Abstract
BACKGROUND AND PURPOSE:
Ventricular dilation and sulcal enlargement are common sequelae after aSAH. Our aim was to quantify the late ventricular dilation and volumes of the CSF spaces after aSAH and to determine if they correlate with neurologic and cognitive impairments frequently detected in these patients.
MATERIALS AND METHODS:
3D T1-weighted images needed for volumetry were available in 76 patients 1 year after aSAH, along with 75 neuropsychological assessments. Volumes of CSF segments and ICV were quantified by SPM in 76 patients and 30 control subjects to determine CSF/ICV ratios. The mCMI was calculated to roughly evaluate the ventricular dilation. The contributing factors for enlarged ventricles and CSF volumes were reviewed from radiologic, clinical, and neuropsychological perspectives.
RESULTS:
The mCMI was higher in patients with aSAH (0.23 ± 0.06) compared with control subjects (0.20 ± 0.04; P = .020). In line with these planimetric measurements, the SPM-based CSF/ICV ratios were higher in patients with aSAH (35.58 ± 7.0) than in control subjects (30.36 ± 6.25; P = .001). Preoperative hydrocephalus, higher HH and Fisher grades, and focal parenchymal lesions on brain MR imaging, but not the treatment technique, were associated with ventricular enlargement. The clinical outcome and presence of neuropsychological deficits correlated significantly with CSF enlargement.
CONCLUSIONS:
Ventricular and sulcal enlargement, together with reduced GM volumes, after aSAH may indicate general atrophy rather than hydrocephalus. Enlarged CSF spaces correlate with cognitive deficits after aSAH. A simple measure, mCMI proved to be a feasible tool to assess the diffuse atrophic brain damage after aSAH.
Many patients surviving aSAH are left with severe persisting neurologic and cognitive impairments.1–4 Ventricular dilation has been reported in patients surviving aSAH,5–7 often interpreted to represent chronic hydrocephalus. In our clinical work, we had observed that by visual estimation, ventricular dilation after aSAH is usually also combined with sulcal dilation. This finding might indicate diffuse atrophic injury after aSAH, similar to the diffuse atrophy detected in patients who survive a severe traumatic brain injury.8 No reports have quantified the degree of whole brain atrophy by modern volumetric MR imaging techniques in patients recovered from aSAH. Neither has ventricular and sulcal dilation after aSAH correlated with neuropsychological test performance, though diffuse aSAH-induced encephalopathy with impairments in cognitive functions is a well-known phenomenon.9 We have now collected follow-up MR imaging-data 1 year after aSAH including3D morphometric data. This gives us an opportunity to apply modern neuromorphometric methods to statistically analyze structural alterations after aSAH on a group level. The aims of this study were 1) to quantify the possible late ventricular and other CSF space dilation in patients with aSAH with use of both planimetric measurements to calculate mCMI and automated SPM analysis, 2) to assess the contributing factors for enlargement of CSF volumes after aSAH, and 3) to correlate the clinical and cognitive outcomes with the degree of enlarged CSF spaces after aSAH.
Materials and Methods
Between February 1995 and December 1999, a total of 467 patients were admitted to Kuopio University Hospital with acute aSAH and were candidates for a prospective, randomized aSAH study. We obtained institutional review board approval and informed consent. The detailed inclusion criteria of the study patients are described in the Appendix. A total of 138 patients were originally included in the MR imaging study; of them, 76 patients (34 men, 42 women; mean age, 50.2 years ± 14.9; range, 14–75 years) with volumetric MR imaging scans 1 year after aSAH were included in this study. All patients had a CT scan on admission, and Fisher grades and hydrocephalus were visually evaluated. Preoperative HH grade was determined. Focal parenchymal lesions were evaluated on MR imaging 1 year after aSAH. Quantitative planimetric measurements of the ventricular width and volumetric measurements of the CSF were obtained from the 76 patients. In addition, 30 age-matched and sex-matched healthy control subjects (13 men, 17 women; mean age, 54.1 years ± 15.5; range, 22.4–79.3 years) underwent volumetric MR imaging with the same imaging sequence and parameters and the same MR imaging scanner. Volunteers were asked about their medical history, especially any neurologic or cardiovascular disturbances, cranial trauma, or permanent medication they were receiving. Any volunteer who was suspected of having any pathologic medical condition or who was taking permanent medication was excluded. Both the patients and control populations were ethnically homogeneous, and all participants were native Finnish speakers.
