Abstract
BACKGROUND AND PURPOSE:
Intravenous (IV) thrombolysis often fails to achieve recanalization of occluded cerebral arteries, especially in patients with proximal large arterial occlusions. The goal of this study was to assess the feasibility, safety, and efficacy of low-dose intra-arterial (IA) urokinase and aggressive mechanical clot disruption (AMCD) after failure of IV thrombolysis for acute ischemic stroke.
MATERIALS AND METHODS:
We prospectively enrolled 12 patients with acute ischemic stroke who initially received IV recombinant tissue plasminogen activator (rtPA) and were subsequently treated with combined low-dose IA urokinase and AMCD. Time to treatment, urokinase dose, duration of the procedure, recanalization rates, and symptomatic hemorrhage were analyzed. Clinical outcome measures were assessed on admission and at discharge (National Institutes of Health Stroke Scale [NIHSS]), and at 3 months after treatment (modified Rankin Scale [mRS]).
RESULTS:
Median NIHSS score on admission was 17. Median time from symptom onset to IV rtPA was 120 minutes, and median time from symptom onset to IA therapy was 230 minutes. The median duration of IA therapy was 55 minutes. Median dose of urokinase was 300,000 U. Recanalization (thrombolysis in cerebral ischemia grade II or III) was achieved in all patients. No procedure-related complications were observed. There was no symptomatic hemorrhage. At discharge, median NIHSS score was 3. The 3-month outcome was excellent (mRS, 0–1) in 8 patients, good (mRS, 2) in 1 patient, and poor (mRS, 3–5) in 3 patients. There was no hospital or 3-month mortality.
CONCLUSIONS:
In this study, combination therapy with low-dose IA urokinase and AMCD is safe and effective after failed IV thrombolysis in patients with acute ischemic stroke. A high rate of recanalization, low rate of symptomatic hemorrhage, and excellent functional outcome can be achieved.
Administration of intravenous (IV) recombinant tissue plasminogen activator (rtPA) within 3 hours of stroke onset is an established therapy for acute ischemic stroke. However, IV rtPA therapy often fails to achieve recanalization of occluded arteries, especially in patients with proximal large arterial occlusions.1–3
As an alternative, intra-arterial (IA) therapy is used to treat patients in whom recanalization did not occur after IV therapy.4–8 The Interventional Management of Stroke (IMS I) study and the IMS II study used reduced-dose (0.6 mg/kg) of IV rtPA followed by IA rtPA.4,5 The major concern with IA therapy after unsuccessful IV therapy is the development of intracerebral hemorrhage because of an increased dosage of thrombolytic agents. Higher thrombolytic dose is one of the main predictors of symptomatic intracranial hemorrhage after IA thrombolysis.9–11 Aggressive mechanical clot disruption (AMCD) by use of interventional techniques may provide an advantage compared with IA infusion therapy by increasing the recanalization rate and decreasing the time to recanalize.12–14 AMCD can also allow administering lower doses of thrombolytic drugs, thus likely reducing the risk for intracranial hemorrhage.
Several studies have examined the use of rescue IA thrombolysis in case of failure of early clinical response after IV rtPA therapy.6–8 However, the safety and effectiveness of low-dose IA urokinase combined with AMCD after failed IV thrombolysis have not yet been tested. The purpose of this study was to assess the feasibility of such a combination therapy after failure of IV thrombolysis in patients with acute ischemic stroke.
Materials and Methods
From July 2006 to August 2007, we prospectively enrolled 12 consecutive patients with acute stroke who initially received full-dose (0.9 mg/kg) IV rtPA and were subsequently treated with low-dose IA urokinase combined with AMCD. This study was approved by the institutional review board, and informed consent was obtained for combination IA therapy after IV thrombolysis. On admission, neurologic assessments were performed by a stroke neurologist by using the National Institutes of Health Stroke Scale (NIHSS). A cerebral CT scan was obtained in all patients before initiation of IV rtPA infusion. CT exclusion criteria included the presence of intracranial hemorrhage or mass effect and evidence of hypoattenuation in more than one-third of the middle cerebral artery (MCA) territory.
