Abstract
BACKROUND AND PURPOSE:
Radiologic identification of the location of the CSF leakage is important for proper surgical planning and increases the chance of dural repair. This article describes our experience in analyzing clinically suspected cranial CSF fistulas by using MR imaging combined with the intrathecal administration of a gadolinium-based contrast agent.
MATERIALS AND METHODS:
A total of 85 consecutive patients with suspected CSF fistulas who presented with persistent or intermittent rhinorrhea or otorrhea lasting for more than 1 month between 2003 and 2007 were included in this study.
RESULTS:
We observed objective CSF leakage in 64 of 85 patients (75%). The CSF leak was located in the ethmoidal region in 37 patients (58%), in the superior wall of the sphenoid sinus in 8 patients (13%), in the posterior wall of the frontal sinus in 10 patients (15%), in the superior wall of the mastoid air cells in 6 patients (9%), and from the skull base into the infratemporal fossa in 1 patient (2%). Two patients (3%) showed leakage into >1 paranasal sinus.
CONCLUSIONS:
MR cisternography after the intrathecal administration of gadopentate dimeglumine represents an effective and minimally invasive method for evaluating suspected CSF fistulas along the skull base. It provides multiplanar capabilities without risk of radiation exposure and is an excellent approach to depict the anatomy of CSF spaces and CSF fistulas.
CSF leakage implies abnormal communication between the subarachnoid space and the nasal or middle ear cavity. It is generally classified as traumatic, nontraumatic (ie, spontaneous), or postsurgical in origin,1 and most cases are traumatic. Approximately 70% of traumatic CSF fistulas close spontaneously within 1 week after injury without surgical intervention.2–4 However, even in cases of mild CSF rhinorrhea or early spontaneous closure, patients remain at risk of recurrent CSF leakage, pneumocephalus, and infectious meningitis. Precise identification of the location of the CSF fistula allows proper surgical planning, increases the chance of dural repair, and can prevent complications.5,6
Numerous techniques, including plain skull radiography, intraoperative injection of fluorescein dye, positive contrast (iophendylate) studies, and radionuclide cisternography, are all helpful in limited ways.7–12 MR imaging with T2-weighted sequences has been used to localize CSF fistulas. The demonstration of high-signal-intensity fluid extending from the subarachnoid space directly into the adjacent paranasal sinuses or herniation of the brain into a sinus through a bone defect has been the principal diagnostic criterion.2,13–18 However, some or all of these findings can occasionally be observed in the absence of fistula formation on MR images obtained for reasons other than CSF leakage. The most common method for evaluating a patient with suspected CSF rhinorrhea is a combination of thin-section CT and subsequent CT cisternography (CTC). Although high-resolution CT (HRCT) is sufficient to show bony defects in the skull base, the site of the dural tear and active CSF leak can be difficult or impossible to confirm by using this technique.19,20 In addition, the combination of CT and CTC results in additional radiation exposure. Thus, a safer and less invasive method is necessary to detect CSF leakage.
This article describes our experience in analyzing clinically suspected cranial CSF fistulas by using MR imaging combined with the intrathecal administration of a gadolinium (Gd)-based contrast agent.
Materials and Methods
A total of 85 consecutive patients with suspected CSF fistulas who presented with persistent or intermittent rhinorrhea or otorrhea lasting for more than 1 month between 2003 and 2007 were included in this study. The patients ranged from 15 to 72 years of age (mean, 35.3 ± 14.7 years) and included 45 males and 40 females. The study protocol was reviewed and approved by the ethics committee of Istanbul University Cerrahpasa Medical School. Consent was obtained from all of the patients, who were informed before the study that intrathecal Gd is an off-label use.
