial occlusion. Angiography was performed 6, 12, and 24 months after embolization to evaluate stent patency and coil packing.
RESULTS:
The 1-stage procedure by using a combination of balloon and stent was technically successful in all patients. There were no complications related to the procedure, complete occlusion was obtained in 16 patients, and partial occlusion, in 4 patients. All patients recovered well except for 2 who died due to causes unrelated to the procedure. Clinical follow-up was performed in all surviving patients at a mean of 12.3 months (range, 7–24 months), and angiography showed that the patent parent arteries were free of aneurysm recanalization or in-stent stenosis.
CONCLUSIONS:
This 1-stage procedure may provide a feasible and safe treatment strategy for the management of intracranial fusiform aneurysms that are not amenable to deconstructive embolization.
Intracranial fusiform aneurysms (IFAs) are categorized as nonsaccular aneurysms based on their morphology. These nonsaccular or truncal aneurysms are not common, and their origin is unclear. Some authors have even speculated that they may originate as a result of dissection and that the etiology of dissection may be congenital or acquired.1–3
Although the indications for treatment are controversial in these aneurysms, vigorous treatment is mandatory in some circumstances, for example, in ruptured aneurysms or symptomatic lesions. The treatment of IFAs that contain branching vessels is particularly challenging for interventional neuroradiologists or neurosurgeons.
Recently, a few studies have reported endovascular treatment of IFAs by using a stent and balloon simultaneously.4,5 We report the clinical outcomes and 2-year follow-up results of endovascular treatment of 20 IFAs by using a 1-stage procedure that involves the concurrent use of a balloon and stent.
Materials and Methods
Patients
We enrolled 20 patients with 20 IFAs from among patients with 312 aneurysms between September 2005 and September 2008 (mean age, 54 years; male:female ratio, 8:12). Medical information and angiograms were reviewed retrospectively. This study was approved by the institutional review board, and written informed consent was obtained from all 20 patients before the 1-stage procedure.
Five aneurysms were located in the internal carotid artery (ICA); 11, in the distal vertebral artery (VA); 2, in the vertebrobasilar junction (VBJ); and 2, in the basilar artery (BA).
Subarachnoid hemorrhage (SAH) was present in 15 aneurysms, and 5 aneurysms were found incidentally. Using the Hunt and Hess (HH) grading system, we classified aneurysms in 6 patients as HH grades 4–5, 9 patients as HH grades 1–3, and 5 patients as HH grade 0.
Eleven of the VA/VBJ fusiform aneurysms incorporated a branching vessel (posterior inferior cerebellar artery [PICA] or anterior inferior cerebellar artery [AICA]). The contralateral VA was hypoplastic in the remaining 2 aneurysms. IFAs that did not incorporate a branching vessel or had an involved VA that was equivalent to a contralateral VA were excluded from this study.
Endovascular Technique
All the procedures were performed with the patient under general anesthesia. In 15 cases, coil embolization was performed immediately after diagnostic cerebral angiography, and 5 procedures were performed electively. All patients had full heparinization (activated clotting time, >300 seconds) during the coil embolization, and heparinization was maintained for 24 hours after the procedure. In elective cases, dual antiplatelet medication (clopidogrel, 75 mg/day, and aspirin, 325 mg/day) was initiated 3–5 days before the procedure. In emergency cases, clopidogrel, 300 mg, and aspirin, 500 mg, were administered immediately after the procedure, and heparinization was maintained 24 hours after the procedure (activated clotting time, >300 seconds).
