Abstract
BACKGROUND AND PURPOSE:
Particle embolization is widely used in the treatment of meningiomas. We assessed the frequency and outcome of complications of embolization of meningiomas and tried to identify risk factors.
MATERIALS AND METHODS:
Between 1994 and 2009, a total of 198 patients with 201 meningiomas underwent embolization. Indication for embolization was preoperative in 165 meningiomas and adjunctive to radiosurgery in 8. In the remaining 28 meningiomas, embolization was initially offered as a sole therapy. There were 128 women and 70 men with a mean age of 54.4 years (median age, 54 years; range, 15–90 years). Complications were defined as any neurologic deficit or death that occurred during or after embolization. Logistic regression was used to identify the following possible risk factors: age above median, female sex, tumor size above median, meningioma location in 5 categories, use of small particle size (45–150 μm), the presence of major peritumoral edema, and arterial supply in 3 categories.
RESULTS:
Complications occurred in 11 patients (5.6%; 95% confidence interval [CI], 3.0%–9.8%). Ten complications were hemorrhagic, and 1 was ischemic. Six of 10 patients with hemorrhagic complications underwent emergency surgery with removal of the hematoma and meningioma. Complications of embolization resulted in death in 2 and dependency in 5 patients (7/198, 3.5%; 95% CI, 1.6%–2.0%). The use of small particles (45–150 μm) was the only risk factor for complications (odds ratio [OR], 10.21; CI, 1.3–80.7; P = .028).
CONCLUSIONS:
In this series, particle embolization of meningiomas had a complication rate of 5.6%. We believe that the use of small polyvinyl alcohol (PVA) particles (45–150 μm) should be discouraged.
Preoperative embolization of meningiomas is widely used to facilitate surgical removal and reduce intraoperative blood loss.1–5 The potential benefits of preoperative embolization should be balanced against the risk for complications. Little is known about the nature and frequency of complications of embolization of meningiomas in large patient series, and risk factors are unknown. Recently, several authors have reported a considerable hemorrhagic and ischemic complication rate.6–8 In the present study, we assessed the frequency of complications and clinical outcome of 201 embolized meningiomas in 198 consecutive patients treated at our institution for a 15-year period. Moreover, we tried to identify risk factors for these adverse events.
Materials and Methods
General
Between 1994 and 2009, a total of 210 patients with 213 meningiomas received embolization at our institution. Clinical or radiologic data from 12 patients were incomplete. The present study consists of 201 embolized meningiomas in 198 patients. Indication for embolization was preoperative in 165 meningiomas and before radiosurgical treatment in 8 meningiomas. In the remaining 28 meningiomas, embolization was initially offered as a sole therapy. Referral for preoperative embolization in patients with meningiomas was dependent on the personal preference of the neurosurgeon. During the study period, a total of 747 meningiomas (excluding skull base meningiomas) were surgically removed, so approximately one-quarter of the patients who underwent surgical removal received embolization preoperatively.
From our data base, the following patient characteristics were assessed: age, sex, indication for embolization, and meningioma size. Meningioma location was categorized as convexity, falx, sphenoid ridge, posterior fossa, and tentorial. Peritumoral edema was dichotomized in minor and major (with midline shift). Arterial supply to the meningioma was classified as more than 75% external carotid artery supply, equal external and internal carotid artery supply, and more than 75% internal carotid artery supply.
Complications were defined as any neurologic deficit or death that occurred during or after embolization. Most patients underwent surgery the following day, and the postembolization follow-up period for these patients was approximately 24 hours. In patients who were not operated on after embolization, follow-up data were collected from their medical and imaging records. Complications were recorded and categorized as any complication, hemorrhagic complication, and complication leading to death or dependency. When the patient’s clinical condition after a complication demanded a subsequent emergency surgical intervention, a complication of the intervention was considered a complication of the embolization.
For hemorrhagic complications, the location of bleeding was categorized as intratumoral, peritumoral, or subarachnoidal. The onset and nature of any neurologic deficit were assessed as was the therapy for these complications. Angiographic abnormalities during embolization, such as contrast extravasation, were recorded. In patients with complications from embolization, the reports on the histopathologic subtype of the meningioma were collected.
Data Analysis
Logistic regression was used to identify risk factors for both any complications and hemorrhagic complications. The following risk factors were evaluated: age above median, female sex, tumor size above median, meningioma location, the presence of major peritumoral edema, arterial supply in 3 categories, and the use of small particle size (45–150 μm).