Regarding the cause of suspected CSF leakage, 14 patients (16%) had skull base surgery, 65 (77%) experienced cranial trauma, and 6 (7%) had spontaneous CSF rhinorrhea. The time between trauma and imaging ranged from 1 month to 3 years. Rhinorrhea was evaluated due to detection by both the patient and physician. The patients were divided into several groups as follows: group 1 consisted of 12 patients with at least 1 case of bacterial meningitis combined with rhinorrhea, group 2 consisted of 16 cases of continuous rhinorrhea detected by both the patient and the physician, group 3 consisted of 17 cases of intermittent rhinorrhea detected by both the patient and the physician, and group 4 consisted of 40 cases of suspected rhinorrhea. None of the patients had signs of meningitis at the time of intrathecal injection. Previous HRCT studies showed evidence of skull base fractures, defects, or erosions in each patient. The β2 transferrin test was performed in 40 patients with active rhinorrhea, and the results were positive in 30 (75%).
We performed Gd-enhanced MR cisternography (Gd-MRC) to confirm and localize CSF leaks in all patients. All lumbar puncture procedures were performed by using a 22-gauge needle at the L4-L5 level under sterile conditions with the patient sitting in an upright position. Saline (4 mL) was mixed with 0.5 mL of gadopentetate dimeglumine (Gd-DPTA or Magnevist; Schering, Berlin, Germany) to produce a solution of 469.01 mg/mL. This solution was injected into the subarachnoid space, and the needle was removed. The patients remained in the knee-to-elbow position for 15 minutes after injection to maximize accumulation of the contrast medium in the basal cisterns and to facilitate its passage into the CSF fistula. One hour after injection, the patient was moved into a prone position and fat-saturated T1-weighted images were obtained in 3 orthogonal planes. Coronal and sagittal T1-weighted images (TR/TE, 500/17 ms; 2 signals acquired) and axial T1-weighted images (TR/TE, 600/17 ms; 2 signals acquired) were obtained by using a 1.5T MR imaging unit (Symphony; Siemens Medical Systems, Erlangen, Germany). In cases in which the CSF leak could not be accurately visualized on MR images, additional scans were obtained in the third and fifth hours. The authors (H.S. and S.A.) reviewed all images. In the evaluation, the extension of hyperintense Gd-enhanced CSF from the cerebral cisterns into the paranasal sinuses, the nasal cavity, or middle ear cavity on Gd-MRC images was considered a positive indicator of CSF leakage.
All patients were observed in the hospital for 24 hours after the procedure. After returning to the ward, the patients were monitored on an hourly basis for headache progression, gross behavioral alterations, neurologic impairment, changes in mental status, subjective complaints, and vital signs, as well as for more serious events, such as seizure activity and anaphylactoid reactions. Assessments were made in comparison with baseline findings before Gd-MRC. In addition, monthly clinical neurologic follow-up was performed for 6 or 12 months. After the 1-year clinical evaluation, the patients were followed annually. The mean follow-up period was 38.6 months.
Results
In all except 3 of the total patient group, the Gd-DTPA bolus entered the subarachnoid space at the basal cistern and showed enhancement throughout the entire subarachnoid space. In the remaining 3 patients, the contrast agent remained in the subdural space and the lumbar puncture was repeated. The CSF leak was located in the ethmoidal region in 37 patients (58%) (Fig 1 ), in the superior wall of the sphenoid sinus in 8 patients (13%) (Fig 2 ), in the posterior wall of the frontal sinus in 10 patients (15%), in the superior wall of the mastoid air cells in 6 patients (9%) (Fig 3 ), and from the skull base into the infratemporal fossa in 1 patient (2%) (Fig 4 ). Two patients (3%) showed leakage into >1 paranasal sinus. The β2 transferrin test was performed in 40 patients with active rhinorrhea, and the results were positive in 30. All patients in groups 1 and 2 had positive results. Findings in 2 other patients were positive in 12 tested patients in groups 3 and 4.