Conventional MR Imaging
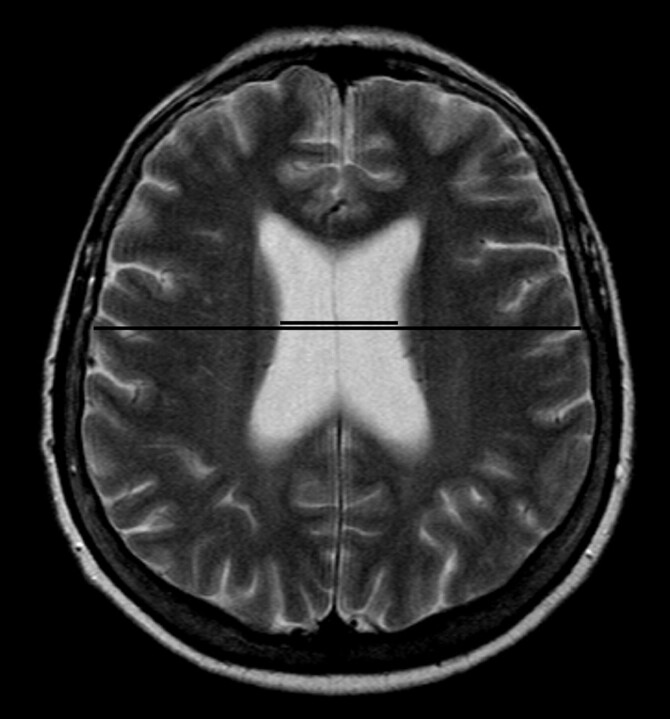
The 1.5T MR imaging protocol (Vision; Siemens, Erlangen, Germany) for the patients and control subjects consisted of T2-weighted and proton attenuation–weighted images. The 3D T1-weighted imaging examinations (TR, 9.7 ms; TI, 20 ms; flip angle, 12°; FOV, 250 mm; matrix, 256 × 256) were scheduled at the end of imaging if the patient was cooperative and willing to stay in the MR scanner for a longer period, and if there were no movement artifacts. All volumetric patients had good or moderate clinical outcome. The 3D T1-weighted images consisted of coronal sections (section thickness, 2.0 mm) covering the entire cerebrum. The presence, localization, and most probable cause of focal parenchymal lesions were analyzed by consensus of an experienced neuroradiologist (R.V.), an experienced neurosurgeon (T.K.), and a resident (P.B.), and the volumes of lesions were further quantified. To determine a rough planimetric estimate of ventricular size, we calculated the mCMI by measuring the maximal ventricular body width and dividing it by the maximal intracranial width measured from the same axial T2-section (Fig 1).
Fig 1.
For determination of a rough planimetric estimate of ventricular size, the mCMI is calculated by measuring the maximal ventricular body width and dividing it by the maximal intracranial width measured from the same axial T2-weighted section.
Volumetric Analysis
To obtain volumes of GM, WM, and CSF segments, we performed volumetric analysis by using the segmentation algorithm provided in VBM5 toolbox (http://dbm.neuro.uni-jena/vbm/) under SPM5 (Wellcome Department of Imaging Neuroscience, London, United Kingdom; www.fil.ion.ac.uk/spm).10,11 The T1-weighted images were first manually reoriented along the commissural line to enhance the segmentation and normalization procedures. The images were segmented into GM, WM, and CSF. The segmented images were visually checked to ensure that there were no major tissue-type misclassifications in the external CSF spaces. The volumes of GM, WM, and CSF were calculated from the segments and were further summarized to obtain the total ICV. The CSF/ICV ratios were then calculated and multiplied by 100 for improved readability.
Age, sex, treatment technique of the ruptured aneurysm, HH grade, Fisher grade, and dichotomized preoperative hydrocephalus were assessed as possible contributing factors for enlarged ventricles and CSF spaces. Age of the patient and control subjects was further categorized into 3 different age groups: young subjects (< 45 years), middle-aged subjects (ages 45–65 years), and elderly subjects (> 65 years), and the volumes of these subjects were separately compared. The presence of focal parenchymal lesions on MR imaging was also correlated with ventricular enlargement. To quantitate the ventricular size at the group level, we calculated an averaged normalized CSF segment image using the MNI template provided by SPM5, both for patients and control subjects. With use of these averaged CSF images, a ventricular region of interest was manually traced (P.B.) for both groups with MriCro (http://www.sph.sc.edu/comd/rorden/mricro.html), and the average size of the ventricles was calculated for patient and control groups.
