Abstract
BACKGROUND AND PURPOSE:
The combination of nonspecific clinical findings and similarities in morphologic appearances on imaging often makes it difficult to distinguish abscesses from other brain lesions. We present a retrospective analysis of in vivo 1H-MR spectroscopy data for characterization of the etiology of the brain abscess based on the established criteria and demonstrate the sensitivity and specificity of metabolite markers assigned to specific bacterial groups defined by the microbial culture in 194 patients.
MATERIALS AND METHODS:
Conventional MR imaging and in vivo 1H-MR spectroscopy data were evaluated from patients with pyogenic brain abscesses, with ages ranging from 3 to 60 years. Imaging and 1H-MR spectroscopy were performed on a 1.5T scanner. After MR imaging was performed and analyzed, pus aspirates were obtained in all patients. The causative organisms were confirmed by pus cultures.
RESULTS:
Resonance of AAs with or without other metabolites on in vivo 1H-MR spectroscopy was observed in 80% of abscesses, with a sensitivity and specificity of 0.72 and 0.30, respectively. Most obligate anaerobes and some facultative anaerobes showed the presence of Lac/Lip, AAs, and Ac with or without Suc. Mostly obligate aerobes or facultative anaerobes showed the presence of Lac and AAs, with or without lipids.
CONCLUSIONS:
The presence of AAs on in vivo 1H-MR spectroscopy is a sensitive marker of pyogenic abscess, but its absence does not rule out a pyogenic etiology. The presence of Ac with or without Suc favors an anaerobic bacterial origin of the abscess; however, this may also be seen in some of the abscesses secondary to facultative anaerobes.
Brain abscess is a focal intracerebral infection, which begins as a localized area of cerebritis and develops into a collection of pus surrounded by a well-vascularized capsule. It is caused by different types of pathogens, but the main organisms that cause brain abscess are of bacterial origin. The bacterial flora involved in this abscess consist of aerobes and anaerobes.1 Infection occurs either by hematogenous spread from a reservoir outside the central nervous system or by direct invasion from a contiguous site of infection in the paranasal sinuses, mastoid, or middle ear.2
The combination of nonspecific clinical findings and similarities in morphologic appearances on imaging often makes it difficult to distinguish abscesses from other brain lesions. Conventional MR imaging also shows similar features in the pyogenic, tubercular, and fungal abscesses and lacks specificity with respect to the identification of the offending microorganisms.3–5 It is important to make a rapid diagnosis of brain abscess to provide appropriate treatment. In vivo 1H-MR spectroscopy, DWI, and DTI are currently being used routinely to assist in the differential diagnosis of focal and diffuse intracranial pathologies.6,7 Bacterial abscesses are completely necrotic lesions, and normal brain metabolites such as N-acetylaspartate, a neuronal and axonal marker; choline, a constituent metabolite of cell membrane and myelin; and creatine, a marker of energy metabolism, are absent. The presence of AAs and Lip/Lac, along with Ac and/or Suc, is considered specific for a brain abscess on in vivo 1H-MR spectroscopy.6,7 On the basis of the presence of these metabolites, an etiologic characterization of the brain abscess has been proposed.8 Combined use of DWI/DTI along with in vivo 1H-MR spectroscopy has been shown to improve the specificity of diagnosis in focal ring-enhancing brain lesions.9–11
We present a retrospective analysis of in vivo 1H-MR spectroscopy data for characterization of the etiology of the brain abscess based on the criteria mentioned in the literature and demonstrate the sensitivity and specificity of metabolite markers assigned to specific bacterial groups defined by the microbial culture in these patients.
Materials and Methods
Subjects
We performed a retrospective study of patients with brain abscesses referred during a span of 7 years. These patients presented to us with various clinical features such as fever, headache, and/or mass lesion. In vivo 1H-MR spectroscopy was performed in a total of 210 patients with brain abscesses; however, only imaging of 194 patients with good spectral quality were included in current study. The remaining 16 patients were excluded due to the noninterpretable quality of spectra, either due to poor shim or motion artifacts. The final population (n = 194) consisted of 140 males and 54 females, with ages ranging from 3 to 60 years. After clinical evaluation, all patients underwent conventional MR imaging and in vivo 1H-MR spectroscopy, followed by aspiration of the abscess cavity. Before imaging, informed consent was obtained from each subject or nearest kin. The study was approved by the institutional ethics committee.