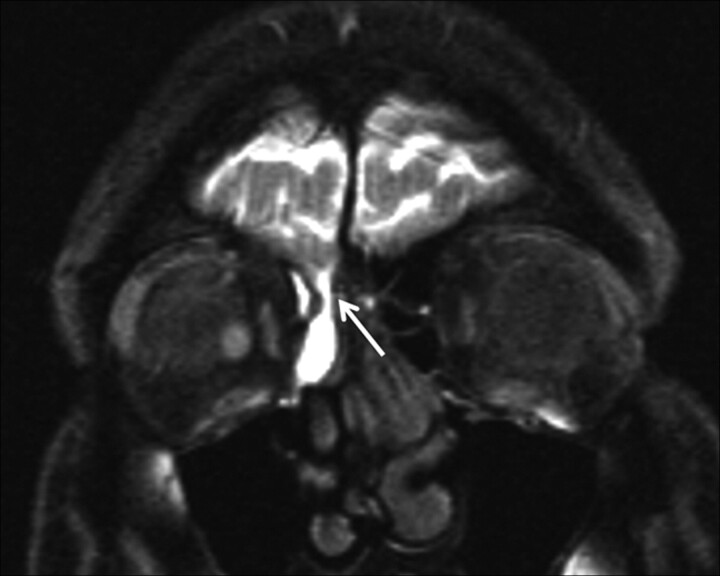
Fig 1.
CSF rhinorrhea following head trauma in a 42-year-old woman. Coronal T1-weighted MR cisternogram obtained after intrathecal administration of gadopentetate dimeglumine (Gd-DTPA) shows contrast leakage (arrow) extending from the cranial subarachnoid space into the ethmoid air cell region from a defect in the right side of the cribriform plate.
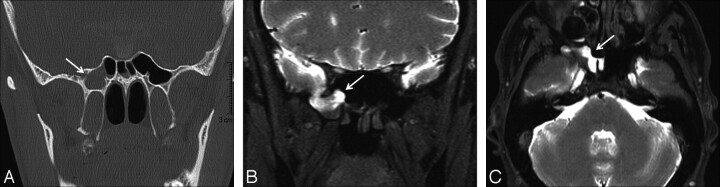
Fig 2.
CSF rhinorrhea in a 39-year-old man after sellar region surgery. A, Coronal thin-section CT scan reveals a defect in the right side of the sphenoid sinus (arrow) and opacification of the right sphenoid sinus. B and C, Coronal and axial T1-weighted fat-saturated MR cisternograms obtained after the intrathecal administration of Gd-DTPA show contrast leakage (arrows) extending from the cranial subarachnoid space into the right sphenoid sinus.
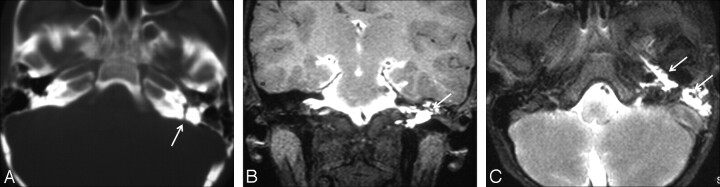
Fig 3.
CSF rhinorrhea and otorrhea following head trauma in a 15-year-old adolescent boy. A, Axial thin-section CT scan shows a defect in the left petrous temporal bone (arrow) and opacification of the left middle ear cavity. B and C, Coronal and axial T1-weighted fat-saturated MR cisternograms show contrast leakage in the middle ear cavity and eustachian tube (arrows).
Fig 4.
CSF rhinorrhea following head trauma in a 32-year-old woman. A–C, Axial, coronal, and left parasagittal T1-weighted fat-saturated MR cisternograms obtained after the intrathecal administration of Gd-DTPA show contrast leakage (arrows) extending from the cranial subarachnoid space into the left infratemporal fossa.
In the remaining 21 patients, no CSF leakage was observed and they showed no symptoms during follow-up. CSF leakage was detected in all patients in groups 1 and 2 and in 12 (70%) and 24 (60%) patients in groups 3 and 4, respectively. Surgical closure of the CSF leak was performed in all 64 patients with positive findings on Gd-MRC, and the site of the leak was confirmed intraoperatively. Postoperatively suspected rhinorrhea occurred in 1 patient (Fig 5 ). With the exception of headache, no acute adverse reaction (ie, seizure, changes in behavior or consciousness, development of focal neurologic signs, or allergic reaction) was observed within 24 hours of the procedure. Vital signs were normal in all patients and remained stable during the 24-hour period after intrathecal injection. No difference was observed between preprocedural and postprocedural neurologic findings within the initial 24-hour follow-up period. The observed increase in orthostatic headache (5 patients or 8%) after the procedure was likely related to the lumbar puncture. At the 1-year follow-up, no patient showed any neurologic symptom or sign that could be attributed to intrathecal Gd injection, and no difference was observed between preprocedural findings and the findings recorded at the annual neurologic examination.
