Abstract
BACKGROUND AND PURPOSE:
In vitro and nonhuman in vivo studies demonstrating impaired fibrinolysis of thrombus by thrombolytic agents in the presence of iodinated contrast media (ICM) have prompted concern regarding the clinical use of ICM. A systematic review and meta-analysis were performed to investigate the proportion of patients with acute stroke experiencing recanalization after thrombolytic therapy in whom ICM were administered compared with those in whom they were not.
MATERIALS AND METHODS:
Embase and Medline searches identified studies reporting recanalization rates in acute ischemic anterior circulation stroke. Pooled proportions of patients who recanalized were calculated with a random-effects model, and studies involving contrast (CS) were compared with those without (NCS).
RESULTS:
Six studies were found in which ICM were administered, and 12 studies, in which they were not. Studies were statistically heterogeneous. Combined pooled proportions and 95% confidence intervals (CI) for recanalization in unselected CS and NCS were 53% (36%–70%) and 61% (52%–71%), respectively. In a subgroup analysis in which only middle cerebral artery occlusions were considered, the pooled proportions in CS (n = 3 studies) and NCS (n = 9 studies) were 66% (95% CI, 49%–82%; I2, 0%) and 63% (CI, 52%–74%; I2, 82.5%).
CONCLUSIONS:
Recanalization rates were not significantly different in patients who received iodinated contrast agents in clinical studies. A randomized trial to test whether ICM affect recanalization would require a prohibitively large number of subjects.
In vitro studies have demonstrated that fibrinolysis by agents including recombinant tissue plasminogen activator (rt-PA) may be impaired by both ionic and nonionic iodinated contrast media (ICM).(1,2) Longer reperfusion delays have also been demonstrated in vivo in a canine coronary artery thrombosis model.(3) This effect may be, in part, due to fibrin-altering properties of ICM, which have been shown to change fiber size, making clots more resistant to fibrinolysis.(4) Despite this, clinical outcomes appear to be favorable in ICM-exposed patients with acute coronary syndrome,(5) and rates of cerebral artery recanalization following intra-arterial thrombolysis and its associated contrast load compare favorably with those following intravenous thrombolysis.(6)
Increasing use of multimodal CT imaging in acute stroke that includes contrast administration for angiography or perfusion studies has raised concerns regarding the potential negative effect of ICM on recanalization.(7) We undertook a systematic review of randomized controlled trials and observational studies to investigate the effects of ICM on the proportion of subjects demonstrating recanalization of an occluded artery after acute ischemic stroke treated with thrombolytic drugs.
Materials and Methods
Literature Search
The Ovid on-line portal was used to search Medline and Embase from inception to week 40, 2006. Searches relevant to “stroke” and “fibrinolysis” were combined (on-line Appendix). Relevant search filters (the Scottish Intercollegiate Guidelines Network) for randomized controlled trials (RCT) and observational studies were applied.(8) The search was performed by 1 author (K.A.D.).
Inclusion Criteria
Human studies published in English were considered. Male and female patients of all ages with anterior circulation stroke were included. In an attempt to extract a homogeneous population of subjects treated with intravenous thrombolysis, subjects with posterior circulation strokes were not considered. Data pertaining to patients with coronary artery occlusion or intra-arterial thrombolysis were excluded. One investigator (K.A.D., Clinical Research Fellow) screened the results and selected studies that reported the absolute number of patients with documented occlusion who recanalized after the administration of intravenous thrombolytic therapy. Only studies that reported data pertaining to the primary culprit vessel were considered (ie, only 1 vessel occlusion per subject). In studies in which only part of the data were relevant to the current question, these data were extracted where possible (eg, data from the treatment arm in an RCT). Care was taken to avoid the use of duplicate data from the same patients in different articles. The end point was either partial or complete recanalization demonstrated on imaging.
Quality Assessment
There was no quality threshold for inclusion into the review. However, assessment of study quality by K.A.D. was made by using a validated checklist(9) assessing whether the following criteria were fulfilled: 1) the question is sufficiently described, 2) the design is evident and appropriate to answer the study question, 3) the method of subject selection is described and appropriate, 4) the subject characteristics are sufficiently described, 5) the randomization is described, 6) blinding of investigators is reported, 7) blinding of subjects is reported, 8) the outcome and exposure measures are well defined, 9) the sample size is appropriate, 10) analysis is described and appropriate, 11) the estimate of variance for the results is reported, 12) confounding is controlled for, 13) the results are reported in sufficient detail, and 14) the results support the conclusions.