Eligible patients who met standard National Institute of Neurological Disorders and Stroke (NINDS) criteria for IV rtPA15 were treated with 0.9 mg/kg of IV rtPA. During the study period, a total of 50 patients received IV thrombolysis. A subsequent noncontrast CT scan and CT angiography with CT perfusion study were obtained in all patients after initiation of IV rtPA. The subsequent IA therapy was considered within 1 hour of IV rtPA if patients had no neurologic improvement, which was determined by an unchanged NIHSS score from baseline or worsening of the neurologic deficit, had no hemorrhage or mass effect or hypoattenuation in more than one-third of the MCA territory at noncontrast CT scan, had major arterial occlusion on CT angiography, or had significant ischemic penumbra on CT perfusion study. Significant ischemic penumbra was defined as time- to-peak (TTP) minus cerebral blood volume (CBV) mismatch (areas of elevated TTP > 30% larger than those of decreased CBV). Of those 50 patients who received IV rtPA, 12 patients met inclusion criteria described above and thus were enrolled in this study. Thirty-eight patients did not undergo subsequent IA therapy because there was no significant ischemic penumbra on CT perfusion study (n = 20), evidence of hypoattenuation in more than one-third of the MCA territory (n = 13), or gyral swelling with mass effect on subsequent noncontrast CT scan (n = 5). CT angiography revealed persistent arterial occlusions in 34 of 38 patients who did not undergo IA therapy.
All IA therapy was performed by 1 interventional neuroradiologist. Cerebral angiography was performed via a femoral approach. The beginning of IA therapy was defined as the needle puncture of the common femoral artery. Angiographic occlusion sites were located in the T-bifurcation of the internal carotid artery (n = 2), M1 segment of the MCA (n = 8), and basilar artery (n = 2). After demonstration of an arterial occlusion on diagnostic angiography, an end-hole microcatheter (Excelsior SL-10; Boston Scientific, Natick, Massachusetts) over a microguide wire (Transend-14; Boston Scientific) was advanced through a 6F guide catheter into the occlusion site. The microcatheter tip was placed into the thrombus, and then a 100,000-U bolus of urokinase diluted in 10 mL of saline was manually infused for 3 to 5 minutes.
AMCD was undertaken immediately after 100,000 U of urokinase was administered. The definition of AMCD has been previously reported.14 Of those techniques, we used aggressive clot maceration with a microcatheter (Excelsior SL-10) and microwire (Agility 10; Cordis, Miami Lakes, Florida) in all patients. Aggressive clot maceration consisted of multiple passes of the microwire through the clot after the tip was manually shaped into a complete J-curve approximating the diameter of the vessel. The microwire was gently rotated clockwise while being advanced. During this process, the microcatheter was often advanced multiple times over the microwire as well.
After withdrawal of the microwire from the microcatheter, an additional 200,000 U of urokinase was continuously administered through the microcatheter at the site of the remaining thrombus by use of an infusion pump for 20 minutes. Control angiography was performed every 5 minutes to evaluate recanalization. Continuous infusion of urokinase was stopped immediately if control angiograms showed complete recanalization. Reattempt of aggressive clot maceration and/or percutaneous angioplasty was performed if recanalization was not achieved after continuous infusion of urokinase. Percutaneous angioplasty with a coronary balloon catheter (Ryujin; Terumo, Tokyo, Japan) was carried out in 2 patients with MCA occlusion. The diameter of the balloon was chosen to be 0.5 mm smaller than that of the adjacent portion of the occluded segment. The balloon was inflated slowly with a screw-type pressure inflation device up to 4 atm for 4 minutes up to 2 times.16 An intracranial stent or snare device was not used in this study.
An additional 50,000 U bolus of urokinase was manually infused up to 3 times if required for further recanalization at the discretion of the treating interventional neuroradiologist. To minimize the risk for intracranial hemorrhage, heparin or glycoprotein IIb/IIIa inhibitor were not administered, either intravenously or intra-arterially, in any patient. The StarClose (Abbott Vascular Devices, Redwood City, California) arteriotomy closure device was used for all patients to obtain hemostasis at the end of the procedure.