For the reconstructive endovascular treatment of IFAs, a 6F guiding catheter system (Envoy; Cordis Neurovascular, Miami Lakes, Florida) or a 6F Shuttle introducer system (Cook, Bloomington, Indiana) was introduced via the femoral artery. In the first step of the 1-stage procedure, a Neuroform stent (Boston Scientific, Natick, Massachusetts), Multilink Zeta (Guidant, Santa Clara, California), or Flexmaster F1 (Abbott Vascular, Rangendingen, Germany) was deployed across the aneurysm neck to preserve the parent artery and incorporating vessel. In the second step, which was performed sequentially, a microcatheter (Prowler 14 microcatheter, Cordis Neurovascular; Excelsior SL-10 microcatheter, Boston Scientific) was advanced carefully into the aneurysm through the stent struts, and an occlusion balloon catheter (HyperGlide [4 × 15/20 mm]; ev3, Irvine, California) was placed inside the stent to protect the parent vessel lumen from protrusion of the coil loop and to stabilize the microcatheter. During temporary inflation of the occlusion balloon within the stent, the coil was delivered into the aneurysm sac via the microcatheter. The temporary occlusion balloon was deflated to verify coil protrusion and in-stent thrombosis of the parent artery and patency of the incorporated branching vessel before coil detachment. This process was repeated until coil embolization was accomplished adequately. A closure device (Angio-Seal; St. Jude Medical, St. Paul, Minnesota) was used to seal off the femoral artery puncture. All treated patients were maintained with heparin for 24 hours after the procedure and clopidogrel, 75 mg/day, and aspirin, 150 mg/day, for 6 months.
Clinical Outcomes and Follow-Up
Patients were evaluated by angiography to confirm the exclusion of the aneurysm and the patency of the incorporated vessels immediately after the procedure. Angiographic results were classified as either complete occlusion (no filling of contrast agent in the aneurysm sac) or partial occlusion (residual filling of contrast agent in the remnant aneurysm sac). Periprocedural and postoperative complications, such as thromboembolism (TE), parent artery occlusion, rebleeding, in-stent thrombosis, and retroperitoneal hematoma, were also evaluated.
Patient Characteristics and Clinical Outcomes
| No | Sex | Age (yr) | Presentation (HH) | Location | Branch Vessel | Size (mm) | Stent (mm) | Occlusion | Procedural Complication | mRS | F/U Angiography | F/U (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | Incidental (0) | LVA | PICA | 8 | Neuroform 4 × 20 | P | None | 0 | IO | 24 |
| 2 | M | 50 | SAH (5) | LVA | N | 8 | Zeta coronary stent 3 × 38 | C | None | Death | NA | |
| 3 | M | 32 | Incidental (0) | LICA | N | 4 | Neuroform 4 × 20 | C | None | 0 | CO | 11 |
| 4 | F | 62 | Incidental (0) | LVA | PICA | 6 | Neuroform 4 × 20 | C | None | 0 | CO | 13 |
| 5 | F | 40 | SAH (4) | LVA | PICA | 6 | Neuroform 4 × 20 | P | Rebleeding due to coil | 1 | IO | 14 |
| Flexmaster 3 × 16 | ||||||||||||
| 6 | F | 55 | SAH (3) | RVA | PICA | 5 | Neuroform 4 × 20 | C | None | 0 | CO | 11 |
| 7 | F | 60 | SAH (4) | LVA | PICA | 7 | Neuroform 4 × 20 | C | None | 1 | CO | 13 |
| 8 | F | 45 | SAH (4) | BA | N | 6 | Neuroform 4 × 20 | C | None | 0 | CO | 11 |
| 9 | F | 42 | SAH (3) | RVA | PICA | 8 | Neuroform 4 × 20 | P | None | 1 | IO | 13 |
| 10 | F | 66 | SAH (3) | RVA | PICA | 6 | Neuroform 4 × 20 | C | None | 0 | CO | 15 |
| 11 | M | 50 | SAH (3) | LVA | PICA | 7 | Neuroform 4 × 20 | C | None | 1 | CO | 12 |
| Neuroform 4 × 20 | ||||||||||||
| 12 | F | 62 | Incidental (0) | RICA | N | 9 | Neuroform 4 × 20 | C | None | 0 | CO | 7 |
| 13 | F | 60 | Incidental (0) | RICA | N | 9 | Neuroform 4 × 20 | C | None | 0 | CO | 8 |
| 14 | F | 45 | SAH (5) | VBJ | AICA | 16 | Neuroform 4.5 × 20 | C | None | Death | NA | 10 |
| 15 | M | 49 | SAH (2) | RICA | N | 3 | Neuroform 4 × 15 | P | None | 1 | CO | 10 |
| Neuroform 4 × 20 | ||||||||||||
| 16 | M | 67 | SAH (3) | VBJ | N | 6 | Neuroform 4.5 × 20 | C | None | 0 | CO | 11 |
| Neuroform 4.5 × 15 | ||||||||||||
| 17 | M | 51 | SAH (4) | RVA | PICA | 4 | Neuroform 4 × 20 | C | None | 1 | CO | 10 |
| Neuroform 4.5 × 20 | ||||||||||||
| 18 | F | 74 | SAH (3) | LICA | N | 5 | Neuroform 4 × 20 | C | None | 0 | CO | 10 |
| 19 | M | 78 | SAH (2) | BA | N | 5 | Neuroform 4 × 20 | C | None | 1 | CO | 10 |
| 20 | F | 49 | SAH (3) | LVA | PICA | 5 | Neuroform 4 × 20 | C | None | 1 | CO | 8 |
Note:—HH indicates Hunt and Hess grading scale; SAH, subarachnoid hemorrhage; LVA, left vertebral artery; RVA, right vertebral artery; VBJ, vertebrobasilar junction; BA, basilar artery; RICA, right intracranial artery; LICA, left intracranial artery; P, partial; C, complete; F/U, follow-up period; mRS: modified Rankin Scale; NA, not available; IO, incomplete occlusion; CO, complete occlusion; PICA, posterior inferior cerebellar artery; AICA, anterior inferior cerebellar artery; N, no branching vessel.
