SUMMARY:
We describe a rare case of SFT existing along the mandibular division of the trigeminal nerve and extending down into the infratemporal fossa through the foramen ovale. The tumor showed heterogeneous hypointensity on T2-weighted images and marked enhancement on CT and MR images.
SFT is an uncommon neoplasm and has been reported with increasing frequency in various anatomic sites, including the central nervous system.1–7 We present an SFT arising along the fifth cranial nerve.
Case Report
The patient was a 49-year-old woman with a several-month history of left-sided mandibular hypoesthesia and trismus. Contrast-enhanced CT demonstrated a large dumbbell-shaped mass extending from the left middle temporal fossa into the infratemporal fossa via an enlarged foramen ovale. The tumor had a well-defined margin and showed heterogeneous enhancement. The mass pushed against the lateral wall of the left maxillary sinus and the pterygoid process without destruction (Fig 1). MR imaging was performed to provide delineation of the location and size of the tumor and the relationship to the surrounding structures. It revealed a solid tumor, 55 × 32 × 84 mm, arising from the middle temporal fossa and extending into the infratemporal fossa. The temporal lobe was pushed aside by intracranial extension and showed hyperintensities on T2-weighted images. The tumor was isointense on T1-weighted imaging with hypointense foci, suggesting flow voids. On T2-weighted images, the tumor showed heterogeneous hypointensity. Most of the tumor showed isointense on T1-weighted imaging with hypointense foci, suggesting flow voids, and heterogeneous hypointensity on T2-weighted imaging. In contrast, the signal intensities of the lateral side of the intracranial component were higher than those of the rest of the tumor on T1- and T2-weighted images. This site showed marked enhancement and early washout on a dynamic contrast study. The rest of the tumor showed early enhancement and poor washout (Fig 2).
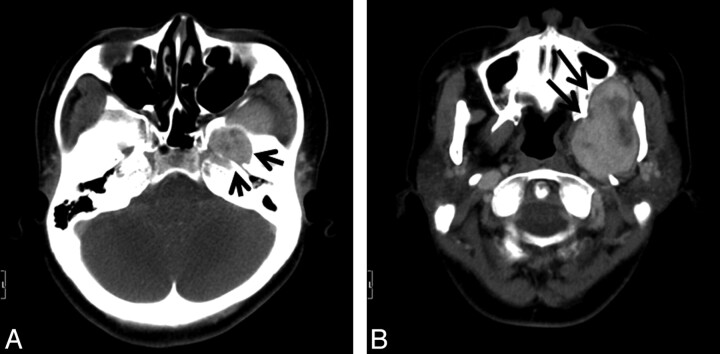
Fig 1.
A, Axial contrast-enhanced CT scan shows an enlarged left foramen ovale (arrows) by the enhanced tumor. B, Axial contrast-enhanced CT scan of the caudal side of A shows the tumor compressing the maxillary bone, left pterygoid plate, and mandible (arrows), without destruction.
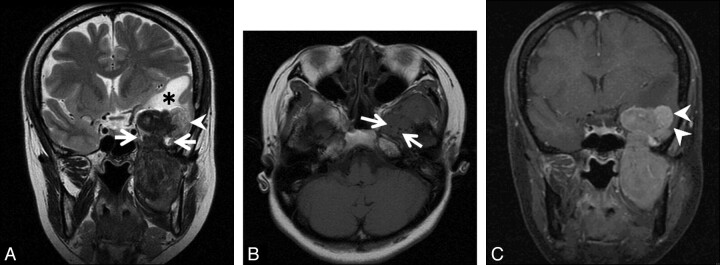
Fig 2.
A, Coronal T2-weighted image shows a dumbbell-shaped tumor passing through left foramen ovale (arrows). The tumor is mainly hypointense to white matter. The lateral part of the intracranial component (arrowhead) shows higher intensities than other parts of the tumor. White matter of the left temporal lobe shows high intensities (asterisk). B, Axial T1-weighted image shows isointensity of the tumor to the brain. The tumor has low-intensity foci, suggesting a flow-void condition (arrows). C, On a coronal contrast-enhanced fat-suppressed T1-weighted image, the tumor shows heterogeneous enhancement. The lateral part of the intracranial component shows different strong enhancement (arrowheads).
Biopsy of the tumor was performed via the left maxillary sinus. The microscopic appearance with hematoxylin-eosin staining revealed spindle cells in a characteristic “patternless pattern,” with amorphous areas of collagen and haphazardly arranged cells. Immunohistochemical testing showed the neoplastic cells to be positive for CD34 and vimentin and negative for SMA, S100, EMA, desmin, and c-kit. On the basis of these findings, a diagnosis of SFT was made. Complete surgical excision was achieved via left frontotemporal craniotomy with zygomectomy. There was no cerebral invasion or adhesion.