MR Imaging Protocol
Imaging was performed on a 1.5T MR imaging scanner (Signa LX EchoSpeed Plus; GE Healthcare, Milwaukee, Wisconsin) by using a standard quadrature birdcage transmit and receive radio-frequency head coil. The routine imaging studies included fast SE T2WI (TR/TE/NEX = 4900 ms/85 ms/3) and SE T1WI (TR/TE/NEX = 650 ms/14 ms/2) sequences with a matrix size of 256 × 256, FOV of 24 × 24 cm, and no intersection gap. We used T1WI or T2WI for voxel localization. Postcontrast T1WIs were acquired after intravenous administration of gadolinium-diethylene-triamine pentaacetic acid-bismethylamide (Omniscan; Amersham Health, Oslo, Norway) at a dose of 0.1 mmol/kg of body weight.
Qualitative Analysis
The size, location, and multiplicity of the abscesses were recorded. When >1 lesion, either in the same section or in some other location, was observed in the brain parenchyma, the patients were considered to have multiple abscesses. In case of multiple abscesses, we selected the lesion with largest dimension on T2WI for spectroscopy. The morphologic features of the lesions were analyzed on conventional MR imaging.
In Vivo 1H-MR Spectroscopy
In vivo 1H-MR spectroscopy was performed in all patients by using a water-suppressed localized single-voxel SE sequence with TR/TE/NEX = 3000 ms/144 ms/8 and a voxel size of 2–3 mL, depending on the size of the lesion. We ensured voxel placement within the cavity of the lesion to avoid contamination from the surrounding brain parenchyma and the wall of the cavity (Fig 1A). After global shimming, we performed voxel shimming and achieved a full width at half maximum of 4–6 Hz in all cases. Only the long-TE technique was used because it has been observed that the metabolites in brain abscesses have intermediate-to-long T2 values and are well assigned at a TE of 144 ms.12 Resonances of AAs (ie, valine, isoleucine, and leucine), Lac, and alanine showed phase reversal at intermediate TEs, whereas the Lip resonances remained unchanged. The spectra with the partial phase reversal at 1.33 ppm were assigned to the presence of both Lip and Lac.
Fig 1.
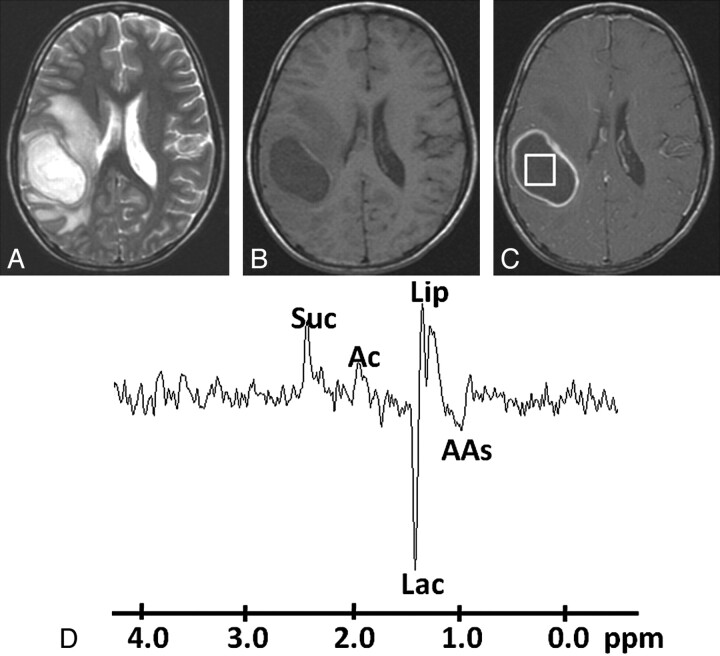
Pyogenic abscess in the right parieto-occipital region of a 34-year-old man. A, Axial T2WI shows a well-defined hyperintense lesion with a hypointense wall and perifocal edema. B, The lesion appears hypointense on the axial T1WI with an isointense wall. C, Postcontrast T1WI shows ring enhancement. D, In vivo 1H-MR spectroscopy by using SE sequence (TR/TE/NEX = 3000 ms/144 ms/128) from the center of the lesion shows resonances of AAs, 0.9 ppm; Lip/Lac, 1.3 ppm; Ac, 1.9 ppm; and Suc, 2.4 ppm. Culture from pus grew B fragilis.
Evaluation of 1H-MR Spectroscopic Data
Two experienced neuroradiologists with >20 years' experience independently reviewed the MR imaging and spectroscopic data on all patients to determine the topographic location, morphology, and metabolites of abscesses. There was no variability in the interpretation of the data between neuroradiologists. For evaluation of all individual spectra, a standard Java-based version of an MR imaging user-interface signal-intensity processing software package (Version 6.0; jMRUI Europe project, Leuven, Belgium) was used for 1H-MR spectroscopy data. For spectral analysis, various spectral peaks of different metabolites in 1H-MR spectroscopy were identified.