Neuropsychological Evaluation
Neuropsychological assessments were performed 12 months after treatment, and the test battery was designed to evaluate 4 cognitive domains: general intellectual functioning, memory, language, and executive functions.12 General intellectual functioning was estimated on the basis of 5 subtests of the Wechsler Adult Intelligence Scale-Revised.13 Language was evaluated with a shortened version of the Boston Naming test and Verbal Fluency test (generation of words beginning with the letters P, A, and S in 60 s).14 Memory was assessed with the Logical Memory and Visual Reproduction subtests (immediate and delayed recall) from the Wechsler Memory Scale.15 Percent retention scores (calculated by dividing the delayed recall score by immediate recall score) were the measures for verbal and visual memory. Executive functions were assessed with the Trail-making test and the Stroop test.16,17 To extract the executive component in these tests, we calculated a difference score by subtracting the time taken in trail- making A from the time taken in trail-making B. A similar calculation was performed for recorded times for color naming and the interference part of the Stroop test. Two experienced neuropsychologists (M.Ä. and T.H.) interpreted neuropsychological test results by using normative data. Impairment was defined as a clinical decision on the basis of the subjects' scores on the whole test battery. Because of the very variable age and education range of the sample, comprehensive normative data could not be obtained for defining absolute age- and education-specific cutoff points (eg, 1.5 or 2 SD below the mean) for each subject. Therefore, scores of each subject were evaluated by following the normal clinical practice. Thus, the cognitive profile in other tests as well as age and education were taken into account when the impairment in different cognitive domains was considered. In the lack of “absolute” cutoff points on the basis of comprehensive normative data, we consider this method accurate to identify subjects with cognitive impairment in 1 or more cognitive domains. Impairment in any of the 4 cognitive domains was defined on the basis of normative data, in which the scores fell below the normal range in either measure of the domain. Performance on neuropsychological testing correlated with enlarged ventricles and CSF spaces. Performance on neuropsychological testing was assessed among the 3 different age groups: young subjects, middle-aged subjects, and elderly subjects.
Statistics
Statistical analyses of demographic and neuropsychological data were performed with SPSS (version 16; SPSS, Chicago, Illinois). Categorical data and dichotomous variables were examined by the χ2 test. Normal and non-normal distribution of the variables were determined by the Kolmogorov-Smirnov test. The Student t test was used to compare normally distributed data (mCMI) between different dichotomized demographic parameters. Non-normally distributed continuous-scale variables (CSF/ICV, GM/ICV, and WM/ICV) were analyzed with Mann-Whitney U tests. The Spearman correlation coefficient was used to analyze correlations between the continuous variables. The level of significance was set at P < .05 and uncorrected P values were used because we did not perform multiple comparisons or post hoc tests in this study. Consequently, Bonferroni correction or other correction procedures were not applied.
Results
Patients and Control Subjects, Preoperative Hydrocephalus and Permanent Shunt Device
The patients and control subjects were balanced in age and sex (On-line Table 1). Preoperative hydrocephalus was detected in 16 (40.0%) of 40 patients in the endovascular group and 11 (30.6%) of 36 patients in the surgical group [χ2(1) = 0.738; P = .390]. A permanent shunt device was present in 4 [1 endovascular, 3 surgical; χ2(1) = 1.293; P = .255] patients. The mean interval between aSAH and shunt operation was 4.75 ± 2.87 weeks (range, 1–8 weeks).
Degree of Ventriculomegaly and Enlarged CSF Spaces
The mCMI proved to be higher in patients with aSAH (0.23 ± 0.06) compared with the age-matched and sex-matched control subjects [0.20 ± 0.038; n = 30; t(104) = 2.359; P = .020]. The more detailed analyses of the CSF volumes in 76 patients were in line with the rough planimetric measurements: the mean volume of the ventricular system (obtained from manually drawn region-of-interest analysis) was 64.14 cm3 in the patients with aSAH, 31.4% larger compared with the volume in control subjects (44.00 cm3). The ICV values were comparable between the patients and control subjects. The CSF/ICV ratios were 35.58 ± 7.00 in the patients compared with 30.36 ± 6.25 in the control subjects (Z = −3.395; P = .001). The mCMI correlated with CSF/ICV ratios (r = 0.495; P < .001). Moreover, the GM/ICV ratios and WM/ICV ratios were lower in the patients (GM/ICV, 37.43 ± 4.97; WM/ICV, 26.99 ± 2.89) compared with the control subjects (GM/ICV, 39.24 ± 4.28; Z = −2.027; P = .043; WM/ICV, 30.40 ± 2.83; Z = −4.79; P < .001). Fig 2 illustrates on a group level that both the ventricles and the cortical sulci are enlarged in the patients compared with the control population.
Fig 2.