Embolization Technique
Angiography and embolization was generally performed the day before surgery. All patients were premedicated with steroids. Apart from heparinized saline in the pressure bags used to flush the catheters (1000 U per 500 mL) continuously, no heparin was given. Vascularization of the meningioma was first assessed by angiography of internal and external carotid arteries and/or the vertebral artery. Embolization was performed via the middle meningeal or occipital artery only. The ophthalmic artery, the meningohypophyseal trunk, or pial feeders were not used as access for embolization. Embolization was performed through a standard microcatheter (TurboTracker 18 or Excel 14; Target Therapeutics/Boston Scientific, Fremont, California). Polyvinyl alcohol particles 45 to 150 μm or 150 to 250 μm (PVA, Contour Emboli; Target Therapeutics, Fremont, California) were used as an embolic agent. Particles were mixed with nonionic contrast medium, and the mixture was diluted to approximately 50% with saline. Under fluoroscopic control, the mixture was slowly injected until stagnation of the contrast agent in the feeding artery was accomplished.
Results
Of the 198 patients, 128 were women and 70 were men (mean age, 54.4 years; median, 54 years; age range, 15–90 years). Mean meningioma size was 50 mm (median size, 50 mm; range, 16–100 mm). Location was convexity in 101, the falx in 50, the sphenoid ridge in 33, the posterior fossa in 9, and tentorial in 8 meningiomas. Minor or no peritumoral edema was seen in 146 meningiomas; major edema with shift of midline structures was present in 55 meningiomas. Arterial supply was more than 75% external carotid artery supply in 131 meningiomas, equal external and internal carotid artery supply in 60 meningiomas, and more than 75% internal carotid artery supply in 10 meningiomas.
Embolization was performed with 45- to 150-μm PVA particles in 108 meningiomas (54%), and 93 meningiomas (46%) were embolized with 150- to 250-μm PVA particles.
Complications occurred in 11 of the 198 patients with 201 embolized meningiomas (5.6%; 95% CI, 3.0%–9.8%). Ten complications were hemorrhagic, and 1 was ischemic. The location of hematomas in 10 hemorrhagic complications was purely intratumoral in 4; intratumoral and peritumoral in 3; peritumoral in 2; and intratumoral, peritumoral, and subarachnoidal in 1 patient (Fig 1). Six of the 10 patients with hemorrhagic complications underwent emergency surgery with removal of the hematoma and meningioma. In 5 of these 6 patients, extravasation of contrast was noted during embolization. This extravasation occurred toward the end of the embolization, during, or after stagnation of the contrast agent in the feeding artery. No overt arterial perforation was noted; extravasation was seen as a slow collecting of contrast in or around the tumor. Complications of embolization or emergency surgery resulted in death in 2 and dependency in 5 patients (7/198, 3.5%; 95% CI, 1.6%–7.2%). Histologic reports on meningioma subtyping were available in 7 of 11 patients with complications, and none were malignant. The characteristics of the 11 patients who had complications of meningioma embolization are listed in the On-line Table.
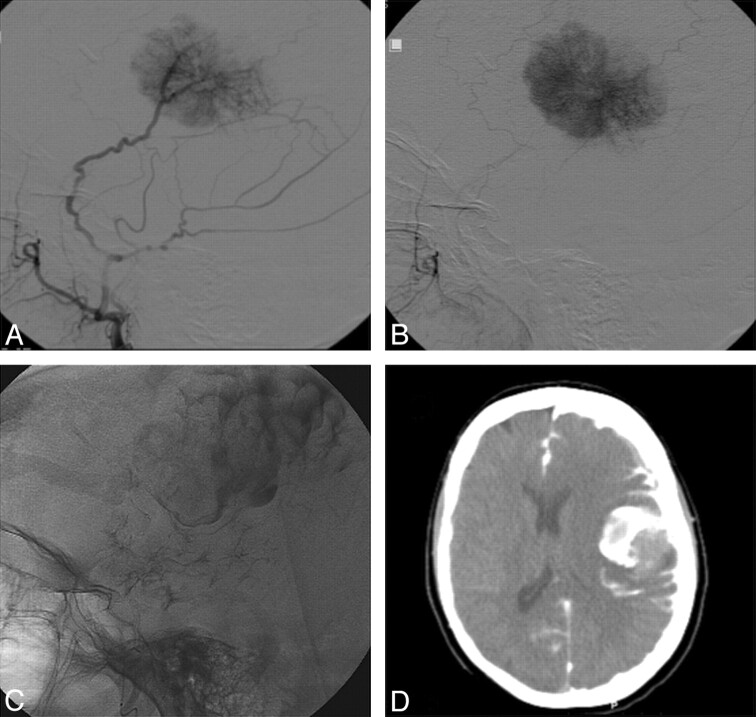
Fig 1.
Patient 7. A and B, Lateral angiography of the left middle meningeal artery showing typical tumor blush of a convexity meningioma. C, During embolization, massive extravasation of contrast is noted around the tumor and in the subarachnoid space. D, Emergency CT scanning showing the extravasation.
Logistic regression analysis identified the use of small particles (45–150 μm) as an embolic agent to be a risk factor for both any complication (OR, 10.2; 95% CI, 1.3–80.7; P = .028) and hemorrhagic complications (OR, 8.0; 95% CI, 1.0–65.9; P = .048). Age, sex, peritumoral edema, meningioma location, and arterial supply were not risk factors for complications.