Angiography was performed 6, 12, and 24 months after embolization to assess recanalization of the coiled aneurysm and the patency of the stent and incorporated vessels. Compared with the initial angiography, these follow-up angiograms were reviewed and classified as a change in the initial angiographic results by 2 senior neuroradiologists (D.I.K., T.-S.C.). Clinical outcome was evaluated according to the modified Rankin Scale (mRS) 6, 12, and 24 months after discharge.
Results
The 1-stage embolization procedure by using a balloon and stent was technically successful in all 20 IFAs of the 20 patients. There were no procedural complications in any patients with the exception of patient 5, and 18 patients recovered well with normal neurologic signs (mRS 0–1). Two patients with a poor initial HH grade died due to causes unrelated to the procedure 1 week after the embolization.
In the 18 surviving patients, complete occlusion was obtained in 14 aneurysms, and 4 aneurysms had partial occlusion because attempts were made to incorporate the branching vessel or because the location and low profile of the aneurysm made it difficult to catheterize. A single stent was used in all except 5 patients. In these 5 patients, a second stent was deployed successively into the stented segment of the parent artery because of aneurysm rupture, unpredictable coil protrusion, or in-stent thrombosis. There was good patency of all the incorporated branching vessels at the conclusion of the procedure.
The follow-up period was 7–24 months (mean, 12.3 months). Follow-up angiographies were performed in all 18 surviving patients and showed incomplete occlusion in 3 patients who had partial occlusion at the initial treatment, stable occlusion in 14 patients, and progressive occlusion with further thrombosis in 1 patient. During the follow-up period, there was no evidence of in-stent stenosis, occlusion of the incorporated branching vessel, or coil compaction; and all the patients performed their usual activities without symptoms (mRS 0–1).
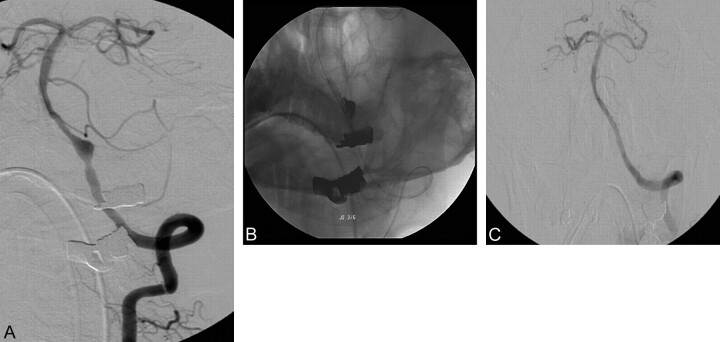
Case 1
A 50-year-old man (patient 2) presented with a comatose mental status. A brain CT revealed attenuated SAH in the prepontine cistern, predominantly in the left side. Diagnostic angiography revealed a fusiform dissecting aneurysm of the left VA with hypoplasia of the contralateral VA (Fig 1A). With the patient under general anesthesia, a 6F Envoy guiding catheter was placed into the distal left VA via the right femoral artery. Initially, a 3 × 38 mm Multilink Zeta coronary stent was deployed across the aneurysm neck, and coil embolization was performed. During the procedure, a remodeling balloon (HyperGlide) was placed within the stent to protect the parent artery (Fig 1B). The final angiography showed complete aneurysm occlusion with a patent VA (Fig 1C). Nevertheless, the patient remained comatose and died 7 days after the procedure.
