SUMMARY:
Lipoid proteinosis is a rare genodermatosis characterized by multisystem involvement due to intracellular deposition of an amorphous hyaline material. Lipoid proteinosis is caused by mutations in the ECM1 gene. In many patients, skin and mucosa abnormalities are the first manifestation. When the CNS is affected, a wide variety of neurologic abnormalities may be present. The hallmark findings are calcifications, mostly occurring in the amygdalae, hippocampus, parahippocampal gyrus, or even the striatum. Present in half of the patients, moniliform blepharosis is considered a pathognomonic finding. In the other half of patients imaging could assist in the diagnosis. The authors present a series of 3 cases of lipoid proteinosis with brief clinical data and imaging findings.
Lipoid proteinosis (LP) or Urbach-Wiethe disease (Online Mendelian Inheritance in Man, 247100; http://www.ncbi.nlm.nih.gov/omim/) is a rare genodermatosis, characterized by multisystem involvement due to intracellular deposition of an amorphous hyaline material. LP is caused by mutations in the extracellular matrix protein 1 (ECM1) gene.1
In many patients, skin abnormalities are the first manifestations. Clinically, the patient may present with hoarseness and thickening of the skin and mucosa. A wide variety of neurologic abnormalities may be present whenever the central nervous system (CNS) is involved.2
We describe a series of 3 patients with skin biopsy−proved LP referred from the dermatology department of our institution for imaging evaluation with brief clinical data and imaging findings.
Case Reports
All 3 patients underwent CT and MR imaging. CT scans were obtained with a 4-channel CT scanner (LightSpeed Plus; GE Healthcare, Waukesha, Wis). 3D and coronal reformatted images were processed in an ADW 4.2 Advantage Workstation, (GE Healthcare). MR images were acquired with a 1.5T MR imaging scanner (Excite HD; GE Healthcare).
Patient 1
A 56-year-old male patient presented with a husky voice and skin and mucous lesions, which had begun at a young age. At 50 years of age, after being referred for a dermatology consultation, he was suspected of having LP on the basis of his eyelid papular lesions (Fig 1). He had experienced several episodes of parotid gland enlargement. Neurologically, he had a mild cognitive deficit but with no signs of parkinsonism, history of seizures, or behavioral abnormalities.
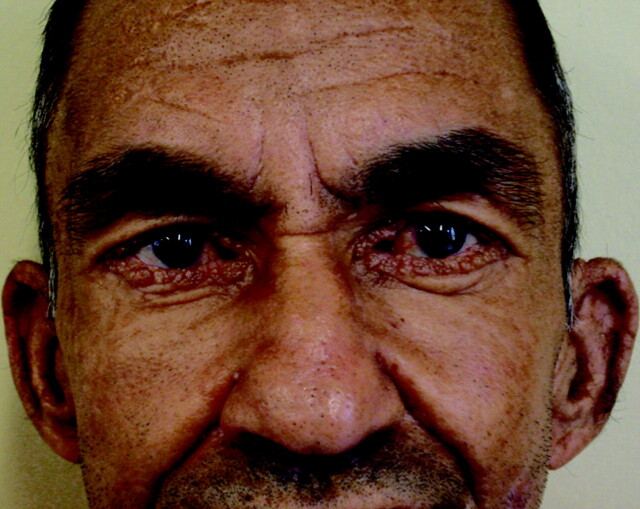
Fig 1.
Close view of patient 1 shows the characteristic beaded papules along the eyelids (moniliform blepharosis) and partially missing eyelashes. These clinically itchy lesions are described in 50% of the patients with LP.
On CT, there were signs of bilateral symmetric amygdaloid and striatum calcifications without mass effect (Fig 2). On T2-weighted images, there were marked hypointense lesions in the mesial temporal lobes immediately anterior to the temporal horns and rounded bilateral striatal lesions (Fig 3). An incidental finding of parotid gland T2 hyperintensity was positively correlated with a history of left parotid gland recurrent inflammation.