Data Extraction
The absolute number and proportion of patients recanalizing was recorded for those patients in studies in which ICM were administered and those in which they were not. Two authors provided further raw data on request, and 1 author advised regarding time periods of patient recruitment ( “Acknowledgments”).
Where studies used Thrombolyis in Myocardial Infarction or Thrombolysis in Brain Ischemia scales to assess vessel-occlusion status at follow-up, grades 0 and 1 on each scale were considered to represent failure of recanalization. No other distinction was made between partial and complete recanalization. When extracting data concerning the upper time limits of administration of thrombolytic therapy from studies in which they were not absolutely stated, the upper time limit was derived from the mean time plus 2 SDs (n = 1 study).
Analysis
Pooled proportions of patients who recanalized were calculated with a random-effects model (DerSimonian-Laird, part of the meta-analysis function StatsDirect Software, Version 2.6.2; http://www.statsdirect.com), and studies involving contrast were compared with those without. In addition to analysis of all data fulfilling the inclusion criteria, the following subgroups were also analyzed: 1) subjects with occlusion of the M1 or M2 segment of the middle cerebral artery (MCA) only, 2) cohort studies only, and 3) subjects in whom recanalization was determined at late time points defined as 24 hours or later. An I2 test(10) (StatsDirect) was used to assess the heterogeneity of studies, with a value of >75% considered to indicate high heterogeneity.
The software package StatMate 2 (Graphpad Software, http://www.graphpad.com/StatMate/statmate.htm) was used to perform a χ2 analysis to calculate the number of subjects needed to perform a clinical trial to answer the question of interest. A meta-regression analysis by using a random-effects model(11) and unrestricted maximum-likelihood estimates for between-trial variance were performed to determine the contribution of differences in the upper time limit of administration of thrombolytics in different studies to the observed heterogeneity (Comprehensive Meta-Analysis, Version 2; Biostat, http://www.biostat.org/).
Results
Medline and Embase yielded 1820 and 2081 studies, respectively. Screening of the title and abstract and, when necessary, the full text of these studies produced a final result of 18 studies (on-line Fig 1). Six studies used contrast agents (CS, n = 135 subjects) and 12 did not (NCS, n = 622 subjects). There was no randomized controlled study that specifically addressed our question. Recanalization was determined by transcranial Doppler sonography, MR angiography, CT angiography, or digital subtraction angiography.
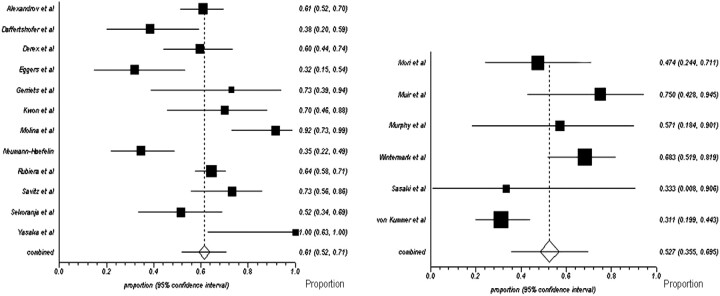
Fig 1.
Proportions of meta-analysis plots (random effects) of noncontrast studies and contrast studies. Study titles are listed on the left-hand side of both plots, and the weighted proportions of subjects who recanalized and their 95% confidence intervals are listed on the right-hand side of the plots. The size of the black squares is proportional to the relative weights of the study in the analysis of pooled proportions. All studies are from on-line Fig 1.