Fig 1.
A 50-year-old man with subarachnoid hemorrhage (SAH). A, The left vertebral artery (VA) angiogram shows a fusiform aneurysm without a branching vessel. The right VA is hypoplastic (not shown). B, Coil embolization is performed by using a Neuroform stent and balloon. C, Control angiogram shows complete occlusion of the aneurysm.
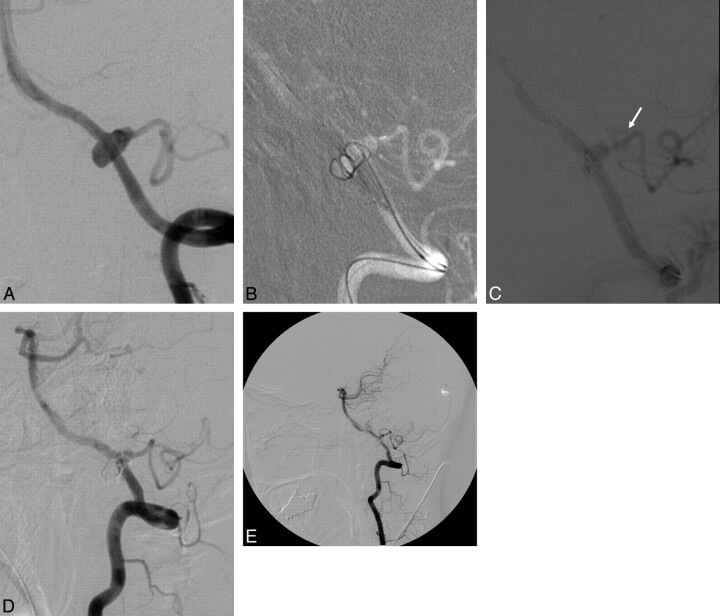
Case 2
A 40-year-old woman (patient 5) was admitted with SAH (HH grade 4). Diagnostic angiography revealed a fusiform dissecting aneurysm of the left VA involving the PICA with antegrade flow (Fig 2A). With the patient under general anesthesia, a 6F Envoy guiding catheter was placed into the distal left VA via the right femoral artery. A 4 × 20 mm Neuroform-2 stent was deployed across the aneurysm neck, and coil embolization was performed by using a balloon and microcatheter (Fig 2A). During the procedure, rebleeding occurred because of aneurysmal rupture by coil prolapse, and the balloon was immediately temporarily occluded to prevent further SAH, followed by reversal of heparinization by using protamine sulfate (Fig 2C). After verifying that the aneurysm was no longer bleeding, we used another stent (Flexmaster, 3 × 16 mm) to replace the additional coil. Final angiography showed partial but satisfactory aneurysm occlusion without bleeding (Fig 2D). At 12 months, the aneurysm remained partially occluded without residual sac growth (Fig 2E).
Fig 2.
A 40-year-old woman with SAH. A, The left VA angiogram shows the circumferential aneurysm incorporated within the left posterior inferior cerebellar artery. B, The roadmap image shows the coil inserted into the circumferential aneurysm by using a Neuroform stent and balloon. C, Aneurysmal rebleeding (arrow) occurs during the procedure, and temporary occlusion of the parent artery is performed by using the balloon. D, After the second stent is placed into the stented parent artery, control angiogram shows partial occlusion of this aneurysm without rebleeding. E, Control angiogram 12 months after the procedure shows partial occlusion of the aneurysm without regrowth of the residual sac or coil compaction.