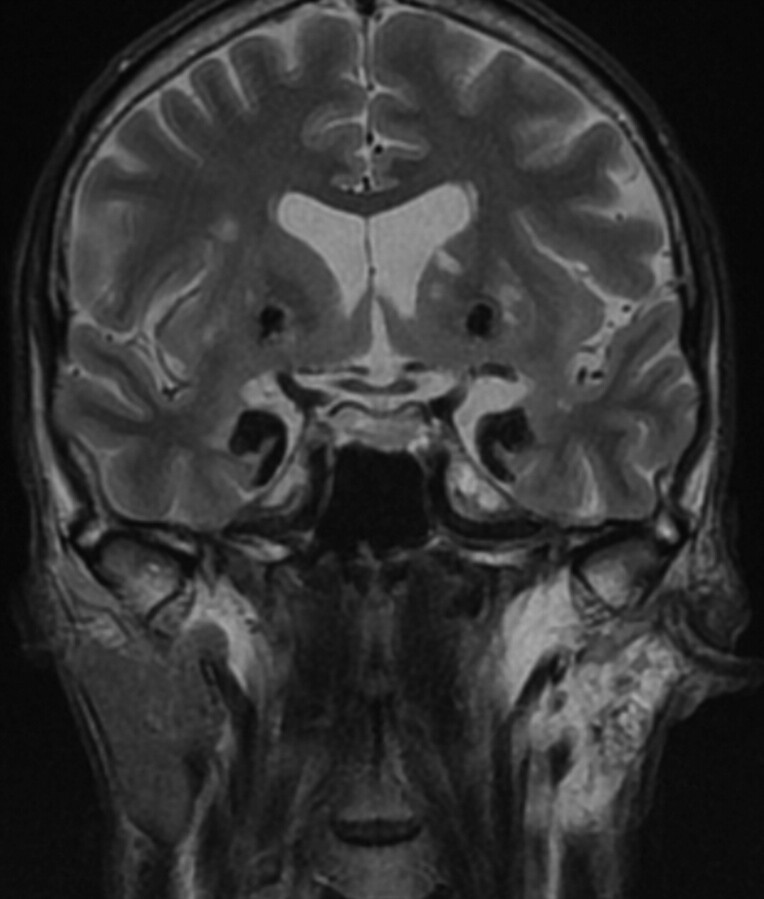
Fig 2.
Patient 1. Coronal T2-weighted MR imaging (TR, 4500 ms; TE, 90 ms) reveals symmetric bilateral markedly hypointense lesions in the temporal lobes. There is involvement of the amygdaloid bodies, parahippocampal gyrus, and uncus as well as symmetric rounded hypointense lesions in the striatum. Note the left parotid gland hyperintensity representing fatty infiltration from recurrent parotid gland inflammation.
Fig 3.
Patient 1. Coronal CT reformatted image shows symmetric bilateral striatal, uncal, and amygdaloid calcifications.
Patient 2
A 58-year-old female patient presented with a long history of throaty voice and very thin fragile hair, requiring the use of a wig for the past 20 years. She also had a history of bilateral submandibular gland enlargement and verrucous lesions throughout the joints, mainly in the groin and elbows. She complained of frequent and easy loss of temper, with a generalized disgust affect, possibly reflecting a degree of depression. There was no history of any seizures or focal neurologic deficits. On imaging, she had symmetric temporal lobe calcifications bilaterally. CT-based 3D-volume rendering demonstrated calcified lesions in the usual bilateral amygdaloid and uncal topography (Fig 4).
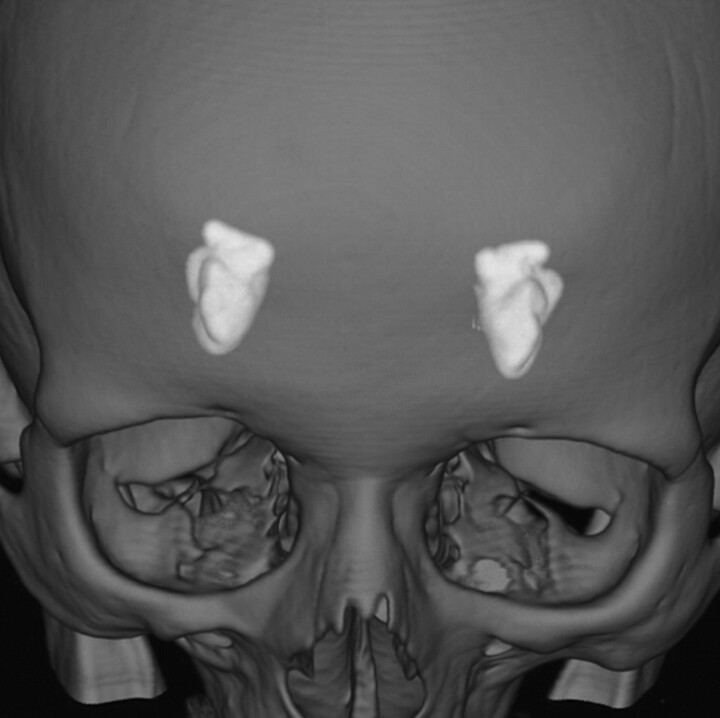
Fig 4.