The presence of cytosolic AAs, such as leucine, isoleucine, valine (0.9 ppm) with or without Lac (1.3 ppm), Ac (1.92 ppm), Suc (2.4 ppm), and Lip (1.3 ppm) peaks, is used as a signature for brain abscess.7,8 When AAs at 0.9 ppm were detected on spectroscopy, it was labeled as a pyogenic abscess.7 Similarly, the abscess was considered to be of anaerobic origin when cytosolic AAs along with Ac and/or Suc were identified on in vivo 1H-MR spectroscopy.8,11 All the resonances were identified with reference to Lac at 1.33 ppm based on literature.12,13 The study in which we could not demonstrate the Lac water peak was taken as a reference at 4.77 ppm.
Culture of Pus Samples
Aspirates from abscesses were obtained in all the patients and immediately applied to BACTEC Plus Aerobic/Anaerobic culture media (Becton Dickinson, Sparks, Maryland) and incubated at 37°C for 5 days and then subcultured on appropriate solid media to isolate the aerobic, anaerobic, and facultative anaerobic bacteria. All the isolates were identified by a standard biochemical test as described elsewhere.14
Statistical Methods
The patients were classified into a 2 × 2 contingency table by using 1H-MR spectroscopy and culture results. Culture was considered the criterion standard, and 1H-MR spectroscopy was evaluated against the culture results. Statistical analysis was performed to test the sensitivity, specificity, and positive and negative predictive values of the 1H-MR spectroscopy method to diagnose pyogenic brain abscesses. Sensitivity, specificity, and positive and negative predictive values of in vivo 1H-MR spectroscopy were calculated by using the following expressions:
where a is the number of patients who were both culture- and 1H-MR spectroscopy–positive, b is the number of patients who were only 1H-MR spectroscopy–positive, c is the number of patients who were only culture–positive, and d is the number of patients who were both culture- and 1H-MR spectroscopy–negative.
Results
MR Imaging
Pyogenic abscesses were located in the parietal lobe in 8 (4%), the frontal lobe in 39 (20%), the temporal lobe in 58 (30%), the occipital lobe in 10 (5%), the frontoparietal lobe in 8 (4%), the parieto-occipital lobe in 9 (5%), and the cerebellum in 31 (16%) patients. However, multiple abscesses were seen in 31 (16%) of the 194 patients. All the lesions appeared hyperintense on T2WI and hypointense on T1WI, with variable perifocal edema, and showed ring enhancement on postcontrast T1WI.
In Vivo 1H-MR Spectroscopy
In vivo 1H-MR spectroscopy characteristics of all patients are summarized in Table 1. Resonance of AAs with or without other metabolites was observed in 155/194 (80%) abscesses on in vivo 1H-MR spectroscopy. On the basis of the presence of AAs, we observed the following sensitivity, specificity, and positive and negative predictive values of in vivo 1H-MR spectroscopy for the diagnosis of pyogenic abscesses: 0.72, 0.30, 0.80, and 0.20, respectively. AAs could not be detected in the remainder of the 39/194 cases (20%). Of the cultures of these 39 patients, 7(17.8%) were anaerobes, 3(7.8%) were aerobes, 18 (46.2%) were facultative anaerobes (Fig 2), and 11 (28.2%) were sterile (Fig 3). Staphylococcus aureus (14/18) was the most common facultative anaerobe that did not show the AA resonances. Acetate with or without Suc was observed in 46 (24%) of 194 cases. Of these 46 patients, 32 cultures (69.5%) were anaerobic: Bacteroides fragilis (n = 10) and Streptococci organisms (n = 22); 11 (24%) were facultative anaerobe: S aureus (n = 8) and Enterococcus species (n = 3); and 3 (6.5%) were sterile. Among 54 anaerobic culture–positive patients, we found that only 32 (59%) showed a peak of Ac with or without Suc (Fig 1). Based on the presence of these metabolites, the sensitivity, specificity, and positive and negative predictive values of in vivo 1H-MR spectroscopy were 0.70, 0.85, 0.60 and 0.90, respectively, for the diagnosis of anaerobic infection. We also found that Suc was always present in conjunction with Ac in these patients.
Table 1:
Summary of the micro-organisms isolated from the culture along with metabolites visible on in-vivo 1H-MR spectroscopy
| Metabolites | Sterile (n = 54) | Aerobic (n = 17) | Anaerobic (n = 54) | Facultative Anaerobic (n = 69) |
|---|---|---|---|---|
| Lac | 9 | 4 | 2 | 6 |
| Lip | 2 | 3 | 1 | 12 |
| AAs + Lac/Lip | 40 | 10 | 19 | 40 |
| Ac + AAs + Lac/Lip | 1 | 0 | 4 | 5 |
| Suc + Ac + AAs + Lac/Lip | 2 | 0 | 28 | 6 |
Fig 2.