Pretreatment and posttreatment angiograms were evaluated by the same interventional neuroradiologist. Recanalization status was classified according to the Thrombolysis in Cerebral Ischemia (TICI) scale (grade 0, no perfusion; grade I, penetration but not perfusion; grade IIa, partial perfusion with incomplete distal filling of < 50% of the expected territory; grade IIb, partial perfusion with incomplete distal filling of 50%–99% of the expected territory; grade IIc, near complete perfusion but with delay in contrast runoff; grade III, full perfusion with normal filling of distal branches in a normal hemodynamic fashion).14 Recanalization was defined as TICI grades II or III.
Age, sex, NIHSS at the time of admission, time to IV rtPA infusion, time to IA therapy, total amount of urokinase, presence or absence of symptomatic hemorrhage, duration of the procedure, recanalization, NIHSS at discharge, and outcomes were recorded and analyzed. The clinical evaluation was done by stroke neurologists who were not blinded to the treatment. A brain CT scan was routinely obtained immediately after the procedure and if the patient showed neurologic deterioration (increase in NIHSS of > 2 points) during admission. CT scans were analyzed for hemorrhagic transformation by the same interventional neuroradiologist. Symptomatic hemorrhage was defined as parenchymal hematoma causing mass effect on CT scan, with clinical deterioration defined as a 4-point or greater increase in NIHSS score or 1-point deterioration in the level of consciousness. Outcome was assessed by a stroke neurologist 3 months after treatment by use of the modified Rankin Scale (mRS).
Results
Twelve consecutive patients (9 men, 3 women) with a median age of 70 years (range, 34–79 years) were treated with low-dose IA urokinase and AMCD after unsuccessful IV rtPA. The clinical and radiologic characteristics of the 12 patients are summarized in Table 1. The median NIHSS score on admission was 17 and ranged from 11 to 23. The median time from symptom onset to IV rtPA was 120 minutes (range, 75–175 minutes), and median time from symptom onset to IA therapy was 230 minutes (range, 170–300 minutes). The time lag between IV rtPA and IA therapy ranged from 55 to 150 minutes (mean, 104.6 minutes). The median duration of IA therapy was 55 minutes (range, 30–80 minutes). The total dose of urokinase ranged from 150,000 to 450,000 U (median, 300,000 U). Recanalization (TICI grade II or III) was achieved in all patients (100%). TICI grade III flow occurred in 3 patients (25%), and TICI grade II occurred in 9 patients (75%). No procedure-related complications such as vessel rupture or dissection were observed. Two patients were treated with carotid stent placement before IA thrombolysis for underlying severe stenosis at the origin site of the internal carotid artery.
Table 1:
Clinical and imaging characteristics and outcome in 12 patients
| Age/Sex | NIHSS Score on Admission | Occlusion Site | Time to IV rtPA (min) | Time to IA Therapy (min) | Duration of Procedure (min) | Urokinase Dose (× 104 U) | TICI Grade | NIHSS at Discharge | Symptomatic Hemorrhage | mRS at 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| 70/M | 13 | MCA | 115 | 170 | 60 | 40 | III | 6 | No | 1 |
| 79/M | 20 | MCA | 123 | 270 | 75 | 20 | IIc | 2 | No | 1 |
| 34/F | 19 | BA | 108 | 170 | 70 | 30 | IIb | 3 | No | 1 |
| 73/M | 20 | ICAT | 120 | 220 | 40 | 40 | IIa | 9 | No | 4 |
| 69/F | 17 | MCA | 127 | 240 | 70 | 30 | IIc | 1 | No | 1 |
| 45/M | 11 | MCA | 90 | 240 | 44 | 15 | IIb | 4 | No | 2 |
| 72/M | 13 | MCA | 97 | 240 | 30 | 30 | III | 0 | No | 0 |
| 72/M | 17 | ICAT | 175 | 300 | 30 | 30 | IIc | 6 | No | 5 |
| 76/M | 13 | MCA | 130 | 190 | 50 | 30 | III | 1 | No | 1 |
| 51/M | 14 | MCA | 130 | 250 | 80 | 40 | IIc | 1 | No | 1 |
| 64/F | 23 | BA | 75 | 190 | 80 | 30 | IIc | 2 | No | 1 |
| 53/F | 18 | MCA | 120 | 185 | 45 | 45 | IIb | 12 | No | 5 |
Note:—NIHSS indicates the National Institutes of Health Stroke scale; M, male; F, female; MCA, middle cerebral artery; BA, basilar artery; ICAT, internal carotid artery T-bifurcation; IV, intravenous; rtPA, recombinant tissue plasminogen activator; IA, intra-arterial; TICI, Thrombolysis in Cerebral Ischemia; mRS, modified Rankin scale.