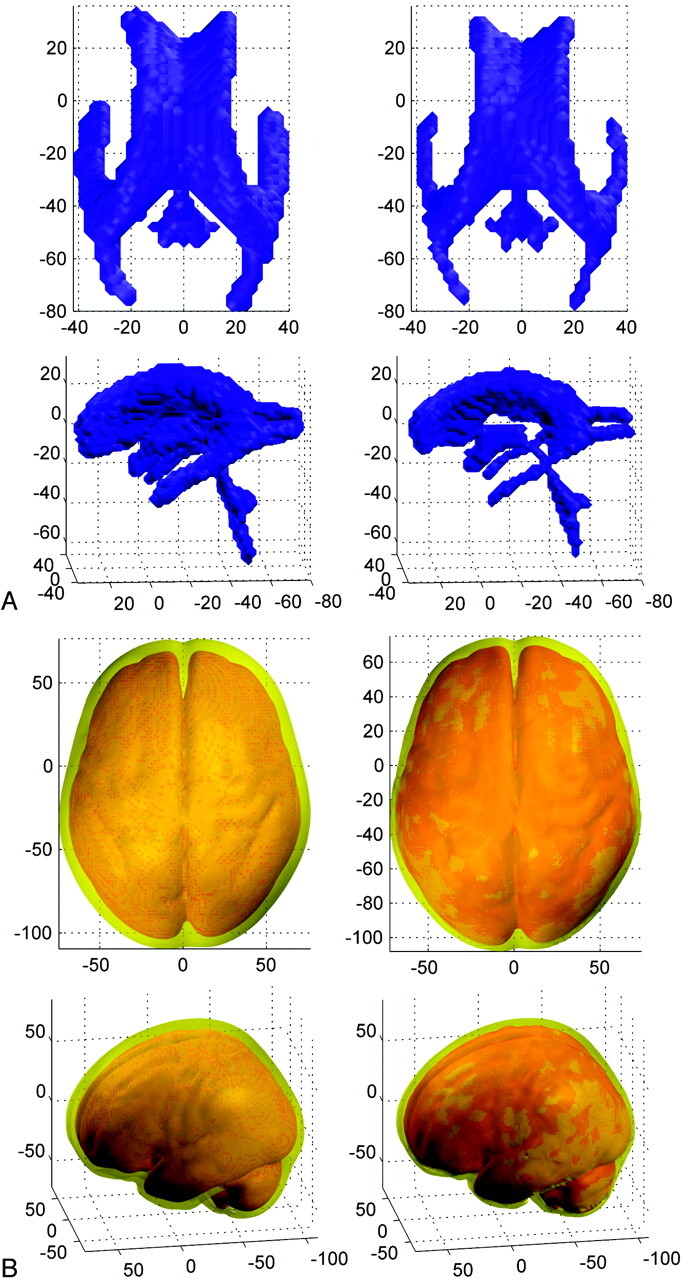
3D rendering images of the averaged ventricular system and the superficial sulcal CSF segments are illustrated in the MNI space. Image axis represents the MNI coordinates. A, The mean ventricular system of patients with aSAH (n = 76) shown on the left side and control subjects (n = 30) shown on the right side, cranial and lateral views. B, The mean superficial CSF segments of patients with aSAH (n = 76) shown on the left side and control subjects (n = 30) shown on the right side, cranial and lateral views.
Associations between Ventricular and Sulcal Enlargement and Clinical Features
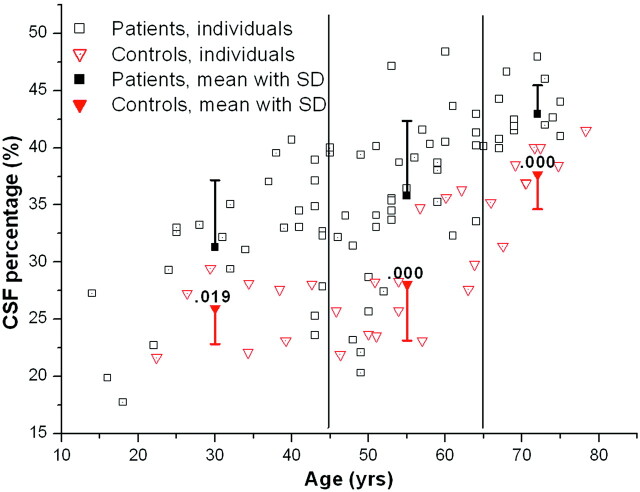
As expected, the age of the patient correlated significantly with larger ventricles and CSF spaces: mCMI, r = 0.416; P < .001, and CSF/ICV, r = 0.718; P < .001. This finding was constant in all 3 age groups compared with matched control subjects. Fig 3 demonstrates the association between the CSF/ICV ratios and the age of the subject.
Fig 3.
CSF/ICV ratios in relationship to the age of the patient and control subjects. The mean CSF/ICV ratios with ± SD are demonstrated in 3 age groups: young patients (< 45 years), middle-aged patients (45–65 years) and elderly patients (> 65 years).
The mCMI was comparable between the treatment groups: 0.23 ± 0.1 after endovascular treatment and 0.23 ± 0.1 after surgical treatment. The treatment method did not affect measured CSF/ICV ratios; CSF/ICV was 34.49 ± 7.7 after endovascular treatment and 36.78 ± 6.0 after surgical treatment (Z = −1.061; P = .289). At the time of MR imaging (mean, 14.6 ± 5.3 months; range, 9–46 months) after aSAH (2 patients had MR imaging studies taken later than 24 months after aSAH), clinical or radiologic evidence of raised intracranial pressure could not be detected among any of the patients studied. There were no differences in mCMI or CSF/ICV ratios when the patients with a permanent shunt device (mCMI, 0.289 ± 0.057; CSF/ICV, 37.18 ± 2.57) or without a permanent shunt device (mCMI, 0.231 ± 0.064, t(74) = −1.752; P = .084 and CSF/ICV, 35.49 ± 7.17; Z = −0.326; P = .745) were compared.