Fig 5.
CSF rhinorrhea following head trauma in a 35-year-old man. A, Coronal thin-section CT scan reveals a defect in the roof of the sphenoid sinus (arrow) and opacification of the right sphenoid sinus. B, A coronal T1-weighted fat-saturated MR cisternogram obtained after the intrathecal administration of Gd-DTPA shows contrast leakage (arrow) extending from the cranial subarachnoid space into the right sphenoid sinus. C, After repair of the dural rupture, suspected CSF rhinorrhea recurred 1 week later and the patient underwent control MR cisternography. Images obtained in the first hour show probable leakage (arrow). D and E, Leakage becomes obvious in late images taken in the third and fifth hours (arrows).
Discussion
Radiologic identification of the site of CSF leakage is important for presurgical planning, because accurate localization of the CSF leak increases the surgical success rate. Diagnostic methods used in the evaluation of CSF leakage have evolved during the past several decades. HRCT with direct coronal and/or axial sections has a sensitivity of 84%–95%12,21; however, this technique relies on indirect signs (eg, fractures of the skull base, bony lesions or defects, mucosa swelling, fluid levels in the paranasal sinus, pneumoencephalos, and meningoencephalocele) to detect the site of the leak. Therefore, the question arises as to whether a defect depicted by HRCT is truly correlated with the site of the CSF fistula. Wenzel and Leppien22 reported a case of CSF rhinorrhea following posterior fossa surgery due to an acoustic schwannoma; in this patient, HRCT suggested a CSF leak laterally into the mastoid, whereas Gd-MRC clearly showed communication into the petrous pyramid adjacent to the internal auditory canal. In cases with multiple defects or fractures of the skull base, it may not be possible to determine the precise localization of the leak via HRCT.
MR imaging provides excellent anatomic depiction of the CSF spaces and surrounding tissues. However, in cases of suspected CSF leakage, further imaging evaluation may be required. Previous studies have indicated that unenhanced coronal T2-weighted MR imaging has the capability of demonstrating CSF fistulas; however, there is a relatively high incidence of false-positive findings (42%), especially in the presence of paranasal sinus disease.21,23,24 False-negative findings have also been reported on nonenhanced MR images from patients who subsequently exhibited skull base fractures and frank CSF leaks during surgery.23 Therefore, T2-weighted images were not included in our imaging protocol due to the high rate of false-negative and false-positive findings.
CTC is accepted as the most accurate method for the investigation of active cranial CSF leakage, but it has a number of disadvantages. The sensitivity of CTC ranges between 72% and 81%,3,25 and CTC may have problems in detecting low-flow fistulas or fistulas with only hairlike communications. This is because iodinated contrast media do not distribute freely in the CSF spaces due to their tendency to form sediment or because the tiny amount of dilute contrast medium that leaks through a fistula cannot be distinguished from the surrounding bone. Wenzel and Leppien22 presented a case in which a CSF fistula was detected only by contrast-enhanced MR cisternography, whereas CTC did not show abnormal CSF communication. In these cases, Gd-DTPA may have certain advantages because it is distributed freely in the subarachnoid space and MR imaging offers much higher contrast resolution. To further increase sensitivity, one could examine patients in the prone position or apply the Valsalva maneuver; however, the Valsalva maneuver remains controversial and is not widely used.11,12
The Biologic Effects of Ionizing Radiation VII report26 published in 2005 by the National Academy of Sciences suggested significant increased risk of developing cancer from a single radiation exposure of 10 mSv. The authors recommended that care should be exercised in the use of CT in adults ≤40 years of age. It is obvious that the dose in CTC is also high with multisection CT when both HRCT and CTC images are obtained. Additional delayed scans are frequently required in slow-flow fistulas, and CT fluoroscopy is required to construct cine images for high-volume fistulas. Considering the high doses of radiation and the lack of criterion-standard sensitivity of the CTC, it is logical that safer and more reliable techniques are required to detect CSF leakage.27