Discussion
The introduction of complex-shaped coils, balloon-assisted techniques, and intracranial self-expandable stents has facilitated the endovascular treatment of complex and surgically intractable aneurysms, but these aneurysms, nevertheless, remain challenging to treat. In particular, immediate treatment of symptomatic circumferential or fusiform aneurysms, which incorporate >180° of the parent vessel and branching vessels such as the PICA or AICA, is mandatory.6–8 Although some authors have treated these complex aneurysms by using surgical techniques, other authors have reported that internal trapping of the dissecting fusiform aneurysm is one of the most reliable treatment options.9–13 Anson et al14 reported surgical treatment of 41 dolichoectatic and fusiform aneurysms in patients with severe disability. Twenty-three percent of patients died, and follow-up results showed 17 unclippable wrapped fusiform aneurysms with 1 minor complication.15 Recently, Fiorella et al5 introduced a novel balloon-in-stent technique for constructive endovascular treatment of circumferential aneurysms.
Our reconstructive endovascular procedure by using a combination of a stent and balloon was technically very successful (100%) with only 1 periprocedural complication observed among 20 patients (patient 5). Although 2 patients died, they presented initially with a poor HH grade of 5, and there was no evidence of postoperative rebleeding or infarction. Rabinov et al10 reported a 20% postprocedural mortality rate for destructive endovascular treatment of vertebrobasilar dissecting aneurysms due to recurrent hemorrhage, vasospasm, global ischemia, or contralateral VA dissection. Fiorella et al5 described 2 significant retroperitoneal hematomas and 2 brain stem infarcts in their constructive endovascular treatment of circumferential aneurysms by using the “balloon-in-stent” technique.
TE is one of the unavoidable complications when using a stent or balloon for endovascular treatment. While some authors have insisted that balloon-assisted techniques increase the risk of symptomatic and asymptomatic TE, others have suggested that procedure-related TE can be decreased with aggressive anticoagulation and antiplatelet therapy.4,16 If this type of therapy is used, it is necessary to maintain antiplatelet and anticoagulation therapy both before and after the procedure and to decrease the procedure time if at all possible. In our symptomatic patients with SAH, we began anticoagulation with heparin immediately after the placement of the balloon and stent and maintained this therapy for 24 hours after the procedure. In addition, loading doses of aspirin and clopidogrel were administered through a nasogastric tube promptly after coil embolization. However, the requirement for dual antiplatelet therapy increases the risk of intracranial hemorrhage and possibly rebleeding from a ruptured aneurysm.17 In our study, 4 patients with SAH required placement of an external ventricular drain, and 1 patient had a minimal intracranial hemorrhage along the catheter. Therefore, intracranial procedures such as ventriculostomy accompanied by dual antiplatelet therapy should be carefully monitored.
Some authors have reported Neuroform stent migration, which would have serious consequences for stent-assisted coiling.18–21 Because most stent migration occurs when the microguidewire and microcatheter are inserted into the aneurysm, some authors prefer using a staged procedure during stent-assisted coiling to allow the stent to stabilize with endothelization.5,22 However, we performed balloon-assisted coiling immediately after placing the stent across the neck of the aneurysm, and there were no complications associated with stent migration. Possible reasons for the lack of complications are the following: 1) most of our cases involved the vertebrobasilar arteries, anatomic structures that are not curved but relatively straight, which may have minimized movement of the indwelling stent when the balloon catheter was manipulated within the stent; and 2) 15 patients presented with SAH and required immediate treatment. Above all, the best approach to prevent stent migration is the use of an oversized self-expandable stent and placement of the microcatheter within the aneurysm before stent deployment. It is also necessary to carefully manipulate the microcatheter and balloon catheter within the stent when catheterizing an aneurysm through the cells of the stent.