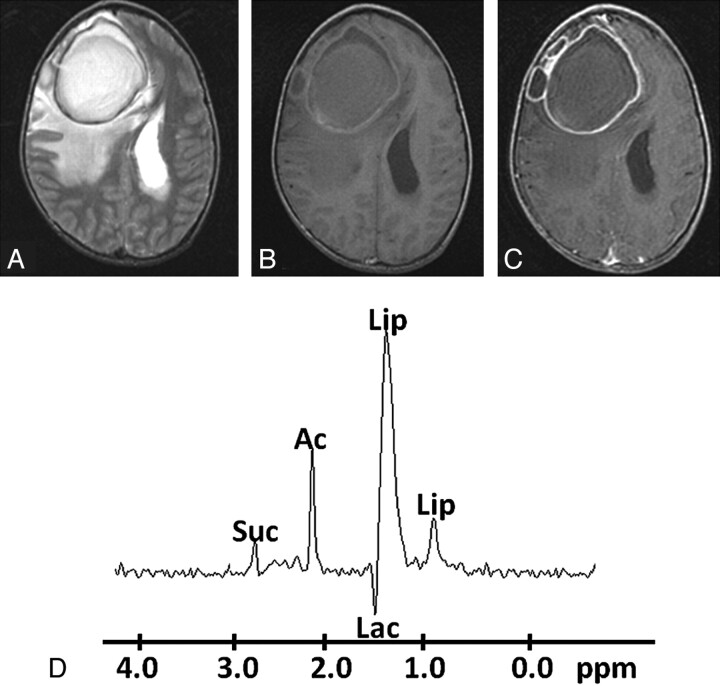
Pyogenic abscess in the right frontal lobe of a 32-year-old man. A, Axial T2WI image shows a large well-defined hyperintense core with a peripheral hypointense rim, perifocal edema, and mass effect. B, The lesion shows mixed intensity on T1WI with a slightly hyperintense wall. C, Axial postcontrast T1WI shows ring enhancement. D, In vivo 1H-MR spectroscopy by using SE sequence (TR/TE/NEX= 3000 ms/144 ms/128) from the center of the lesion shows Lip/Lac, 1.3 ppm; Ac, 1.9 ppm; and Suc, 2.4 ppm. Culture from pus grew E faecalis.
Fig 3.
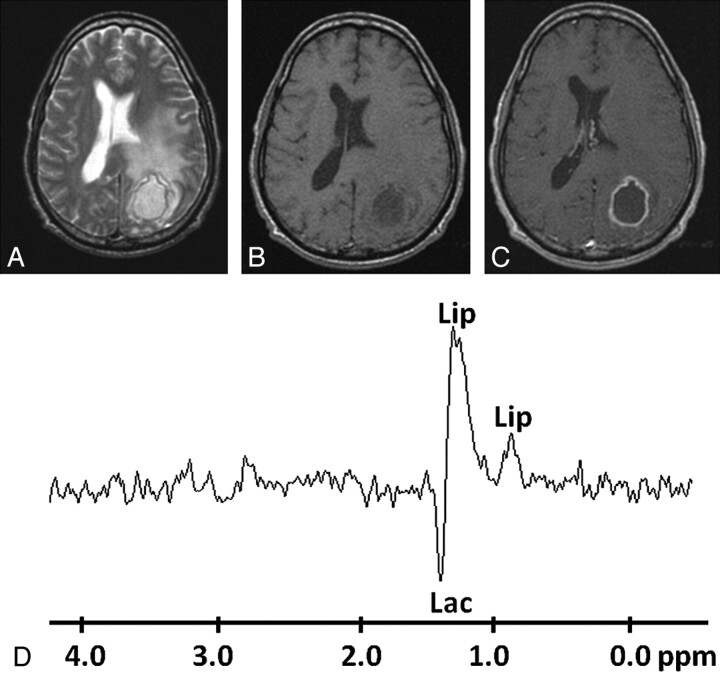
Pyogenic abscess in the left occipital lobe of a 28-year-old man. A, Axial T2WI shows a well-defined hyperintense lesion with a hypointense wall and perifocal edema. B and C, The lesion appears hypointense on T1WI (B) with an isointense wall showing ring enhancement on the postcontrast T1WI (C). D, In vivo 1H-MR spectroscopy by using SE sequence (TR/TE/NEX = 3000 ms/144 ms/128) from the center of the lesion shows only Lip/Lac peaks, 1.3 ppm. Culture from pus showed no bacterial growth.