Many studies have supported the safety of intravenously administered Gd-DTPA. Although some potential adverse systemic effects have been reported, the incidences of these side effects are low.28–33 Nausea or vomiting and headache are the most common side effects, with frequencies ranging from 0.26% to 0.42%.28 Other rare reactions reported after the intravenous administration of Gd products include paresthesia, dizziness, focal convulsions, urticaria, cardiovascular reactions (eg, tachycardia and arrhythmia), laryngospasm, and anaphylactic shock.28–31 In addition, nephrogenic systemic fibrosis characterized by skin thickening resembling scleroderma has been recently described as one of the adverse effects following Gd administration in patients with renal failure.32
Animal studies have demonstrated the practical applicability of Gd-MRC in detecting the sites of surgically induced nasoethmoidal CSF fistulas.34–36
In 2 previous reports, the behavioral and neurologic alterations were observed only after the intraventricular injection of relatively high doses of Gd; no such behavioral or morphologic changes were observed in the same studies when the total dose was limited to <3.3 μmol/g brain in 15 μL.37,38 However, several recent studies reported intrathecal Gd-induced encephalopathy in humans after the accidental intrathecal injection of a high dose of Gd.39,40 In these cases, the patients developed severe neurologic symptoms, such as dysarthria, blurred vision, nystagmus, ataxia, and somnolence, followed by behavioral disturbances and psychotic symptoms.
Intrathecal Gd-DTPA is currently used to identify potential CSF fistulas in patients with rhinorrhea. In the first prospective human trial, no significant gross neurologic abnormalities, CSF changes, electroencephalographic alterations, MR morphologic evidence, or MR signal intensity changes were observed during the initial examination or during follow-up clinicoradiologic studies.41 With a total volume of 0.5–1.0 mL, the estimated intrathecal dose per gram of brain in this trial was much lower than the dose used in animal experiments (0.07–0.36 versus 2.5–15 μmol/g brain).37,38,42,43 Reiche et al44 and Aydin et al45 used the same technique to evaluate CSF fistulas, and a multicenter human study performed by Tali et al43 evaluated the safety and clinical response to Gd-DTPA in 95 patients who presented clinically with a variety of cranial or spinal signs and symptoms. A recent study demonstrated the safety of low-dose intrathecal Gd application in pediatric patients.46 In addition, a large case series published by Aydin et al47 demonstrated the relative safety and tolerability of low-dose (0.5 mL) intrathecal Gd administration in the evaluation of CSF fistulas. However, the long-term effects of intrathecal Gd in large numbers of patients are not known.
In our series, CSF fistulas were not observed in 21 patients (25%), mostly in groups 3 and 4. One possible explanation is that the patients included in the study were clinically suspected of having CSF leakage, but their symptoms were, in fact, related to other causes. In addition, 10 of these patients showed no rhinorrhea during imaging, so there was probably no leakage during that period. Negative findings may also have been related to slow-flow fistulas. Thus, rhinorrhea must be present at the time of imaging to obtain good results. The patients without demonstrable CSF fistulas showed no persistent symptoms, and during the follow-up period, they showed no fistula complications.
Furthermore, we encountered an interesting case in which the patient developed a leak from a defect in the medial wall of the middle cranial fossa into the infratemporal fossa following trauma; to our knowledge, no such case has been reported in previous series. In addition, leakage into the middle ear cavity from a defect in the mastoid bone was detected in 6 patients with otorrhea following trauma. Finally, leakage into the sphenoid sinus was demonstrated in 8 patients who had undergone sellar surgery.
Dural repair was performed in 64 patients diagnosed with CSF leakage. Probable rhinorrhea occurred in only 1 patient approximately 1 week after treatment. Control MR cisternography was performed in this patient, and images obtained in the first hour showed probable leakage, which became obvious in images from the third and fifth hours. These observations emphasize the importance of late control images. In patients with strongly suspected leakage, combining early images with the late images increases the diagnostic value of the procedure.