In 1 patient (patient 5) with SAH, aneurysm rebleeding occurred due to coil prolapse and inadequate manipulation of the microcatheter. As a rescue remedy, the previously equipped balloon catheter was inflated to the same diameter as the parent vessel for 10 minutes and reversion of heparinization was performed by using protamine sulfate (50 mg) through the venous route. When the aneurysm was confirmed by angiography to have stopped bleeding, an additional balloon-expandable stent was placed rather than a coil. If we had not used a preloaded balloon catheter in this case, the parent vessel and combined branching vessel would have been sacrificed; the preloaded balloon catheter has the unique advantage of allowing control of intraprocedural aneurysmal bleeding.4,23,24 For ruptured and uncoilable aneurysms, placement of an additional stent within the stent may reconstruct the affected aneurysm and dissecting vessel wall by decreasing wall shear stress, inflow momentum, velocity, and vorticity, thereby diverting the blood flow into the aneurysm or to the weakened vascular wall.25–29 Some authors have reported multiple and overlapping stent therapy for treatment of uncoilable aneurysms, such as intradural pseudoaneurysms, blood-blister-like aneurysms, and dissection.23,30–33
Our study has some limitations. First, the number of patients enrolled in this study was too small to evaluate the efficacy of our procedure, but fusiform aneurysms are not common and their management is restricted technically. Second, coil compaction may be observed in the long-term follow-up despite good midterm follow-up results. Therefore, it is necessary to perform further hemodynamic studies of fusiform aneurysms. Hoi et al34 reported that hemodynamics play a major role in the pathogenesis of BA enlargement. Finally, additional trials of new therapy to treat IFAs are required, because incomplete coiling is sometimes necessary to preserve the parent artery and incorporated vessels. Recently, sole stent therapy has showed promising results in animal models.35–40 A newly designed stent with low porosity such as a pipeline embolization device may have a major role in the treatment of circumferential and fusiform aneurysms as well as a saccular aneurysms.31,38
Conclusions
A 1-stage procedure by using a combination of balloon and stent may provide a feasible and safe treatment strategy for the reconstructive treatment of IFAs that are not amenable to deconstructive embolization.
References
- 1. Mizutani T, Miki Y, Kojima H, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 1999; 45: 253–59, discussion 59–60 [DOI] [PubMed] [Google Scholar]
- 2. Mizutani T.. A fatal, chronically growing basilar artery: a new type of dissecting aneurysm. J Neurosurg 1996; 84: 962–71 [DOI] [PubMed] [Google Scholar]
- 3. Flemming KD, Wiebers DO, Brown RD, Jr, et al. The natural history of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis 2005; 20: 270–79 [DOI] [PubMed] [Google Scholar]
- 4. Ross IB, Dhillon GS.. Balloon assistance as a routine adjunct to the endovascular treatment of cerebral aneurysms. Surg Neurol 2006; 66: 593–601 discussion 601–02 [DOI] [PubMed] [Google Scholar]
- 5. Fiorella D, Albuquerque FC, Masaryk TJ, et al. Balloon-in-stent technique for the constructive endovascular treatment of “ultra-wide necked” circumferential aneurysms. Neurosurgery 2005; 57: 1218–27 [DOI] [PubMed] [Google Scholar]
- 6. Anxionnat R, de Melo Neto JF, Bracard S, et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery 2003; 53: 289–300 discussion 300–01 [DOI] [PubMed] [Google Scholar]
- 7. Albuquerque FC, Fiorella DJ, Han PP, et al. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus 2005; 18: E3. [PubMed] [Google Scholar]
- 8. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997; 87: 141–62 [DOI] [PubMed] [Google Scholar]
- 9. Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 1999; 90: 853–56 [DOI] [PubMed] [Google Scholar]
- 10. Rabinov JD, Hellinger FR, Morris PP, et al. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 2003; 24: 1421–28 [PMC free article] [PubMed] [Google Scholar]
- 11. Peluso JP, van Rooij WJ, Sluzewski M, et al. Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol 2008; 29: 102–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurata A, Ohmomo T, Miyasaka Y, et al. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol 2001; 22: 11–18 [PMC free article] [PubMed] [Google Scholar]
- 13. Kai Y, Hamada JI, Morioka M, et al. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheter technique in the acute stage. Acta Neurochir (Wien) 2003; 145: 447–51 [DOI] [PubMed] [Google Scholar]