Culture
The bacteriologic data obtained from the pus cultures are summarized in Table 2. On the basis of the results of bacterial culture, abscesses were grouped as sterile (no bacterial growth), anaerobic, aerobic, and facultative anaerobic. There were 54 sterile (27.8%), 54 anaerobic (27.8%), 17 aerobic (8.8%), and 69 facultative anaerobic (35.6%) abscesses. Fifty of 54 patients with sterile abscesses had been on broad-spectrum antibiotics for 7–10 days before MR imaging and 1H-MR spectroscopy. Of remaining 140 culture–positive patients, 59 had also been on antibiotic therapy for 3–4 days before MR imaging. Anaerobic cultures were positive for anaerobic Streptococci organisms (n = 39) and B fragilis (n = 15); aerobic cultures were positive for Nocardia species (n = 10) and Pseudomonas aeruginosa (n = 7); and facultative anaerobic cultures were positive for S aureus (n = 38), Streptococcus uberis (n = 7), Streptococcus intermedius (n = 5), Klebsiella pneumoniae (n = 1), Eikenella corrodens (n = 2), Enterococcus faecalis (n = 8), Proteus mirabilis (n = 1), and Escherichia coli (n = 7).
Table 2:
Summary of micro-organisms isolated in cultures from the patients with brain abscess
| Group | No. of Patients | Specific Isolates |
|---|---|---|
| Anaerobic | 54 | Anaerobic Streptococci organisms (39), B fragilis (15) |
| Aerobic | 17 | Nocardia species (10), P aeruginosa (7) |
| Facultative anaerobic | 69 | S aureus (38), S uberis (7), S intermedius (5), K pneumonia (1), E corrodens (2), E faecalis (8), P mirabilis (1), E coli (7) |
| Sterile | 54 |
Discussion
The presence of the resonance of cytosolic AAs is considered as a sensitive marker of pyogenic brain abscess7; however, in our study, we found that AAs were not always present in pyogenic abscesses. We found that AAs were present in 80% of the pyogenic abscesses with a sensitivity and specificity of 0.72 and 0.30 on in vivo 1H-MR spectroscopy. It is known that the abscess cavity contains a large amount of neutrophils and protein. Proteolytic enzymes are released due to the breakdown of the neutrophils. These enzymes hydrolyze the proteins into AAs, which are detected at 0.9 ppm on in vivo 1H-MR spectroscopy in pyogenic brain abscesses.15–19 However; we could not find AAs at 0.9 ppm in some of the patients having aerobes, anaerobes, facultative anaerobes, and sterile cultures. The absence of AAs in sterile abscesses was probably due to the fact that these patients were undergoing treatment with antibiotics20–24 before the in vivo 1H-MR spectroscopy, probably resulting in the lack of bacterial growth on the culture. Earlier studies have also reported the disappearance of all resonances except those of Lac within the abscess cavity on in vivo 1H-MR spectroscopy following antibiotic therapy.10,25 The absence of AAs in patients with aerobic and anaerobic culture–positive brain abscesses on in vivo 1H-MR spectroscopy could be explained by the lower inflammatory cell count in the pus of these patients, resulting in a low concentration of metabolites, which was below the sensitivity of the 1.5T MR imaging.26 On the basis of these data, it appears that the presence of AAs is a sensitive marker of pyogenic brain abscess; however, its absence does not rule it out.
The selective presence of Ac with or without Suc resonances on in vivo 1H-MR spectroscopy of abscesses has been described as a signature for anaerobic infection.8 We observed these resonances in 59% of patients who showed anaerobic growth on culture. These metabolite resonances are probably the result of enhanced glycolysis and formation of the pyruvate, which follows the fermentative route in anaerobes, where it may go through carboxylation to form Suc via oxaloacetate, malate, and fumarate; part of the pyruvate also forms Ac.8 Garg et al8 in an in vivo and in vitro study of 75 patients with brain abscesses also demonstrated that Ac or Suc or both were present in anaerobic infections. Suc has also been described as a marker for anaerobic infections in clinical pus samples on gas-liquid chromatography.27 Nonvisibility of the resonance of Ac with or without Suc on in vivo 1H-MR spectroscopy in 41% of anaerobic culture–positive brain abscesses in our study could be explained by the relatively low concentration of these metabolites, which might be below the sensitivity of the current scanner.28
Suc and Ac are usually not observed in brain abscesses secondary to aerobic (Fig 4) and facultative anaerobic micro-organisms because the pyruvate formed during glycolysis enters the tricarboxylic acid cycle in these bacteria in the presence of oxygen.8 In accord with the literature, we did not observe these resonances in any of the aerobic brain abscesses.8 However, we found these resonances in 11 patients demonstrating facultative anaerobes (Fig 5) on culture:11 S aureus (n = 8) and E faecalis (n = 3). E faecalis organisms are usually considered strict fermenters, and they lack a Kreb cycle respiratory chain29; this may be the reason for the resonances of Ac with or without Suc on in vivo 1H-MR spectroscopy in 3 patients in this study. The presence of Suc and Ac in patients showing no bacterial growth on culture may be attributed to the antibiotics that these patients received before MR imaging and MR spectroscopy, resulting in degeneration of anaerobic bacteria and persistence of metabolites in the lesion.