3D-volume rendering reformation from a volume CT acquisition shows, in perspective, the 2 “almond-shaped” symmetrically and mesially located calcifications in patient 2.
Patient 3
A 16-year-old female patient presented with a history of long-standing hoarseness that began at 2 years of age. She developed skin lesions after 5 years of age, which started at the extremities and progressed to the chest and face. She also had thin fragile hair. She had no history of seizures, though there was a positive familial history of “fits” in an aunt on her mother’s side, who had similar voice and skin lesions. On examination, she spontaneously expressed an inability to perceive fear in people’s faces and was unable to process simple addition or subtraction tasks.
Axial gradient refocused-echo (GRE) T2* images (Fig 5) demonstrated the typical bilateral horn-shaped lesions. They were characteristically hypointense and involved the amygdaloid complex.
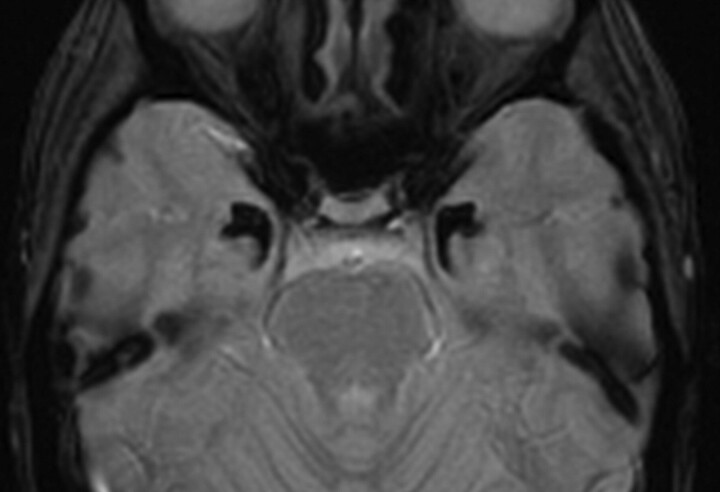
Fig 5.
Patient 3. Axial T2* GRE image (fast low-angle shot; TR, 600 ms; TE, 18 ms) depicts well the bilaterally located horn-shaped marked hypointense lesions in both amygdaloid bodies. This marked hypointensity reflects the high calcium content in both uncal regions.
Discussion
LP was initially described by Urbach and Wiethe in 1929 as “hyalinosis cutis et mucosae.”3 It is a rare autosomal recessive genodermatosis due to a mutation in the ECM1 gene on chromosome 1q21, with approximately 300 cases described in the medical literature.4 ECM1 is a glycoprotein with 3 varieties: ECM1a, ECM1b, and ECM1c. EMC1 is expressed in the dermis, basal keratinocytes, endothelial cells, and developing bones, linked to keratinocyte differentiation, basement membrane regulation, collagen composition, and growth-factor binding.1 Neurologic features do not show any specific genotype-phenotype correlation.
The mutated ECM1 gene gives rise to hyaline material deposition in the dermis and thickening of the skin and mucous basement membrane around blood vessels and adnexal epithelia. The patient may present with abnormal scarring and wound healing and premature skin aging.1,5 Hoarseness is present at birth or in the early infancy in two-thirds of patients, due to early hyaline material larynx infiltration, progressing with time.6
As a multisystem disease, LP involves the CNS infrequently. CNS infiltration occurs predominantly around the hippocampal capillaries, resulting in wall thickening, which later progresses to perivascular calcium deposition. Microscopic findings include gross amorphous calcifications encompassed by gliotic tissue and calcified thickened capillary walls.7 Subsequent medial temporal lobe architectural distortion with gliotic tissue and calcium accumulation can lead to a constellation of reported neurologic manifestations, which range from migraine, variable degrees of mental retardation, seizures, depression, anxiety, and panic attacks to disturbances in decision making, memory, and abnormal social interaction patterns.8,9 These varied symptoms frequently lead to radiologic evaluation by CT or MR imaging, which, in unsuspected patients, may indicate the proper diagnosis.