The sex of the patient did not affect the measured mCMI or CSF/ICV ratios. Patients with originally higher HH and Fisher grades showed more pronounced mCMIs vs patients with lower HH and Fisher grades. Higher Fisher grade was also associated with higher CSF/ICV ratios, and patients with preoperative hydrocephalus tended to have higher CSF/ICV ratios. Associations between enlarged CSF spaces and contributing factors (eg, preoperative hydrocephalus and HH and Fisher grades) are demonstrated in On-line Table 2.
Associations between Ventricular and Sulcal Enlargement and MR Imaging Features
Higher mCMIs and CSF/ICVs were detected in patients with focal parenchymal lesions. The prevalence of different MR imaging lesions of the study populations is also presented in On-line Table 2. MR imaging findings are presented in On-line Table 3 to characterize the endovascular vs the surgical patient populations completing the neuropsychological examination.
Associations between Ventricular Size and Clinical and Cognitive Outcome
Our volumetric study population mostly consisted of patients with good recovery; 66 patients had good outcome and 10 patients had moderate disability. Patients with GOS of 2 to 4 had larger ventricles and CSF spaces (mCMI, 0.28 ± 0.07; CSF/ICV, 38.16 ± 5.81) compared with patients with GOS of 5 (mCMI, 0.23 ± 0.06; t(74) = −2.24; P <.028 and CSF/ICV, 35.19 ± 7.12; Z = −1.18; P = .237).
The neuropsychological evaluation was available in 75 (98.7%) of 76 patients, as 1 endovascular patient refused to undergo the neuropsychological examination. Most patients (44/75; 58.7%) showed neuropsychological impairments in at least 1 of the 4 cognitive domains measured. Cognitive impairment tended to be more common after surgical treatment (n = 25; 69.4%) than after endovascular treatment [n = 19/39; 52.0%; χ2(1) = 3.32; P = .069]. Eight patients in the endovascular group and 13 patients in the surgical group showed impairment in 1 of the 4 cognitive domains. Impairment in 2 domains was detected in 6 endovascular and 5 surgical patients. Four endovascular and 2 surgical patients were impaired in 3 cognitive domains, and 1 patient in the endovascular group and 5 patients in the surgical group were impaired in all 4 neuropsychological domains [χ2(4) = 7.12; P = .130]. Clinical outcome and neuropsychological outcome between endovascularly vs surgically treated patients are shown in On-line Table 3. Patients with neuropsychological deficits were older (mean age, 55.56 ± 13.12 years) than patients with a normal cognitive profile (mean age, 42.32 ± 14.06 years; t(73) = −4.18; P < .001). The age of the patient was significantly associated with neuropsychological deficits in general [t(2) = 15.81; P < .001]. Furthermore, in a separate analysis in the 4 cognitive domains, the age of the patient was found to be strongly associated with a deficit in general intellectual functioning [t(2) = 15.81; P < .001], memory deficits [t(2) = 13.34; P = .001], verbal deficits [t(2) = 11.15; P = .004], and deficits in executive functions [t(2) = 18.37; P <.001].
In the group of patients with at least 1 neuropsychological deficit detected, the mCMIs (0.248 ± 0.07) and CSF/ICV ratios (37.45 ± 6.1) were higher compared with those patients without any neuropsychological deficits (mCMI, 0.215 ± 0.05; t(73) = −2.24; P = .028 and CSF/ICV, 32.66 ± 7.25; Z = −2.94; P = .003). In line with the higher CSF/ICV ratios, the patients with neuropsychological deficits showed lower GM/ICV ratios (36.00 ± 4.29) and WM/ICV ratios (26.55 ± 2.76) than did patients without neuropsychological deficits (GM/ICV, 39.59 ± 5.19; Z = −3.02; P = .003 and WM/ICV, 27.75 ± 2.93; Z = −1.32; P = .186, nonsignificant.) In separate analyses of each cognitive domain, higher mCMIs and CSF/ICV ratios were detected in the patients with a deficit (On-line Table 4).
In our study population, the 25 of the 76 (13 male, 12 female) patients without focal parenchymal lesions were significantly younger (mean age, 44.6 ± 13.75 years) compared with the control subjects (54.1 ± 5.5 years; t(53) = −2.36; P = .022). To compare the late atrophy in patients without focal MR imaging abnormalities with age-matched and sex-matched control subjects, we selected a balanced subgroup of younger control subjects (n = 18; 9 male, 9 female; mean age, 44.0 ± 11.3 years) and found that significantly higher CSF/ICV ratios were detected in the patients (31.39 ± 7.52) compared with CSF/ICV ratios in control subjects (26.56 ± 4.05; Z = −2.21; P = .027). There was no significant difference in mCMIs when comparing the patients without focal parenchymal deficit (mCMI, 0.21 ± 0.07) and the subgroup of younger control subjects [mCMI, 0.19 ± 0.03; t(41) = 1.13; P = .264].