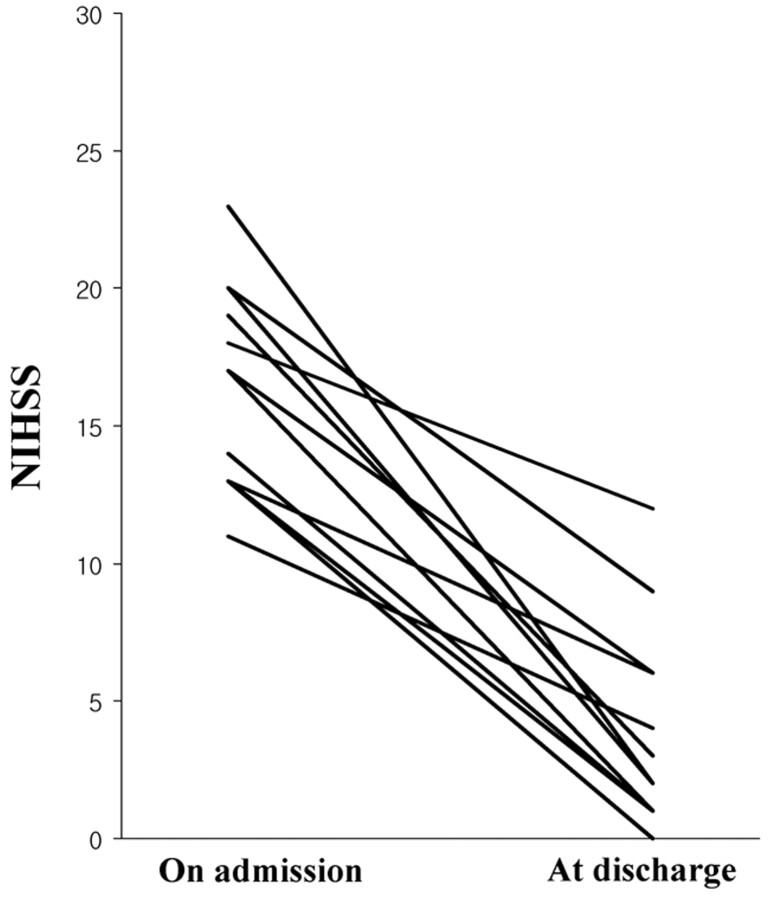
No symptomatic hemorrhagic transformation occurred during the hospital stay. At discharge, the NIHSS score was improved (decrease ≥ 4 points) in all patients and ranged from 1 to 12 (median, 3), as shown in Fig 1. At the 3-month follow-up, the functional outcome was excellent (mRS, 0 or 1) in 8 of the 12 patients, good (mRS, 2) in 1 patient, and poor (mRS, 3–5) in 3 patients. There was no hospital or 3-month mortality.
Fig 1.
The NIHSS score on admission and at discharge. Median scores were 17 and 3, respectively.