As in previous studies, no early or late complications due to the intrathecal administration of Gd were observed in the present study. However, 5 patients experiencing postprocedural postural headache lasting <24 hours were treated with conservative measures. The mean incidence for postural headache reported here (8%) falls within the lower range of values reported in previous studies (4%–49%).48 Furthermore, the cases of postprocedural headache encountered here were consistent in type and frequency with those observed after either water-soluble iodinated contrast material−enhanced myelographic procedures or simple diagnostic lumbar puncture. Thus, this subjective complaint is likely due to the lumbar tap (ie, iatrogenic CSF space postural hypotension associated with needle puncture−related transient CSF leakage) and not Gd-MRC.
In conclusion, MRC after the intrathecal administration of Gd-DTPA represents an effective and minimally invasive method for evaluating suspected CSF fistulas along the skull base. It provides multiplanar capabilities without risk of radiation exposure and an excellent approach to depict the anatomy of CSF spaces and CSF fistulas.
References
- 1. Park J, Strelzow V, Friedman W. Current management of cerebrospinal fluid rhinorrhea. Laryngoscope 1983; 93: 1924–300 [DOI] [PubMed] [Google Scholar]
- 2. Johnson DB, Brennan P, Toland J, et al. Magnetic resonance imaging in the evaluation of the cerebrospinal fluid fistula. Clin Radiol 1996; 51: 837–41 [DOI] [PubMed] [Google Scholar]
- 3. Colquhoun IR. CT cisternography in the investigation of cerebrospinal rhinorrhea. Clin Radiol 1993; 47: 403–08 [DOI] [PubMed] [Google Scholar]
- 4. Wakhloo AK, van Velthoven V, Shumacher M, et al. Evaluation of MR imaging, digital subtraction cisternography, and CT cisternography in diagnosing CSF fistula. Acta Neurochir (Wien) 1991; 111: 119–27 [DOI] [PubMed] [Google Scholar]
- 5. Manelfe C, Cellerier P, Sobel D, et al. Cerebrospinal fluid rhinorrhea: evaluation with metrizamide cisternography. AJR Am J Roentgenol 1982; 138: 471–76 [DOI] [PubMed] [Google Scholar]
- 6. Nickaus P, Dutcher PO, Kido DK, et al. New imaging techniques in the diagnosis of cerebrospinal fluid fistula. Laryngoscope 1998; 98: 1065–68 [DOI] [PubMed] [Google Scholar]
- 7. Ozgen T, Tekkok IH, Cila A, et al. CT cisternography in evaluation of cerebrospinal fluid rhinorrhea. Neuroradiology 1990; 32: 481–84 [DOI] [PubMed] [Google Scholar]
- 8. Lloyd MNH, Kimber PM, Burrows EH. Posttraumatic cerebrospinal fluid rhinorrhea: modern high-resolution computed tomography is all that is required for effective demonstration of the site of leakage. Clin Radiol 1994; 49: 100–03 [DOI] [PubMed] [Google Scholar]
- 9. Ahmadi J, Weiss MH, Segall HD, et al. Evaluation of cerebrospinal fluid rhinorrhea by metrizamide computed tomography. Neurosurgery 1985; 16: 54–59 [PubMed] [Google Scholar]
- 10. Drayer BP, Wilkins RH, Boehnke M, et al. Cerebrospinal fluid rhinorrhea demonstrated by metrizamide CT cisternography. AJR Am J Roentgenol 1977; 129: 149–51 [DOI] [PubMed] [Google Scholar]
- 11. Curnes JT, Vicent LM, Kowalsky RJ, et al. CSF rhinorrhea: detection and localization using overpressure cisternography with TC-99m-DTPA. Radiology 1985; 154: 795–99 [DOI] [PubMed] [Google Scholar]
- 12. Stone JA, Castillo M, Neelon B, et al. Evaluation of CSF leaks: high-resolution CT compared with contrast-enhanced CT and radionuclide cisternography. AJNR Am J Neuroradiol 1999; 20: 706–12 [PMC free article] [PubMed] [Google Scholar]