Fig 4.
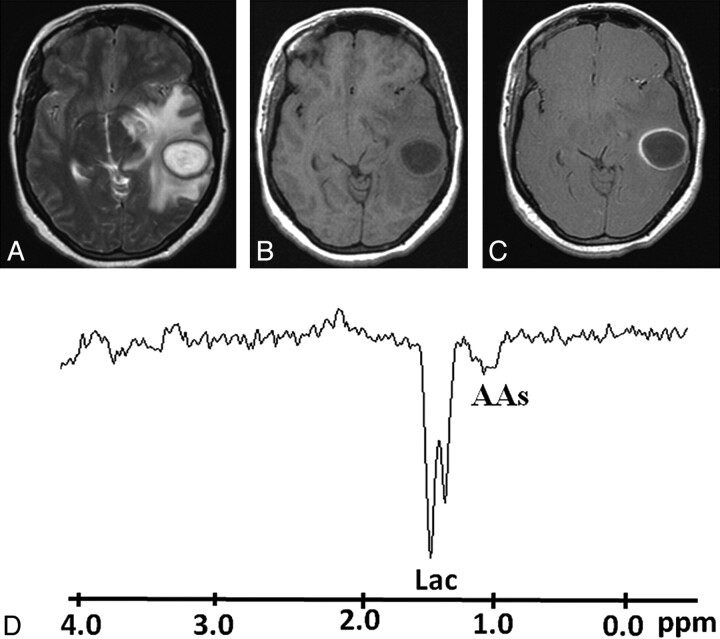
Pyogenic abscess in the left superior temporal lobe of a 30-year-old woman. A, Axial T2WI shows a well-defined hyperintense lesion with a hypointense wall and perifocal edema. B and C, The lesion appears hypointense on the corresponding T1WI (B) with an isointense wall showing ring enhancement on the postcontrast T1WI (C). D, In vivo 1H-MR spectroscopy by using SE sequence (TR/TE/NEX = 3000 ms/144 ms/128) from the center of the lesion shows AAs, 0.9 ppm; and a predominant Lac peak, 1.3 ppm. Culture from pus grew P aeruginosa.
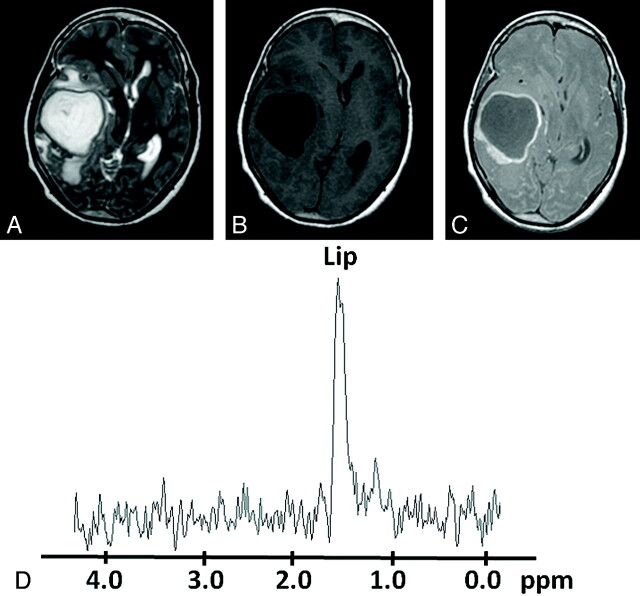
Fig 5.
Pyogenic abscess in the right temporal region of a 27-year-old woman. A, Axial T2WI shows a well-defined hyperintense lesion with a hypointense wall and perifocal edema. B and C, The lesion appears hypointense on the T1WI (B) with an isointense wall showing ring enhancement on the postcontrast T1WI (C). D, In vivo 1H-MR spectroscopy by using SE sequence (TR/TE/NEX= 3000 ms/144 ms/128) from the center of the lesion shows only a Lip peak, 1.3 ppm. Culture from pus grew S aureus.
Although most of the strains of S aureus produce proteolytic enzymes, a few strains may not produce these due to genetic variability. The absence of these enzymes may result in nonproteolysis of the proteins within the abscess cavity and consequential absence of AA resonance on in vivo 1H-MR spectroscopy as we observed in our study.30 This observation is also supported by Himmelreich et al.31,32 Occasionally, facultative anaerobes like S aureus produce Ac with or without Suc in anaerobic environments by anaerobic glycolysis.33 This could explain the presence of these resonances on in vivo 1H-MR spectroscopy in S aureus abscesses. Another probable reason could be that the patients while on treatment might have had mixed obligate and facultative anaerobic infections initially, which had shown resonances of Ac and/or Suc on in vivo 1H-MR spectroscopy and yielded only a few obligate anaerobes and thus remained undetectable on culture.