Discussion
In this study, we quantified ventricular and sulcal enlargement after aSAH and related it to the neuropsychological outcome of the patients. To our knowledge, this is the first time modern 3D image analysis methods were used in patients who have recovered from aSAH. Diffuse atrophic brain damage after aSAH has been recognized in clinical practice and was mentioned in the literature in 1928.18 Numerous studies have reported ventricular dilation after aSAH. However, most of these studies were performed in the acute or subacute phase after aSAH, when ventricular dilation is caused by active hydrocephalus.5,19–21 In the present late MR imaging study, performed 1 year after aSAH, all patients with clinical and radiologic findings suggesting active hydrocephalus had already been treated with a permanent shunt device. Furthermore, there were no differences in mCMI or CSF/ICV ratios when the patients with or without the permanent shunt device were compared, suggesting that patients with symptomatic hydrocephalus had been successfully treated. In the patients with aSAH, the cortical sulci were also wider than those in the control subjects (Fig 2). Therefore, we suggest that the ventricular dilation, together with reduced GM and WM volumes of the brain, observed in the chronic phase after aSAH should be interpreted to represent diffuse brain atrophy rather than chronic hydrocephalus. In the comparative sense, a recent study concluded that posttraumatic ventriculomegaly is a frequent finding in patients surviving traumatic brain injury.8 The mechanism of the diffuse brain atrophy after aSAH cannot be ischemia only. It is more likely a result of the recently described concept of EBI22,23; a number of critical pathways initiating acutely after bleeding such as inflammation, hypoxia, oxidative stress, and excitotoxicity are interrelated with a similar end result: cell death. We speculate that the diffuse brain atrophy after aSAH detected in this study may be related to these mechanisms in EBI.
We performed an assessment of ventricular enlargement using 2 different methods: a simple planimetric measurement of ventricular dilation by measuring mCMI, and a more laborious SPM-based measurement, which covers both the ventricular, cisternal, and sulcal CSF-filled spaces. These 2 measurements correlated significantly with each other. Therefore, measuring the individual mCMI can be advocated in everyday clinical practice when assessing the central type of atrophy after aSAH in cases where hydrocephalus can be excluded.
The volumetric sequence was scheduled at the end of the imaging as an extra sequence if the patient was cooperative and willing to stay in the scanner for a longer period, a fact favoring the good-grade patients to be included to this volumetric study. Furthermore, the clinical outcome was better in our volumetric population than in most of the aSAH population.2,24 Our study consisted of only patients with good or moderate clinical outcome. Therefore, our results cannot be generalized in a straightforward manner to all consecutive patients with aSAH. However, the atrophic damage that aSAH causes to the brain is probably more severe in patients with poorer clinical outcomes than detected in the current study. Higher Fisher grades and preoperative hydrocephalus were found to be associated with mCMIs and higher CSF/ICV ratios. It is noteworthy that a recent study reported that the severity of cognitive impairment 1 year after SAH is best predicted by the volume of blood in the subarachnoid space assessed by the Fisher score.25
Late structural focal brain damage is often detected on MR imaging after aSAH26–29 and explains some of the neurologic and neuropsychological deficits, though absence of pathologic findings in our subjects' MR images does not exclude the possibility of cognitive difficulties.30 Diffuse brain atrophy may partly explain this phenomenon. General atrophy may also enhance the neuropsychological deficits caused by focal lesions on brain MR imaging. In the present study, most of the patients had focal parenchymal lesions. As expected, patients with focal lesions detected on MR imaging showed more pronounced atrophy measured both by the mCMI and CSF/ICV ratios than those patients without these lesions.
In our study, older age was strongly associated with the neuropsychological deficits detected, though the dichotomous neuropsychological classifications were based on normative data of healthy age-matched control subjects. This is in accordance with previous studies evaluating the cognitive outcome after aSAH.4,25,31
There were some limitations in our study. Our data were fairly old. However, modern whole brain volumetric techniques were not available at the time our data were collected. The MR imaging sequence we used for this study is widely used nowadays for volumetric purposes and has not been dramatically changed. Moreover, despite recent advances in the treatment of patients after aSAH, morbidity and mortality rates have failed to improve significantly; thus, it is not probable that the results would be different now.
An optimal way to report development of atrophy would be the comparison of 2 longitudinal MR imaging sessions. We did not include MR imaging at the acute phase of aSAH in our study protocol because of the multiple sources of error: many patients have acute hydrocephalus, brain parenchyma is swollen, ventricles and sulci can be displaced by acute hematomas or infarction edema, and the cisternal and sulcal blood obscures delineation of anatomic structures. Furthermore, an ethical concern may emerge when most of these critically ill patients with acute aSAH would need general anesthesia to enable sufficient image quality for volumetric analyses. To minimize this limitation, we balanced the control group with age and sex, though neuropsychological data were not available in the control group. Another limitation is that the possible comorbid medical conditions were not meticulously recorded in the aSAH patient population.