- 13. Murata Y, Yamada I, Suzuki S, et al. MRI in spontaneous cerebrospinal fluid rhinorrhea. Neuroradiology 1995; 37: 453–55 [DOI] [PubMed] [Google Scholar]
- 14. Eljamel MS, Pidgeon CN, Toland J, et al. MRI cisternography and the localization of CSF fistula. Br J Neurosurg 1994; 8: 433–37 [DOI] [PubMed] [Google Scholar]
- 15. Eberhardt KE, Hollenbach HP, Deimling M, et al. MR cisternography: a new method for the diagnosis of CSF fistulae. Eur Radiol 1997; 7: 1485–91 [DOI] [PubMed] [Google Scholar]
- 16. El Gammal T, Brooks BS. MR cisternography: initial experience in 41 cases. AJNR Am J Neuroradiol 1994; 15: 1647–56 [PMC free article] [PubMed] [Google Scholar]
- 17. Zlab MK, Moore GF, Daly DT, et al. Cerebrospinal fluid rhinorrhea: a review of the literature. Ear Nose Throat J 1992; 71: 314–17 [PubMed] [Google Scholar]
- 18. Gupta V, Goyal M, Mishra N, et al. MR evaluation of CSF fistulae. Acta Radiol 1997; 38: 603–09 [DOI] [PubMed] [Google Scholar]
- 19. Sand T, Myhr G, Stovner LJ, et al. Side effects after lumbar iohexol myelography: relation to radiological diagnosis, sex and age. Neuroradiology 1990; 31: 523–28 [DOI] [PubMed] [Google Scholar]
- 20. Van de Kelft E, Bosmans J, Paziel P, et al. Intracerebral hemorrhage after lumbar myelography with iohexol: report of a case and review of the literature. Neurosurgery 1991; 28: 570–74 [PubMed] [Google Scholar]
- 21. Shetty PG, Shroff MM, Sahani DV, et al. Evaluation of high-resolution CT and MR cisternography in the diagnosis of cerebrospinal fluid fistula. AJNR Am J Neuroradiol 1998; 19: 633–39 [PMC free article] [PubMed] [Google Scholar]
- 22. Wenzel R, Leppien A. Gadolinium-myelocisternography for cerebrospinal fluid rhinorrhoea. Neuroradiology 2000; 42: 874–80 [DOI] [PubMed] [Google Scholar]
- 23. Hegarty SE, Millar JS. MRI in the localization of CSF fistulae: is it of any value? Clin Radiol 1997; 52: 768–70 [DOI] [PubMed] [Google Scholar]
- 24. El Gammal T, Sobol W, Wadlington VR, et al. Cerebrospinal fluid fistula: detection with MR cisternography. AJNR Am J Neuroradiol 1998; 19: 627–31 [PMC free article] [PubMed] [Google Scholar]
- 25. Chow JM, Goodman D, Mafee F. Evaluation of CSF rhinorrhea by computerized tomography with metrizamide. Otolaryngol Head Neck Surg 1989; 100: 99–105 [DOI] [PubMed] [Google Scholar]
- 26. The National Academies Press. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2. 2005Available at: http://books.nap.edu/catalog/11340.html. Accessed February 1, 2006 [PubMed]
- 27. Albayram S, Kilic F, Ozer H, et al. Gadolinium-enhanced MR cisternography to evaluate dural leaks in intracranial hypotension syndrome. AJNR Am J Neuroradiol 2008; 29: 116–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niendorf HP, Haustein J, Cornelius I, et al. Safety of gadolinium-DTPA: extended clinical experience. Mag Reson Med 1991; 22: 222–28 [DOI] [PubMed] [Google Scholar]
- 29. Niendorf HP, Haustein J, Louton T, et al. Safety and tolerance after intravenous administration of 0.3 mmol/kg Gd-DTPA: results of a randomized, controlled clinical trial. Invest Radiol 1991; 26: 221–23 [DOI] [PubMed] [Google Scholar]
- 30. Goldstein HA, Kashanian FK, Blumetti RF, et al. Safety assessment of gadopentetate dimeglumine in US clinical trials. Radiology 1990; 174: 17–23 [DOI] [PubMed] [Google Scholar]
- 31. Tardy B, Guy C, Barral G, et al. Anaphylactic shock induced by intravenous gadopentetate dimeglumine. Lancet 1992; 339: 494. [DOI] [PubMed] [Google Scholar]