The number of subjects analyzed from each study varied from 8 to 219 patients, with the number of relevant subjects fulfilling our inclusion criteria in each study ranging from 3 to 219. The mean upper time limit for administration of thrombolytic therapy was 6.5 hours and 5.0 hours for CS and NCS studies, respectively. Study characteristics are described in Table 1
Table 1:
Characteristics of included studiesa
| Article | Year | Journal | Study Type | rtPA Dose/NINDS | Site of Occlusion | Recan. Assessment Tool | Recan. Times | No. of Patients With Recan. | Total No. of Relevant Patients |
|---|---|---|---|---|---|---|---|---|---|
| Noncontrast Studiesb | |||||||||
| Alexandrov et al | 2004 | Stroke | Prospective cohort | Yes <180 min | M1, M2 | TCD | 2 h (Post-rtPA) | 73 | 120 |
| Daffertshofer et al | 2005 | Stroke | NRCT | Yes <360 min | ICA, M1, M2, M3 | MRA | 24 h | 10 | 26 |
| Derex at al | 2004 | Neuroradiology | Prospective cohort | No <360 min | ICA, M1, M2, ACA | MRA | 24 h | 28 | 47 |
| Eggers at al | 2003 | Ann Neurol | RCT | Yes <180 min | M1, M2 | TCD | 1 h (Post-rtPA) | 8 | 25 |
| Gerriets et al | 2002 | Stroke | RCT (substudy of DIAS 1) | Yes <360 min | ICA, M1, M2 | TCD | 24 h | 8 | 11 |
| Kwon et al | 2004 | Arch Neurol | Retrospective cohort | Yes <360 min | ICA, MCA | MRA | 72 h | 14 | 20 |
| Molina et al | 2001 | Stroke | Case control | Yes <180 min | M1, M2 | TCD | 48 h | 22 | 24 |
| Neumann-Haefelin et al | 2004 | Stroke | Retrospective cohort | NS <360 min | M1 | MRA | 24 h | 18 | 52 |
| Rubiera et al | 2006 | Stroke | Prospective cohort | Yes <360 min | ICA, M1, M2 | TCD | 2 h (Post-rtPA) | 141 | 219 |
| Savitz et al | 2005 | Stroke | Retrospective cohort | Yes <360 min | ICA, MCA | MRA | 72 h | 27 | 37 |
| Sekoranja et al | 2006 | Stroke | Prospective cohort | Yes <180 min | ICA, MCA | TCD | 30 min (Post-rtPA) | 17 | 33 |
| Yasaka et al | 1998 | Neurology | RCT | No 210 ± 36 min | MCA; origin, trunk, distal | TCD | 24 h | 8 | 8 |
| Contrast Contrast studiesb | |||||||||
| Muir et al | 2005 | JNNP | Prospective cohort | Yes <200 min | M1, M2 | CTA, TCD, DSA | 48 h | 9 | 12 |
| Murphy et al | 2006 | Stroke | Prospective cohort | NS <420 min | MCA syndrome | CTA | 24 h | 4 | 7 |
| Wintermark et al | 2006 | Stroke | Prospective cohort | No <420 min | Large-artery occlusion | MRA | 2–7 d | 28 | 41 |
| von Kummer et al | 1995 | Stroke | Prospective cohort | No <360 min | ICA, M1, M2 | DSA | Immediately post-rtPA | 19 | 61 |
| Sasaki et al | 1996 | AJNR | Prospective cohort | No <480 min | M1, M2 | DSA | 24 h | 1 | 3 |
| Mori et al | 1992 | Neurology | RCT | No <360 min | ICA, M1, M2, AM | DSA | Immediately post-rtPA | 9 | 19 |
Note:—Recan. indicates recanalization, JNNP, J Neurol Neurosurg Psychiatry; RCT, randomized controlled trial; NRCT, non-randomized controlled trial; CTA, CT angiography; DSA, digital subtraction angiography; MRA, MR angiography; TCD, transcranial Doppler sonography; ICA, internal carotid artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; M1, M1 branch of the MCA; M2, M2 branch of the MCA; M3, M3 branch of the MCA; AM, A2 branch of the ACA; NS, not specified; rtPA, recombinant tissue plasminogen activator; DIAS I, Desmoteplase in Acute Ishemic Stroke Trial; NINDS, National Institute of Neurological Disorders and Stroke.
Included are data indicating whether rtPA was administered as per the protocol used in the NINDS trial.24
All studies are listed in on-line Fig 1.
Eight studies were judged to have achieved all quality checklist requirements, and in 6 studies, only 1 point was subtracted. However in 3 studies, 2 points were subtracted, and in 1 study, 3 points were subtracted (Table 2.).