Discussion
Our study showed that combined IA therapy of low-dose IA urokinase and AMCD is feasible in case of failed IV thrombolysis in properly selected patients with acute ischemic stroke. The early recanalization rate after IV rtPA use is very low in cases with large proximal arterial occlusions. Lee et al3 reported a 12.5% recanalization rate after full-dose IV rtPA in patients with internal carotid artery or proximal MCA occlusion. Our study showed that the present method resulted in an excellent recanalization rate in such patients. We were able to achieve recanalization (TICI grades II or III) in all patients. Complete or near-complete recanalization (TICI grades IIc or III) was observed in 8 (67%) of 12 patients.
The rapidity as well as the degree of recanalization has been shown to predict good outcome in IV or IA thrombolysis of acute ischemic stroke.11,17,18 In this study, with use of AMCD and low-dose IA urokinase, we achieved the recanalization in a very short time. The median duration of IA therapy (from the start of angiography to the completion of the IA therapy) was 55 minutes (range, 30–80 minutes), and the median time from the onset of symptom to completion of the IA therapy was 270 minutes (range, 230–345 minutes). An excellent outcome (mRS score 0 or 1) at 3 months was achieved in 67% of the treated patients. There was no hospital or 3-month mortality, despite relatively high initial NIHSS scores (median, 17) and the fact that all patients had a proximal large arterial occlusion. Early recanalization without symptomatic hemorrhage may be responsible for the excellent clinical outcome in this study.
In comparison with controlled trials of combined IV and IA approaches and the NINDS rtPA stroke trial, our study showed a very low mortality rate and rate of symptomatic intracerebral hemorrhage (Table 2). Symptomatic intracerebral hemorrhage is the most feared complication of combined IV-IA thrombolysis. In this study, there was no symptomatic hemorrhage in our patients, though the IA therapy was performed after full-dose IV rtPA. Previous studies of IA thrombolysis with urokinase suggest that higher doses of IA urokinase lead to higher symptomatic hemorrhage rates.9,10 A higher dose of IA urokinase is also associated with poor clinical outcome.19 In our study, we could use a minimal amount of urokinase because of the combined use of AMCD. To our knowledge, a median dose of urokinase of 300,000 U is the lowest of any study regarding IA thrombolysis with urokinase to date. No use of heparin or glycoprotein IIb/IIIa inhibitor either intravenously or intra-arterially may be additional contributing factors to the low rate of symptomatic hemorrhage in this study.
Table 2:
Clinical outcomes
| NINDS rtPA (n = 182) | NINDS Placebo (n = 211) | IMS I Study (n = 80) | IMS II Study (n = 81) | Present Study (n = 12) | |
|---|---|---|---|---|---|
| Mortality rate at 3 months (%) | 21 | 24 | 16 | 16 | 0 |
| Symptomatic ICH (%) | 6.6 | 1 | 6.3 | 9.9 | 0 |
| Asymptomatic ICH (%) | 6.0 | 5.7 | 42.5 | 32.1 | 41 |
| mRS 0–2 at 3 months (%) | 39 | 28 | 43 | 46 | 75 |
Note:—NINDS indicates National Institute of Neurological Disorders and Stroke; IMS, Interventional Management of Stroke; ICH, intracerebral hemorrhage.
AMCD can provide an advantage vs pharmacologic IA thrombolysis by increasing recanalization rate and speed and reducing the total thrombolytic dose.12–14 Noser et al14 reported a 38% immediate recanalization rate in 32 patients with acute ischemic stroke who were treated with AMCD in conjunction with IA infusion of reteplase. Of the 32 patients, 22 received combined IV-IA thrombolysis. Favorable outcome was noted in 59% of the patients, with a rate of symptomatic cerebral hemorrhage of 9.4% and a mortality rate of 12.5%. Currently, there are several techniques for AMCD, including aggressive microcatheter-microwire clot maceration, intracranial angioplasty, stent deployment, use of a snare device, and clot disruption with a deflated balloon catheter.14,20,21 Using the aggressive microcatheter-microwire clot maceration method, we achieved successful recanalization in most (83%) of our patients. A more aggressive method, percutaneous angioplasty, was needed in only 2 of our patients. Limitations of this study included the small number of patients and the lack of a control group.