In this study, we never observed Suc without Ac in any of the patients. This finding is supported by the fact that biochemical reactions leading to Ac production are more favorable than those that lead to Suc production in anaerobes and that Suc sometimes may be produced in concentrations that are beyond the sensitivity of in vivo 1H-MR spectroscopy.8
In the initial studies on brain abscesses, 1H-MR spectroscopy with short, intermediate, and long TEs has been used for metabolite identification.15,34 Subsequently, it has been observed that the metabolites in brain abscesses have intermediate-to-long T2 values and can be well assigned at a TE of 135 ms or 144 ms.5,8 We did not have any difficulty in the assignment of metabolites at 144 ms TE.
Conclusions
In conclusion, though the presence of AAs on in vivo 1H-MR spectroscopy is a sensitive marker of pyogenic abscess, its absence does not rule out an abscess of pyogenic origin. Second, even though resonances of Ac with or without Suc on in vivo 1H-MR spectroscopy favor an abscess of anaerobic bacterial origin, these may also be seen in some abscesses due to facultative anaerobic microbes. Hence, caution should be exercised in categorizing brain abscesses on the basis of in vivo MR spectroscopy.
Abbreviations
- AAs
amino acids
- Ac
acetate
- DWI
diffusion-weighted imaging
- DTI
diffusion tensor imaging
- 1H-MR spectroscopy
proton MR spectroscopy
- Lac
lactate
- Lip
lipid
- SE
spin-echo
- Suc
succinate
- T1WI
T1-weighted imaging
- T2WI
T2-weighted imaging
Footnotes
This work was supported by grant 5/4–5/12/Neuro/2005-NCD-I from the Indian Council of Medical Research, New Delhi, India.
References
- 1. Chaudhry R, Dhawan B, Laxmi BVJ, et al. The microbial spectrum of brain abscess with special reference to anaerobic bacteria. Br J Neurosurg 1998;12:127–30 [DOI] [PubMed] [Google Scholar]
- 2. Osenbach RK, Loftus CM. Diagnosis and management of brain abscess. Neurosurg Clin North Am 1992;3:403–20 [PubMed] [Google Scholar]
- 3. Whiteman MLH, Bowen BC, Post MJ, et al. Intracranial infection. In: Atlas SW. eds. Magnetic Resonance Imaging of the Brain and Spine. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002:1099–177 [Google Scholar]
- 4. Kastrup O, Wanke I, Maschke M. Neuroimaging of infections. NeuroRx 2005;2:324–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta RK, Vatsal DK, Husain N, et al. Differentiation of tuberculous from pyogenic brain abscesses with in vivo proton MR spectroscopy and magnetization transfer MR Imaging. AJNR Am J Neuroradiol 2001;22:1503–09 [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta RK, Nath K, Prasad A, et al. In vivo demonstration of neuroinflammatory molecule expression in brain abscess with diffusion tensor imaging. AJNR Am J Neuroradiol 2008;29:326–32. Epub 2007 Nov 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mishra AM, Gupta RK, Jaggi RS, et al. Role of diffusion-weighted imaging and in vivo proton magnetic resonance spectroscopy in the differential diagnosis of ring-enhancing intracranial cystic mass lesions. J Comput Assist Tomog 2004;28:540–47 [DOI] [PubMed] [Google Scholar]
- 8. Garg M, Gupta RK, Husain M, et al. Brain abscesses: etiologic categorization with in vivo proton MR spectroscopy. Radiology 2004;230:519–27 [DOI] [PubMed] [Google Scholar]
- 9. Shukla DA, Gupta RK, Roy R, et al. Prospective evaluation of in vivo proton MR spectroscopy in differentiation of similar-appearing intracranial cystic lesions. Magn Reson Imaging 2001;19:103–10 [DOI] [PubMed] [Google Scholar]
- 10. Lai PH, Ho JT, Chen WL, et al. Brain abscess and necrotic brain tumors: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol 2002;23:1369–77 [PMC free article] [PubMed] [Google Scholar]
- 11. Rowley HA, Grant PE, Roberts TP. Diffusion MR imaging: theory and applications. Neuroimaging Clin N Am 1999;9:343–61 [PubMed] [Google Scholar]