Table 2:
Quality assessment for included studies
| Studya | Question | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Noncontrast studies | |||||||||||||||
| Alexandrov et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | 2 | * | 2 | 2 | 20/20 |
| Daffertshofer et al | 2 | 2 | 1 | 2 | * | * | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 18/20 |
| Derex et al | 2 | 2 | 2 | 2 | * | * | * | 1 | 2 | 2 | 2 | * | 2 | 2 | 19/20 |
| Eggers et al | 2 | 2 | 2 | 2 | 2 | * | * | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 23/24 |
| Gerriets et al (DIAS) | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | * | * | 2 | 2 | 18/18 |
| Kwon et al | 1 | 1 | 2 | 2 | * | * | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 17/20 |
| Molina et al | 2 | 2 | 2 | 2 | * | 2 | * | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 24/24 |
| Neumann-Haefelin et al | 2 | 2 | 2 | 1 | * | * | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 18/20 |
| Rubeira et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | 2 | * | 2 | 2 | 20/20 |
| Savitz et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | 2 | * | 2 | 2 | 20/20 |
| Sekoranja et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 1 | 1 | * | * | 2 | 2 | 16/18 |
| Yasaka et al (ASK) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 27/28 |
| Contrast studies | |||||||||||||||
| Muir et al | 2 | 2 | 2 | 2 | * | 2 | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 21/22 |
| Murphy et al | 2 | 2 | 2 | 2 | * | 2 | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 21/22 |
| Wintermark et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | 2 | * | 2 | 2 | 20/20 |
| von Kummer et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 2 | 2 | 2 | * | 2 | 2 | 20/20 |
| Sasaki et al | 2 | 2 | 2 | 2 | * | * | * | 2 | 1 | 2 | 2 | * | 2 | 2 | 19/20 |
| Mori et al | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | * | 2 | 2 | 25/26 |
Note:—ASK indicates Australian Streptokinase Trial.
These are the same studies as cited in Table 1.
Combined pooled proportions and 95% confidence intervals (CIs) for recanalization in unselected CS and NCS were 53% (36%–70%) and 61% (52%–71%), respectively (Fig 1 ). When only studies assessing late recanalization (24 hours or later) were evaluated, the weighted pooled proportions with recanalization were similar in each group; the proportion recanalizing in NCS (n = 8 studies) and CS (n = 4 studies) was 67% (95% CI, 51%–82%; I2 84.5%) and 66% (95% CI, 54%–77%; I2, 0%), respectively. Again, weighted proportions were similar between groups when considering only those subjects with isolated MCA (M1 or M2) occlusions; proportions in NCS (n = 9 studies) and CS (n = 3 studies) were 63% (95% CI, 52%–74%; I2, 82.5%) and 66% (95% CI, 49%–82%; I2, 0%), respectively. Meta-regression analysis did not reveal a statistically significant influence of the upper time limit for the administration of thrombolysis in each study on the proportion of subjects experiencing recanalization (slope = −0.001, P = .2).
The 3% difference in recanalization of MCA occlusions which was noted in NCS compared to CS has a 95% confidence interval of between 16% reduction, and 10% increase in recanalisation in NCS compared to CS. We calculate that an RCT to detect a 3% or 16% reduction in recanalization would require a total of 10,000 or 240 subjects respectively, assuming a power of 80% and a significance level of 0.05.
Discussion
Although head-to-head studies are still unavailable, it is believed by many that the benefits of multimodal over plain CT—including improved sensitivity for diagnostic confirmation, prediction of viable tissue, and identification of occlusion site—may confer advantages in patient selection for thrombolytic treatment.(12,13) However, because CT angiography and perfusion studies involve moderately large quantities of ICM, experimental data on ICM impairing fibrinolysis are of concern. More recent studies have also raised the possibility of contrast toxicity predisposing to intracranial hemorrhage.(14)
This study was conducted to ascertain whether there was any evidence that such concerns are vindicated on the basis of current clinical evidence. We found no evidence that ICM in patients with acute ischemic stroke undergoing thrombolysis were associated with an unreasonably low rate, or reduced probability, of recanalization. More specifically, the rates of recanalization in cohorts used in studies that administered ICM were comparable with those in which ICM were not administered. This study, however, does not necessarily contradict previous in vitro and in vivo studies that suggest impaired fibrinolysis by ICM. For example, the delay to optimal perfusion of approximately 45 minutes, which was noted in a coronary artery canine study,(3) would not necessarily be detected by our analysis.