Conclusions
Our study showed that combination therapy with low-dose IA urokinase and AMCD is safe and effective after failure of IV thrombolysis in selected patients with acute ischemic stroke. A high rate of recanalization, low rate of symptomatic hemorrhage, and excellent functional outcome can be achieved.
References
- 1. del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992; 32: 78–86 [DOI] [PubMed] [Google Scholar]
- 2. von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 1992; 23: 646–52 [DOI] [PubMed] [Google Scholar]
- 3. Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 2007; 38: 192–93 [DOI] [PubMed] [Google Scholar]
- 4. The IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the interventional management of stroke study. Stroke 2004; 35: 904–12 [DOI] [PubMed] [Google Scholar]
- 5. The IMS II Trial Investigators. The interventional management of stroke (IMS) II study. Stroke 2007; 38: 2127–35 [DOI] [PubMed] [Google Scholar]
- 6. Lee KY, Kim DI, Kim SH, et al. Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. AJNR Am J Neuroradiol 2004; 25: 1470–75 [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DJ, Kim DI, Kim SH, et al. Rescue localized intra-arterial thrombolysis for hyperacute MCA ischemic stroke patients after early non-responsive intravenous tissue plasminogen activator therapy. Neuroradiology 2005; 47: 616–21 [DOI] [PubMed] [Google Scholar]
- 8. Shaltoni HM, Albright KC, Gonzales NR, et al. Is intra-arterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke 2007; 38: 80–84 [DOI] [PubMed] [Google Scholar]
- 9. Vora NA, Gupta R, Thomas AJ, et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR Am J Neuroradiol 2007; 28: 1391–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brekenfeld C, Remonda L, Nedeltchev K, et al. Symptomatic intracranial hemorrhage after intra-arterial thrombolysis in acute ischemic stroke: assessment of 294 patients treated with urokinase. J Neurol Neurosurg Psychiatry 2007; 78: 280–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke 2007; 38: 431–40 [DOI] [PubMed] [Google Scholar]
- 12. Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery 2002; 51: 1319–29 [DOI] [PubMed] [Google Scholar]
- 13. Sorimachi T, Fujii Y, Tsuchiya N, et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol 2004; 25: 1391–402 [PMC free article] [PubMed] [Google Scholar]
- 14. Noser EA, Shaltoni HM, Hall CE, et al. Aggressive mechanical clot disruption. A safe adjunct to thrombolytic therapy in acute stroke? Stroke 2005; 36: 292–96 [DOI] [PubMed] [Google Scholar]
- 15. The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–87 [DOI] [PubMed] [Google Scholar]
- 16. Yoon W, Seo JJ, Cho KH, et al. Symptomatic middle cerebral artery stenosis treated with intracranial angioplasty: experience in 32 patients. Radiology 2005; 237: 620–26 [DOI] [PubMed] [Google Scholar]
- 17. Labiche LA, Al-Senani F, Wojner AW, et al. Is the benefit of early recanalization sustained at 3 months? A prospective cohort study. Stroke 2003; 34: 695–98 [DOI] [PubMed] [Google Scholar]
- 18. Alexandrov AV, Burgin WS, Demchuk AM, et al. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation 2001; 103: 2897–902 [DOI] [PubMed] [Google Scholar]
- 19. Mandava P, Kent TA. Intra-arterial therapies for acute ischemic stroke. Neurology 2007; 68: 2132–39 [DOI] [PubMed] [Google Scholar]
- 20. Nakano S, Iseda T, Yoneyama T, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion. An alternative option to intra-arterial thrombolysis. Stroke 2002; 33: 2872–76 [DOI] [PubMed] [Google Scholar]
- 21. Ikushima I, Ohta H, Hirai T, et al. Balloon catheter disruption of middle cerebral artery thrombus in conjunction with thrombolysis for the treatment of acute middle cerebral artery embolism. AJNR Am J Neuroradiol 2007; 28: 513–17 [PMC free article] [PubMed] [Google Scholar]