- 12. Luthra G, Parihar A, Nath K, et al. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol 2007;28:1332–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sweatman BC, Farrant RD, Holmes E, et al. 600 MHz 1H-NMR spectroscopy of human cerebrospinal fluid: effects of sample 20 manipulation and assignment of resonances. J Pharm Biomed Anal 1993;11:651–64 [DOI] [PubMed] [Google Scholar]
- 14. Prasad KN, Mishra AM, Gupta RK, et al. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect 2006;53:221–27 [DOI] [PubMed] [Google Scholar]
- 15. Poptani H, Gupta RK, Jain VK, et al. Cystic intracranial mass lesions: possible role of in vivo MR spectroscopy in its differential diagnosis. Magn Reson Imaging 1995;13:1019–29 [DOI] [PubMed] [Google Scholar]
- 16. Grand S, Lai ES, Esteve F, et al. In vivo 1H MRS of brain abscesses versus necrotic brain tumors. Neurology 1996;47:846–48 [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Chang KH, Song IC, et al. Brain abscess and brain tumor: discrimination with in vivo H-1 MR spectroscopy. Radiology 1997;204:239–45 [DOI] [PubMed] [Google Scholar]
- 18. Martinez PI, Moreno A, Alonso J, et al. Diagnosis of brain abscess by magnetic resonance spectroscopy. J Neurosurg 1997;86:708–13 [DOI] [PubMed] [Google Scholar]
- 19. Habib AA, Mozaffar T. Brain abscess. Arch Neurol 2001;58:1302–04 [DOI] [PubMed] [Google Scholar]
- 20. Harada M, Tanouchi M, Miyoshi H, et al. Brain abscess observed by localized proton magnetic resonance spectroscopy. Magn Reson Imaging 1994;12:1269–74 [DOI] [PubMed] [Google Scholar]
- 21. Sabatier J, Tremoulet M, Ranjeva JP, et al. Contribution of in vivo 1H spectroscopy to the diagnosis of deep-seated brain abscess. J Neurol Neurosurg Psychiatry 1999;66:120–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dureux J, Voiriot P, Auque J, et al. Bases of antibiotherapy in neuromeningeal infections [in French]. Neurochirurgie 1988;34:72–82 [PubMed] [Google Scholar]
- 23. Akutsu H, Matsumura A, Isobe T, et al. Chronological change of brain abscess in 1H magnetic resonance spectroscopy. Neuroradiology 2002;44:574–78 [DOI] [PubMed] [Google Scholar]
- 24. Chang KH, Song IC, Kim SH, et al. In vivo single-voxel proton MR spectroscopy in intracranial cystic masses. AJNR Am J Neuroradiol 1998;19:401–05 [PMC free article] [PubMed] [Google Scholar]
- 25. Burtscher IM, Holtas S. In vivo proton MR spectroscopy of untreated and treated brain abscesses. AJNR Am J Neuroradiol 1999;20:1049–53 [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra AM, Gupta RK, Saksena S, et al. Biological correlates of diffusivity in brain abscess. Magn Reson Med 2005;54:878–85 [DOI] [PubMed] [Google Scholar]
- 27. Gorbach SL, Mayhew JW, Bartle JG, et al. Rapid diagnosis of anaerobic infection by direct gas-liquid chromatography of clinical specimens. J Clin Invest 1976;57:478–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remy C, Grand S, Lai ES, et al. 1H MRS of human brain abscess in vivo and in vitro. Magn Reson Med 1995;34:508–14 [DOI] [PubMed] [Google Scholar]
- 29. Willett HP. Energy metabolism. In: Willett HP, Amons DB, Wilfert CM. eds. Zinsser Microbiology. 20th ed. East Norwalk, Connecticut: Appleton and Lange; 1992:53–75 [Google Scholar]
- 30. Francois P, Schrenzel J. Rapid diagnosis and typing of Staphylococcus aureus. In: Lindsay J. ed. Staphylococcus: Molecular Genetics. Wymondham, UK: Caister Academic Press; 2008:71–90 [Google Scholar]
- 31. Himmelreich U, Gupta RK. Application of magnetic resonance for the diagnosis of infective brain lesions. In: Webb GA. ed. Modern Magnetic Resonance (part 4). Springer Netherlands; 2006:1005–13 [Google Scholar]
- 32. Himmelreich U, Accurso R, Malik R, et al. Identification of staphylococcus aureus brain abscesses: rat and human studies with 1H MR spectroscopy. Radiology 2005;236:270–61 [DOI] [PubMed] [Google Scholar]
- 33. Garg M, Misra MK, Chawla S, et al. Fingerprinting of bacteria from pus for its identification by 1H MR spectroscopy. Eu J Clin Invest. 2003;33:518–24 [DOI] [PubMed] [Google Scholar]
- 34. Dev R, Gupta RK, Poptani H, et al. Role of in vivo proton magnetic resonance spectroscopy in the diagnosis and management of brain abscesses. Neurosurgery 1998;42:37–43 [DOI] [PubMed] [Google Scholar]