- 32. Perez-Rodriguez J, Lai S, Ehst BD, et al. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment: report of 33 cases. Radiology 2009; 250: 371–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Chiro G, Knop RH, Girton MR, et al. MR cisternography and myelography with GD-GTPA in monkeys. Radiology 1985; 157: 373–77 [DOI] [PubMed] [Google Scholar]
- 34. Di Chiro G, Girton ME, Frank JA, et al. Cerebrospinal fluid rhinorrhea: depiction with MR cisternography in dogs. Radiology 1986; 160: 221–22 [DOI] [PubMed] [Google Scholar]
- 35. Jinkins JR, Williams RF, Xiong L. Evaluation of gadopentetate dimeglumine magnetic resonance cisternography in an animal model. Invest Radiol 1999; 34: 156–59 [DOI] [PubMed] [Google Scholar]
- 36. Ibarra R, Jinkins JR, Korvick D, et al. Evaluation of intrathecal gadolinium-enhanced MR cisternography in a rabbit model of traumatic nasoethmoidal CSF fistula. J Magn Reson Imaging 2000; 11: 20–24 [DOI] [PubMed] [Google Scholar]
- 37. Ray DE, Cavanagh JB, Nolan CC, et al. Neurotoxic effects of gadopentetate dimeglumine: behavioral disturbance and morphology after intracerebroventricular injection in rats. AJNR Am J Neuroradiol 1996; 17: 365–73 [PMC free article] [PubMed] [Google Scholar]
- 38. Ray DE, Holton JL, Nolan CC, et al. Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. AJNR Am J Neuroradiol 1998; 19: 1455–62 [PMC free article] [PubMed] [Google Scholar]
- 39. Arlt S, Cepek L, Rustenbeck HH, et al. Gadolinium encephalopathy due to accidental intrathecal administration of gadopentetate dimeglumine. J Neurol 2007; 254: 810–12 [DOI] [PubMed] [Google Scholar]
- 40. Li L, Gao FQ, Zhang B, et al. Overdosage of intrathecal gadolinium and neurological response. Clin Radiol 2008; 63: 1063–68 [DOI] [PubMed] [Google Scholar]
- 41. Zeng QY, Xiong L, Jinkins JR, et al. Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced MR myelography: a pilot study in human patients. AJR Am J Radiol 1999; 173: 1109–15 [DOI] [PubMed] [Google Scholar]
- 42. Jinkins JR, Rudwan M, Krumina G, et al. Intrathecal gadolinium-enhanced MR cisternography in the evaluation of clinically suspected cerebrospinal fluid rhinorrhea in humans: early experience. Radiology 2002; 222: 555–59 [DOI] [PubMed] [Google Scholar]
- 43. Tali ET, Ercan N, Krumina G, et al. Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol 2002; 7: 152–59 [DOI] [PubMed] [Google Scholar]
- 44. Reiche W, Komenda Y, Schick B, et al. MR cisternography after intrathecal Gd-DTPA application. Eur Radiol 2002; 12: 2943–49 [DOI] [PubMed] [Google Scholar]
- 45. Aydin K, Guven K, Sencer S, et al. MRI cisternography with gadolinium-containing contrast medium: its role, advantages and limitations in the investigation of rhinorrhoea. Neuroradiology 2004; 46: 75–80 [DOI] [PubMed] [Google Scholar]
- 46. Muñoz A, Hinojosa J, Esparza J. Cisternography and ventriculography gadopentate dimeglumine-enhanced MR imaging in pediatric patients: preliminary report. AJNR Am J Neuroradiol 2007; 28: 889–94 [PMC free article] [PubMed] [Google Scholar]
- 47. Aydin K, Terzibasioglu E, Sencer S, et al. Localization of cerebrospinal fluid leaks by gadolinium-enhanced magnetic resonance cisternography: a 5-year single-center experience. Neurosurgery 2008; 62: 584–89 [DOI] [PubMed] [Google Scholar]
- 48. Woodcock RJ, Marx WF, Johnson RM, et al. Needle diameter in outpatient myelography: rates of adverse effects and current practice trends. Neuroradiol 2000; 42: 371–74 [DOI] [PubMed] [Google Scholar]