The essential imaging finding in LP is the appearance of atypical intracranial calcifications, mostly occurring in the medial temporal lobes. Amygdalae involvement is considered pathognomonic, being more prominent with longer disease duration. The most commonly affected sites are the amygdalae, hippocampus, parahippocampal gyrus, or even the striatum. Curvilinear hyperattenuated horn-shaped lesions are well depicted by CT in the amygdaloid bodies. On MR imaging, such lesions are hypointense in all pulse sequences, especially in GRE T2* weighted images. CT or MR imaging findings may be unremarkable in patients with LP in the absence of brain calcifications.7
Moniliform blepharosis is considered a pathognomonic finding present in 50% of patients. In this group, the diagnosis is straightforward after dermatologic consultation. In the other half of patients with LP, imaging assists in the diagnosis because there are few diseases that can manifest such a calcification pattern. There have been reports of calcified gliomas located in the amygdalohippocampal region in pediatric patients, but they are neither bilateral nor symmetrically distributed. Raine syndrome, a rare autosomal recessive osteosclerotic bone dysplasia, is characterized, among other findings, by intracranial calcifications, with only 9 reported cases. In this disease, the mineralization rarely occurs in blood vessel walls and mainly affects the basal ganglia. In those cases, there were no records of calcifications in the Ammon horn or in the amygdala.10
The amygdalae as part of the limbic system play a key role in mediating emotions (mainly fear recognition related to possible danger and threat), modulation of attention, perception, learning, and emotional long-term memory. Patients with amygdala damage may show impaired decision-making under ambiguity and stress/risk conditions.8,9,11 Generalized dystonia has been described in patients with striatal calcification,12 which was not present in our 3 patients.
Conclusions
Selective brain parenchymal calcification is the hallmark of LP, with involvement of very specific sites such as the amygdalae, hippocampus, parahippocampal gyrus, and the striatum. Such lesions can explain many of the varied neurologic symptoms in these patients. The recognition of this pathognomonic lesion pattern can support the correct diagnosis in unsuspected patients.
References
- 1. Hamada T, McLean WH, Ramsay M, et al. Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1). Hum Mol Genet 2002; 11: 833–40 [DOI] [PubMed] [Google Scholar]
- 2. Van Hougenhouck-Tulleken W, Chan I, Hamada T, et al. Clinical and molecular characterization of lipoid proteinosis in Namaqualand, South Africa. Br J Dermatol 2004; 151: 413–23 [DOI] [PubMed] [Google Scholar]
- 3. Urbach E, Wiethe C. Lipoidosis cutis et mucosae. Wirch Arch Pathol Anat 1929; 273: 285–319 [Google Scholar]
- 4. Oz F, Kalekoğlu N, Karakullukçu B, et al. Lipoid proteinosis of the larynx. J Laryngol Otol 2002; 116: 736–39 [DOI] [PubMed] [Google Scholar]
- 5. Ringpfeil F. Selected disorders of connective tissue: pseudoxanthoma elasticum, cutis laxa, and lipoid proteinosis. Clin Dermatol 2005; 23: 41–46 [DOI] [PubMed] [Google Scholar]
- 6. Acar A, Eryılmaz A, Gocer C, et al. Lipoid proteinosis of larynx: review of four cases. Int J Pediatr Otorhinolaryngol 2004; 68: 1557–61 [DOI] [PubMed] [Google Scholar]
- 7. Appenzeller S, Chaloult E, Velho P, et al. Amygdalae calcifications associated with disease duration in lipoid proteinosis. J Neuroimaging 2006; 16: 154–56 [DOI] [PubMed] [Google Scholar]
- 8. Thornton HB, Nel D, Thornton D, et al. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsychiatry Clin Neurosci 2008; 20: 86–92 [DOI] [PubMed] [Google Scholar]
- 9. Wiest G, Lehner-Baumgartner E, Baumgartner C. Panic attacks in an individual with bilateral selective lesions of the amygdala. Arch Neurol 2006; 63: 1798–801 [DOI] [PubMed] [Google Scholar]
- 10. Rickert CH, Rieder H, Rehder H, et al. Neuropathology of Raine syndrome. Acta Neuropathol 2002; 103: 281–87. Epub 2001 Dec 5 [DOI] [PubMed] [Google Scholar]
- 11. Claeys KG, Claes LR, Van Goethem JW, et al. Epilepsy and migraine in a patient with Urbach-Wiethe disease. Seizure 2007; 16: 465–68. Epub 2007 Apr 2 [DOI] [PubMed] [Google Scholar]
- 12. Teive HA, Pereira ER, Zavala JA, et al. Generalized dystonia and striatal calcifications with lipoid proteinosis. Neurology 2004; 63: 2168–69 [DOI] [PubMed] [Google Scholar]