Our study would also have been unlikely to detect the small yet significant impairment of recanalization by ioxaglate, a low-osmolar ionic contrast agent, compared with iodixamol, a nonionic hyposmolar contrast agent (85.9% versus 92.2%), demonstrated after acute coronary syndrome in human subjects.(5) In addition, our understanding of the nature of recanalization following coronary artery occlusion cannot be easily extrapolated to that following cerebral artery occlusion; while recanalization after acute coronary syndrome is predominantly due to in situ plaque rupture and formation of platelet-rich thrombus,(15) the varied nature of clot formation in stroke may lead to a differential pattern of recanalization of cerebral arteries that is dependent on stroke subtype.(16)
This study reports an indirect comparison of heterogeneous studies, and the findings should be regarded cautiously. Nevertheless, it seems probable that a study to address the question directly would be prohibitively large. Analysis of the unselected group included subjects with a number of different occlusion sites. Given that recanalization rates differ dramatically according to occlusion site,(17,18) particularly between occlusions of the carotid-T and the M2 segment of the MCA, a subgroup analysis restricted to subjects with MCA occlusions was performed. Here the weighted pooled proportions of subjects recanalizing were almost identical between groups.
Both the significant heterogeneity among studies and the small number of studies that assessed subjects who received contrast media limit the interpretation of this meta-analysis. The statistical heterogeneity may be explained by the methodologic heterogeneity, with differences seen between studies with respect to the following variables: time to administration of thrombolytic therapy, preparation and regimen of administration of thrombolytic therapy, and criteria and tools to determine recanalization. In addition, unlike the in vitro and in vivo studies in which the contrast agent was unchanged throughout the study, the agent varied among the studies included in this analysis; different agents vary in their osmolality and ionic nature and exert different effects on fibrinolysis,(2,3,5)thus making this an important issue and compounding the issues surrounding heterogeneity of data. A further meta-regression analysis with respect to the use of sonothrombolysis and sex would have been useful should the raw data have been available because these factors have been shown to influence recanalization.(19,20)
A further limitation is the restriction of the search strategy to include only English language studies, a decision based on the high cost involved with the translation of non-English language articles. Although there was no quality threshold for inclusion of studies in this analysis, it is unlikely that this in itself introduced bias; all studies were of reasonable quality and the data extracted from the studies were in raw format. In addition, as to the influence on clinical outcome, it is not only whether recanalization occurs but also the speed(21,22) and degree of recanalization,(22) which were not assessed here. Indeed, most of the available data related to recanalization at or beyond 24 hours, whereas we know that thrombolytic therapy has the most substantial effect on recanalization in the first few hours.(23) Therefore, assessment of recanalization at late time points will be confounded by the effects of both spontaneous recanalization and reocclusion, thus limiting the ability to detect differences between groups.
Conclusions
This study has failed to demonstrate lower recanalization rates in those subjects in whom contrast agents were administered. Therefore, on the basis of the current literature, we cannot recommend withholding contrast agents in scenarios in which the treating physician deems it to be clinically useful. The question can only be answered more definitively with an RCT, which would require the recruitment of a large number of subjects. Although we calculated that as few as 240 subjects would be required, both wide confidence intervals and the need for a noninferiority design mean that the numbers required in practice are likely to be much larger.
Acknowledgments
We thank Magdy Selim and Max Wintermark for providing further raw data and Andrei Alexandrov for providing information regarding timing of recruitment to studies.
Footnotes
K.A. Dani was funded by a Patrick Berthoud Research Fellowship.
Previously presented as a poster at: European Stroke Conference, May 29–June 1, 2007; Glasgow, Scotland, UK.
Conflicts of Interest: The authors are currently involved in studies using CT perfusion and angiography.
Indicates article with supplemental on-line appendix.