Discussion
In 1931, Klemperer and Rabin8 reported SFT as a localized fibrous tumor arising in the pleura. The further development of electron microscopy and immunohistochemistry clarified that this tumor was not from mesothelial but rather from mesenchymal origin, and it is now referred to as a SFT, different from a mesothelial tumor. Although initially most SFTs were reported to arise within the thoracic cavity, recently they have been more frequently reported in other sites and organs. The diagnosis of SFT rests on a combination of histopathologic and immunohistochemical features. Because SFT shows such a variety of patterns on radiologic imaging, it would be difficult to make a correct presurgical diagnosis.9 Most SFTs present as well-circumscribed and often encapsuled lesions of 1–25 cm. On sections, they frequently show a multinodular, whitish, and firm appearance. In addition, myxoid and hemorrhagic changes are occasionally observed. Histopathologically, SFTs typically exhibit a patternless architecture characterized by the coexistence of hypo- and hypercellular areas, which are separated by fibrous stroma having hemangiopericytoma-like branching blood vessels. Immunohistochemically, SFTs tend to be positive for CD34, CD99, and BCL2. They are usually negative for S100 protein, cytokeratin, and/or desmin.9
On CT scans, SFTs usually appear as well-circumscribed, round, or lobulated masses with attenuation of soft tissue.9,10 On T1-weighted MR images, they are usually isointense to the muscle.9 On T2-weighted images, SFTs are mainly hypointense, corresponding to fibrous stroma intermixed with prominent vascular channels.2,9,10 In our case, SFT had rich collagenous stroma with hyalinization. After intravenous contrast administration, the tumor usually shows homogeneous enhancement but also sometimes heterogeneous enhancement, corresponding to the cellularity, stromal components, and degeneration. The enhancement can be from moderate to strong, presumably depending on the cellularity, stromal component, and vascularity. In a tumor with strong enhancement, the vascular bed may be seen as serpiginous signal-intensity voids on MR imaging.10–12
The typical presentation of intracranial SFT strongly mimics that of a dural-based meningioma, on the basis of the likely mesenchymal origin of SFT. When located in the spine or in close relationship with the central nervous system, it may be difficult to distinguish from a schwannoma.3 Meningioma usually appears isointense relative to brain parenchyma on both T1- and T2-weighted images, and schwannoma usually appears hyperintense on T2-weighted images. On T2-weighted and postcontrast T1-weighted images, the intracranial component of jugular foramen meningioma has higher intensities and stronger enhancement than its extracranial component.13 These findings represent a clue to the possibility of a jugular foraminal tumor being a meningioma.13 In our case of SFT, different signal intensities and contrast enhancements were noted between intra- and extracranial components. Although SFT could show variable intensities on T2-weighted images, many SFTs are reported as mainly hypointense on T2-weighted images.
Conclusions
This is the first report of an intra- and extracranial SFT in the foramen ovale arising from a branch of the trigeminal nerve. SFT should be considered in the differential diagnosis of hypervascular and hypointense soft-tissue tumors on T2-weighted images.
Abbreviations
- EMA
epithelial membrane antigen
- SFT
solitary fibrous tumor
- SMA
smooth muscle antigen.
References
- 1. Carneiro SS, Scheithauer BW, Nascimento AG, et al. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma—a clinicopathologic and immunohistochemical study. Am J Clin Pathol 1996;106:217–24 [DOI] [PubMed] [Google Scholar]
- 2. Bohinski RJ, Mendel E, Aldape KD, et al. Intramedullary and extramedullary solitary fibrous tumor of the cervical spine: case report and review of the literature. J Neurosurg 2004;100:358–63 [DOI] [PubMed] [Google Scholar]
- 3. Endo K, Komagata M, Ikegami H, et al. Dumbbell-type solitary fibrous tumor in the cervical spine. J Orthop Sci 2003;8:428–31 [DOI] [PubMed] [Google Scholar]
- 4. Kocak A, Cayli SR, Sarac K, et al. Intraventricular solitary fibrous tumor: an unusual tumor with radiological, ultrastructural, and immunohistochemical evaluation—case report. Neurosurgery 2004;54:213–17 [DOI] [PubMed] [Google Scholar]
- 5. Waldron JS, Tihan T, Parsa AT. Solitary fibrous tumor arising from cranial nerve VI in the prepontine cistern: case report and review of a tumor subpopulation mimicking schwannoma. Neurosurgery 2006;59:E939–40 [DOI] [PubMed] [Google Scholar]
- 6. Badion ML, Lim CC, Teo J, et al. Solitary fibrous tumor of the hypoglossal nerve. AJNR Am J Neuroradiol; 24:343–45 [PMC free article] [PubMed] [Google Scholar]
- 7. Kim KA, Gonzalez I, McComb JG, et al. Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 2004;54:1004–09 [DOI] [PubMed] [Google Scholar]
- 8. Klemperer P, Rabin CG. Primary neoplasms of the pleura. Arch Pathol 1931;11:385–412 [Google Scholar]
- 9. Ganly I, Petel SG, Stambuk HE, et al. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg 2006;132:517–25 [DOI] [PubMed] [Google Scholar]
- 10. Kim TA, Brunberg JA, Pearson JP, et al. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol 1996;17:1767–72 [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong AK, Lee HK, Kim SY, et al. Solitary fibrous tumor of the parapharyngeal space: MR imaging findings. AJNR Am J Neuroradiol 2002;23:473–75 [PMC free article] [PubMed] [Google Scholar]
- 12. Cardillo G, Facciolo F, Cavazzana AO, et al. Localized (solitary) fibrous tumors of the pleura: an analysis of 55 patients. Ann Thorac Surg 2000;70:1808–12 [DOI] [PubMed] [Google Scholar]
- 13. Shimono T, Akai F, Yamamoto A, et al. Different signal intensities between intra- and extracranial components in jugular foramen meningioma: an enigma. AJNR Am J Neuroradiol 2005;26:1122–27 [PMC free article] [PubMed] [Google Scholar]