Discussion
We found that serious complications of particle embolization of meningiomas are not infrequent. All but 1 complication were hemorrhagic and occurred during embolization or within a few hours. In the literature, reports on complications after particle embolization in large patient groups are scarce. In a recent study, Bendszus6 reported a comparable complication rate of 6.4% in a series of 185 patients. In this study, hemorrhagic and ischemic complications were equally frequent, whereas the complications in our patients were almost exclusively hemorrhagic. The pathophysiology of hemorrhage in meningiomas that are embolized is not fully understood. It has been postulated that necrosis as a result of deep penetration of the particles causes the tissue to be more vulnerable to bleeding.6–11 It is possible that penetration of particles into the draining veins of the tumor may block the outflow, increasing the risk for hemorrhage. Some authors suggested that malignant meningiomas are more prone to hemorrhage; however, in our study, all hemorrhagic complications developed in meningiomas of a benign histologic subtype. In the present study, the use of small particles (45–150 μm) proved to be the only significant risk factor for complications.
That small particles may carry a potential higher risk in the embolization of meningiomas is not new; several authors have referred to these small particles as being “aggressive” or “dangerous” despite lack of hard evidence to substantiate this assumption.8,11 On the other hand, small particles induce a better devascularization of the meningioma compared with larger particles, with an improved surgical treatment demonstrated in 1 study and a positive effect on blood loss during surgery in another.9,10 These contradictory results outline a clinical dilemma: small particles are more effective but cause more complications, but the use of larger particles is less effective but safer. More precisely, with small particles, the possible benefit of a better devascularization may well be outweighed by the higher risk for periprocedural tumoral hemorrhage.
The results of our study have changed our policy in the embolization of meningiomas: To reduce complications, we are more reluctant in the indication for embolization of meningiomas. For example, we now refrain from embolization when the arterial supply by the internal carotid artery is substantial and the benefits are likely to be low. When arterial supply is largely by external carotid branches, we now only embolize those meningiomas that are hypervascular with hypertrophic feeders. We no longer use small particles: with larger particles, the complication rate is likely to be low, and the larger particles will probably penetrate sufficiently into the feeders of these hypervascular tumors to reduce blood loss during surgery. If embolization of a meningioma is performed as a sole therapy and maximal infarction is desired, we still consider the use of small particles.
Conclusions
In this series, particle embolization of meningiomas had a complication rate of 5.6%. On the basis of our experience, we believe that the use of small PVA particles (45–150 μm) should be discouraged.
Supplementary Material
Footnotes
indicates article with supplemental on-line table.
References
- 1. Richter HP, Schachenmayr W.. Preoperative embolization of intracranial meningiomas. Neurosurgery 1983; 13: 261–68 [DOI] [PubMed] [Google Scholar]
- 2. Manelfe C, Lasjaunias P, Ruscalleda J.. Preoperative embolization of intracranial meningiomas. AJNR Am J Neuroradiol 1986; 7: 963–72 [PMC free article] [PubMed] [Google Scholar]
- 3. Macpherson P.. The value of pre-operative embolisation of meningioma estimated subjectively and objectively. Neuroradiology 1991; 33: 334–37 [DOI] [PubMed] [Google Scholar]
- 4. Dean B, Flom RA, Wallace RC, et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol 1993; 15: 1675–80 [PMC free article] [PubMed] [Google Scholar]
- 5. Gruber A, Killer M, Mazal P, et al. Pre-operative embolization of intracranial meningiomas: a 17-year single center experience. Minim Invasive Neurosurg 2000; 43: 18–29 [DOI] [PubMed] [Google Scholar]
- 6. Bendszus M, Monoranu CM, Schütz A, et al. Neurologic complications after particle embolization of intracranial meningiomas. AJNR Am J Neuroradiol 2005; 26: 1413–19 [PMC free article] [PubMed] [Google Scholar]
- 7. Yu SC, Boet R, Wong GK, et al. Postembolization hemorrhage of a large and necrotic meningioma. AJNR Am J Neuroradiol 2004; 25: 506–08 [PMC free article] [PubMed] [Google Scholar]
- 8. Kallmes DF, Evans AJ, Kaptain GJ, et al. Hemorrhagic complications in embolization of a meningioma: case report and review of the literature. Neuroradiology 1997; 39: 877–80 [DOI] [PubMed] [Google Scholar]
- 9. Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993; 14: 571–82 [PMC free article] [PubMed] [Google Scholar]
- 10. Bendszus M, Rao G, Burger R, et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery 2000; 47: 1306–12 [PubMed] [Google Scholar]
- 11. Latchaw RE.. Preoperative intracranial meningioma embolization: technical considerations affecting the risk-to-benefit ratio. AJNR Am J Neuroradiol 1993; 14: 583–86 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.