The limitation of SPM5 segmentation is that it is mainly designated for segmenting GM and WM, whereas the differentiation between the external CSF spaces and the skull may not be as optimal. Furthermore, there is no model for bone or soft tissue outside the skull, which may affect the overall segmentation procedure and normalization. However, the segmentation algorithm in SPM5 is an automated method and, thus, is not operator dependent. The segmentation process takes into account previous knowledge of contextual signal intensity information with use of previous probability maps of GM, WM, and CSF. VBM5-toolbox extends the core segmentation algorithm of SPM5 by removing isolated voxels of 1 tissue class, resulting in less noisy tissue segments. Therefore, in this study the segmented images were visually checked to ensure that there were no major tissue-type misclassifications. In addition, we performed a manual drawing of the ventricle regions of interest using the mean images of normalized T1-images for both the patients and control subjects and not the automated CSF segments, thus minimizing the possible problems of automated segmentation. The results of the automated SPM-segmented full-brain CSF calculations are in line with the manually drawn region-of-interest results.
The current study shows that diffuse atrophic ventricular and sulcal enlargement are common sequelae after aSAH. Furthermore, enlarged CSF spaces correlate with neuropsychological test performance. It is probable that diffuse brain atrophy, together with focal parenchymal lesions such as cortical infarctions and retraction lesions and previously reported temporomesial volume loss,32 all contribute to the development of neurocognitive deficits in patients recovering from aSAH. The mCMI proved to be a simple and feasible tool to assess the diffuse atrophic brain damage after aSAH. Correct detection and quantitation of general atrophy on follow-up MR imaging examinations may be of clinical importance.
Conclusions
Ventricular and sulcal enlargement, together with reduced brain parenchymal volumes, are common sequelae after aSAH and indicate general atrophy rather than hydrocephalus. Higher Fisher scores, preoperative hydrocephalus, and older age were found to be associated with ventricular and sulcal enlargement. Enlarged CSF spaces correlated with cognitive deficits after aSAH. A simple measure, mCMI proved to be a feasible tool to assess diffuse atrophic brain damage after aSAH.
Supplementary Material
Abbreviations
- aSAH
aneurysmal subarachnoid hemorrhage
- EBI
early brain injury
- GM
gray matter
- GOS
Glasgow Outcome Scale
- HH
Hunt and Hess
- ICV
total intracranial volume
- mCMI
modified cella media index
- MNI
Montreal Neurologic Institute
- MRI
MR imaging
- SPM
statistical parametrical mapping
- WM
white matter.
Appendix, Study Design
The ethical committee at our hospital approved the study design. During the study period (February 1, 1995 to December 31, 1999), all patients who were admitted to our university hospital because of primary SAH were evaluated as potential candidates for the study. After informed consent was obtained from the patient or the patient's closest relative, all patients with a ruptured aneurysm that was considered to be suitable for both surgical clipping and endovascular treatment were consecutively included, provided that the following exclusion criteria were not fulfilled: 1) age older than 75 years, 2) bleeding for more than 3 days before the procedure, 3) presence of a large hematoma necessitating surgery, 4) aneurysm associated with mass effect causing a neurologic deficit, or 5) previous surgery for the ruptured aneurysm.
The aneurysm was not considered to be suitable for endovascular treatment, and the patient was not considered for random assignment to a treatment group if the following findings from diagnostic angiography were present: 1) neck of the aneurysm wider than the fundus, 2) fusiform aneurysm, 3) neck and its relationship to the parent vessel and adjacent branches not distinguishable, or 4) diameter of the aneurysm smaller than 2 mm (< the smallest coil available). The patient's suitability for random assignment and endovascular treatment was always considered according to the morphologic features of the aneurysm that had most probably ruptured (aneurysm irregularity, size, and findings seen on CT scan).
To avoid selection bias, random assignment was performed separately for patients with a HH grade of I to II, for those with a grade of III, and for those with a grade of IV to V. After the procedure, both the patients who underwent surgery and those who underwent endovascular treatment received care in a similar manner in the intensive care unit.
Neuropsychological tests were scheduled after surgical or endovascular treatment of the ruptured aneurysm during the primary hospital stay and 3 and 12 months after SAH.
Footnotes
This study has been supported by Kuopio University Hospital, EVO grant 5063515 and the Finnish Cultural Foundation (North Savo Regional Fund; Natalia and Frederik Trube Foundation).
Indicates article with supplemental on-line table.