References
- 1. Dehmer GJ, Gresalfi N, Daly D, et al. Impairment of fibrinolysis by streptokinase, urokinase and recombinant tissue-type plasminogen activator in the presence of radiographic contrast agents. J Am Coll Cardiol 1995; 25: 1069–75 [DOI] [PubMed] [Google Scholar]
- 2. Jones CI, Goodall AH. Differential effects of the iodinated contrast agents ioxaglate, iohexol and iodixanol on thrombus formation and fibrinolysis. Thromb Res 2003; 112: 65–71 [DOI] [PubMed] [Google Scholar]
- 3. Pislaru S, Pislaru C, Szilard M, et al. In vivo effects of contrast media on coronary thrombolysis. J Am Coll Cardiol 1998; 32: 1102–08 [DOI] [PubMed] [Google Scholar]
- 4. Carr ME, Jr, Carr SL, Merten SR. Effects of ionic and nonionic contrast media on clot structure, platelet function and thrombolysis mediated by tissue plasminogen activator in plasma clots. Haemostasis 1995; 25: 172–81 [DOI] [PubMed] [Google Scholar]
- 5. Davidson CJ, Laskey WK, Hermiller JB, et al. Randomized trial of contrast media utilization in high-risk PTCA: the COURT trial. Circulation 2000; 101: 2172–77 [DOI] [PubMed] [Google Scholar]
- 6. Wardlaw JM, Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003: CD000213, update of Cochrane Database Syst Rev 2000:CD000213 [DOI] [PubMed] [Google Scholar]
- 7. Warlow C, van Gijn J, Dennis M, et al. Stroke, Practical Management, 3rd ed., Oxford, UK: Blackwell Publishing; 2008: 203 [Google Scholar]
- 8. Scottish Intercollegiate Guidelines Network. Search Filters. Available at: http://www.sign.ac.uk/methodology/filters.html. Accessed September 2006
- 9. Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Alberta Heritage Foundation for Medical Research; 2004 [Google Scholar]
- 10. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58 [DOI] [PubMed] [Google Scholar]
- 11. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21: 1559–73 [DOI] [PubMed] [Google Scholar]
- 12. Nabavi DG, Kloska SP, Nam EM, et al. MOSAIC: Multimodal Stroke Assessment using Computed Tomography—novel diagnostic approach for the prediction of infarction size and clinical outcome. Stroke 2002; 33: [DOI] [PubMed] [Google Scholar]
- 13. Kloska SP, Nabavi DG, Gaus C, et al. Acute stroke assessment with CT: do we need multimodal evaluation? Radiology 2004; 233: 79–86 [DOI] [PubMed] [Google Scholar]
- 14. Khatri P, Broderick JP, Khoury JC, et al. Microcatheter contrast injections during intra-arterial thrombolysis may increase intracranial hemorrhage risk. Stroke 2008; 39: 3283–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies MJ, Thomas AC. Plaque fissuring–the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 1985; 53: 363–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molina CA, Montaner J, Arenillas JF, et al. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke 2004; 35: 486–90 [DOI] [PubMed] [Google Scholar]
- 17. del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992; 32: 78–86 [DOI] [PubMed] [Google Scholar]
- 18. Saqqur M, Uchino K, Demchuk AM, et al. for the CLOTBUST Investigators.. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007; 38: 948–54 [DOI] [PubMed] [Google Scholar]
- 19. Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004; 351: 2170–78 [DOI] [PubMed] [Google Scholar]
- 20. Savitz SI, Schlaug G, Caplan L, et al. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 2005; 36: 1447–51 [DOI] [PubMed] [Google Scholar]
- 21. Alexandrov AV, Burgin WS, Demchuk AM, et al. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation 2001; 103: 2897–902 [DOI] [PubMed] [Google Scholar]
- 22. Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, et al. Effect of incomplete (spontaneous and postthrombolytic) recanalization after middle cerebral artery occlusion: a magnetic resonance imaging study. Stroke 2004; 35: 109–14 [DOI] [PubMed] [Google Scholar]
- 23. Molina CA, Montaner J, Abilleira S, et al. Time course of tissue plasminogen activator-induced recanalization in acute cardioembolic stroke: a case-control study. Stroke 2001; 32: 2821–27 [DOI] [PubMed] [Google Scholar]
- 24. Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581–87 [DOI] [PubMed] [Google Scholar]