- 14. Anson JA, Lawton MT, Spetzler RF.. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg 1996; 84: 185–93 [DOI] [PubMed] [Google Scholar]
- 15. Deshmukh VR, Kakarla UK, Figueiredo EG, et al. Long-term clinical and angiographic follow-up of unclippable wrapped intracranial aneurysms. Neurosurgery 2006; 58: 434–42 [DOI] [PubMed] [Google Scholar]
- 16. Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003; 24: 127–32 [PMC free article] [PubMed] [Google Scholar]
- 17. Tumialan LM, Zhang YJ, Cawley CM, et al. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: a cautionary report. J Neurosurg 2008; 108: 1122–29 [DOI] [PubMed] [Google Scholar]
- 18. Lylyk P, Ferrario A, Pasbon B, et al. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg 2005; 102: 235–41 [DOI] [PubMed] [Google Scholar]
- 19. Kis B, Weber W, Berlit P, et al. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery 2006; 58: 443–50 [DOI] [PubMed] [Google Scholar]
- 20. Broadbent LP, Moran CJ, Cross DT, 3rd, et al. Management of Neuroform stent dislodgement and misplacement. AJNR Am J Neuroradiol 2003; 24: 1819–22 [PMC free article] [PubMed] [Google Scholar]
- 21. Yahia AM, Gordon V, Whapham J, et al. Complications of Neuroform stent in endovascular treatment of intracranial aneurysms. Neurocrit Care 2008; 8: 19–30 [DOI] [PubMed] [Google Scholar]
- 22. Lubicz B, Collignon L, Lefranc F, et al. Circumferential and fusiform intracranial aneurysms: reconstructive endovascular treatment with self-expandable stents. Neuroradiology 2008; 50: 499–507 [DOI] [PubMed] [Google Scholar]
- 23. Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg 2009; 110: 431–36 [DOI] [PubMed] [Google Scholar]
- 24. Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results—apropos of 56 cases [in French]. J Neuroradiol 1997; 24: 30–44 [PubMed] [Google Scholar]
- 25. Canton G, Levy DI, Lasheras JC, et al. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005; 103: 891–902 [DOI] [PubMed] [Google Scholar]
- 26. Kim M, Levy EI, Meng H, et al. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery 2007; 61: 1305–12, discussion 12–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lieber BB, Stancampiano AP, Wakhloo AK.. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng 1997; 25: 460–69 [DOI] [PubMed] [Google Scholar]
- 28. Liou TM, Li YC.. Effects of stent porosity on hemodynamics in a sidewall aneurysm model. J Biomech 2008; 41: 1174–83 [DOI] [PubMed] [Google Scholar]
- 29. Yu SC, Zhao JB.. A steady flow analysis on the stented and non-stented sidewall aneurysm models. Med Eng Phys 1999; 21: 133–41 [DOI] [PubMed] [Google Scholar]
- 30. Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery 2006; 59: 291–300 [DOI] [PubMed] [Google Scholar]
- 31. Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery 2008; 62: 1115–20 discussion 11120–21 [DOI] [PubMed] [Google Scholar]
- 32. Kim BM, Chung EC, Park SI, et al. Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique: case report. J Neurosurg 2007; 107: 1211–13 [DOI] [PubMed] [Google Scholar]
- 33. Kim BM, Suh SH, Park SI, et al. Management and clinical outcome of acute basilar artery dissection. AJNR Am J Neuroradiol 2008; 29: 1937–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoi Y, Gao L, Tremmel M, et al. In vivo assessment of rapid cerebrovascular morphological adaptation following acute blood flow increase. J Neurosurg 2008; 109: 1141–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geremia G, Brack T, Brennecke L, et al. Occlusion of experimentally created fusiform aneurysms with porous metallic stents. AJNR Am J Neuroradiol 2000; 21: 739–45 [PMC free article] [PubMed] [Google Scholar]
- 36. Geremia G, Haklin M, Brennecke L.. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol 1994; 15: 1223–31 [PMC free article] [PubMed] [Google Scholar]
- 37. Hans FJ, Krings T, Moller-Hartmann W, et al. Endovascular treatment of experimentally induced aneurysms in rabbits using stents: a feasibility study. Neuroradiology 2003; 45: 430–34 [DOI] [PubMed] [Google Scholar]
- 38. Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–52 [DOI] [PubMed] [Google Scholar]
- 39. Turjman F, Acevedo G, Moll T, et al. Treatment of experimental carotid aneurysms by endoprosthesis implantation: preliminary report. Neurol Res 1993; 15: 181–84 [DOI] [PubMed] [Google Scholar]
- 40. Wakhloo AK, Schellhammer F, de Vries J, et al. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am J Neuroradiol 1994; 15: 493–502 [PMC free article] [PubMed] [Google Scholar]