References
- 1. Bellebaum C, Schafers L, Schoch B, et al. Clipping versus coiling: neuropsychological follow up after aneurysmal subarachnoid haemorrhage (SAH). J Clin Exp Neuropsychol 2004;26:1081–92 [DOI] [PubMed] [Google Scholar]
- 2. Hackett ML, Anderson CS. Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The Australian Cooperative Research on Subarachnoid Hemorrhage Study Group. Neurology 2000;55:658–62 [DOI] [PubMed] [Google Scholar]
- 3. Kreiter KT, Copeland D, Bernardini GL, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 2002;33:200–08 [DOI] [PubMed] [Google Scholar]
- 4. Ogden JA, Mee EW, Henning MA. Prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery 1993;33:572–86; discussion 586–87 [DOI] [PubMed] [Google Scholar]
- 5. Jartti P, Karttunen A, Isokangas JM, et al. Chronic hydrocephalus after neurosurgical and endovascular treatment of ruptured intracranial aneurysms. Acta Radiol 2008;49:680–86 [DOI] [PubMed] [Google Scholar]
- 6. Vilkki J, Holst P, Ohman J, et al. Cognitive deficits related to computed tomographic findings after surgery for a ruptured intracranial aneurysm. Neurosurgery 1989;25:166–72 [DOI] [PubMed] [Google Scholar]
- 7. Vilkki J, Holst P, Ohman J, et al. Social outcome related to cognitive performance and computed tomographic findings after surgery for a ruptured intracranial aneurysm. Neurosurgery 1990;26:579–84; discussion 584–85 [DOI] [PubMed] [Google Scholar]
- 8. Poca MA, Sahuquillo J, Mataro M, et al. Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma 2005;22:1303–10 [DOI] [PubMed] [Google Scholar]
- 9. Ljunggren B, Sonesson B, Saveland H, et al. Cognitive impairment and adjustment in patients without neurological deficits after aneurysmal SAH and early operation. J Neurosurg 1985;62:673–79 [DOI] [PubMed] [Google Scholar]
- 10. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 11. Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36 [DOI] [PubMed] [Google Scholar]
- 12. Koivisto T, Vanninen R, Hurskainen H, et al. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000;31:2369–77 [DOI] [PubMed] [Google Scholar]
- 13. Wechsler D Wechsler. Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 14. Lezak HD, Loring DW. Neuropsychological Assessment. New York: 2004. [Google Scholar]
- 15. Wechsler D. Wechsler Memory Scale Manual. San Antonio: The Psychological Corporation; 1974. [Google Scholar]
- 16. Golden C. Stroop Color and Word Tests. Chicago: Stoerting; 1978. [Google Scholar]
- 17. Reitan R. Validity of the trail making tests as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–76 [Google Scholar]
- 18. Bagley C. Blood in the cerebrospinal fluid. Resultant functional and organic alterations in the central nervous system. A. Experimental data. Arch Surg 1928;17:18–38 [Google Scholar]
- 19. Dehdashti AR, Rilliet B, Rufenacht DA, et al. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg 2004;101:402–07 [DOI] [PubMed] [Google Scholar]
- 20. Sethi H, Moore A, Dervin J, et al. Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg 2000;92:991–94 [DOI] [PubMed] [Google Scholar]
- 21. Vassilouthis J, Richardson AE. Ventricular dilatation and communicating hydrocephalus following spontaneous subarachnoid hemorrhage. J Neurosurg 1979;51:341–51 [DOI] [PubMed] [Google Scholar]
- 22. Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2006;26:1341–53 [DOI] [PubMed] [Google Scholar]
- 23. Cahill J, Zhang JH. Subarachnoid hemorrhage: is it time for a new direction? Stroke 2009;40:S86–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hernesniemi J, Vapalahti M, Niskanen M, et al. One-year outcome in early aneurysm surgery: a 14 years experience. Acta Neurochir (Wien) 1993;122:1–10 [DOI] [PubMed] [Google Scholar]
- 25. Orbo M, Waterloo K, Egge A, et al. Predictors for cognitive impairment one year after surgery for aneurysmal subarachnoid hemorrhage. J Neurol 2008;255:1770–76 [DOI] [PubMed] [Google Scholar]
- 26. Bendel P, Koivisto T, Kononen M, et al. MR imaging of the brain 1 year after aneurysmal subarachnoid hemorrhage: randomized study comparing surgical with endovascular treatment. Radiology 2008;246:543–52 [DOI] [PubMed] [Google Scholar]
- 27. Hadjivassiliou M, Tooth CL, Romanowski CA, et al. Aneurysmal SAH: cognitive outcome and structural damage after clipping or coiling. Neurology 2001;56:1672–77 [DOI] [PubMed] [Google Scholar]
- 28. Kivisaari RP, Salonen O, Servo A, et al. MR imaging after aneurysmal subarachnoid hemorrhage and surgery: a long-term follow-up study. AJNR Am J Neuroradiol 2001;22:1143–48 [PMC free article] [PubMed] [Google Scholar]
- 29. Rabinstein AA, Weigand S, Atkinson JL, et al. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2005;36:992–97 [DOI] [PubMed] [Google Scholar]
- 30. Romner B, Sonesson B, Ljunggren B, et al. Late magnetic resonance imaging related to neurobehavioral functioning after aneurysmal subarachnoid hemorrhage. Neurosurgery 1989;25:390–96; discussion 396–97 [DOI] [PubMed] [Google Scholar]
- 31. Mayer SA, Kreiter KT, Copeland D, et al. Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology 2002;59:1750–58 [DOI] [PubMed] [Google Scholar]
- 32. Bendel P, Koivisto T, Hanninen T, et al. Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology 2006;67:575–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.