Abstract
BACKGROUND AND PURPOSE:
With a 64-channel multidetector row CT, imaging acquisition during speech, swallowing, or phonation has become feasible. However, the actual benefit of these additional focused images should be critically evaluated with respect to radiation dose. The purpose of this study was to determine if dedicated laryngeal CT using breath-holding and straw-blowing improved the accuracy of TNM-staging for patients with biopsy-proved laryngeal carcinomas in comparison with a standard neck CT.
MATERIALS AND METHODS:
A total of 27 patients underwent a standard neck CT and a laryngeal CT with additional images acquired while patients held their breath or blew through a straw. Two radiologists interpreted the neck CT and later the laryngeal CT and assigned a TNM-stage for each case. These interpretations were compared with a TNM-stage determined by surgery and/or clinical examination for the individual patients. The accuracy of standard neck CT was compared with the accuracy of laryngeal CT.
RESULTS:
The overall accuracy was not significantly different between standard neck CT and the additional laryngeal CT and was, in fact, lower in cases with additional larynx images. The accuracy of staging was slightly improved with the additional laryngeal CT for glottic cancers; however, it was decreased for supraglottic cancers. The accuracy of a dichotomous diagnosis of early-versus-advanced-stage cancer was 0.86 for the standard neck CT and 0.80 for the laryngeal CT. The readers' confidence levels did not improve with the use of the additional images.
CONCLUSIONS:
In the era of isovoxel multidetector CT technology and judicious monitoring of radiation dose, a standard neck CT with coronal and sagittal reformats should suffice for the staging of laryngeal cancer.
The NCI estimates that there were 12,250 new cases of laryngeal cancer and 3670 deaths from laryngeal cancer in 2008 (NCI cancer statistics: www.cancer.gov). More than 95% of laryngeal tumors are squamous cell carcinomas arising from the mucosal surface of the aerodigestive tract. Tobacco and alcohol use are the most important risk factors for head and neck cancer, including laryngeal cancer. Patients often present with voice changes, sore throat, swallowing difficulty, or a lump in the neck. Clinical mirror examination and endoscopy are used to evaluate the larynx and hypopharynx. Once a mucosal lesion is identified on mirror examination or laryngoscopy, a biopsy is taken to confirm the diagnosis histologically. The treatment plan for an individual patient with laryngeal cancer depends on a number of factors, including location of the tumor, stage of the tumor, histology, and the patient's age and other medical comorbidities. The aim of therapy is to conserve the laryngeal function while achieving the best life expectancy and quality of life for patients with laryngeal cancer. Various types of surgery and radiation with adjuvant chemotherapy are generally accepted treatments.
The extent of the tumor has substantial impact on treatment decisions for laryngeal cancer. For example, early T1 and T2 tumors of the glottis and supraglottis can be treated with laser excision. In more advanced tumors, a total or partial laryngectomy may be indicated.1,2 The specific procedure performed depends on the extent of the tumor into the glottis or subglottis: either superficially or deeply through the paraglottic space and pre-epiglottic space, presence or absence of cartilage invasion, or extralaryngeal spread. Because of the variety of therapeutic options for laryngeal tumors and their associated indications and contraindications based on tumor extension, imaging plays a key role in the staging of laryngeal cancer.
At our institution, dedicated laryngeal CT has been performed to visualize the mobility of the vocal cords and the extent of disease involving the piriform sinuses. The larynx protocol includes standard neck CT obtained during quiet breathing, plus repeat scanning through the larynx during breath-holding and straw-blowing. The vocal cords normally adduct during breath-holding, while abducting in a paramedian location during quiet breathing. Blowing through a small-caliber straw increases intralaryngeal pressure, thus dilating the piriform sinuses. A smaller FOV was used to improve the spatial resolution of the larynx for breath-holding and straw-blowing.
However, it was not clear if adding 2 sets of CT images would improve our ability to evaluate the extent of tumor and thereby improve the accuracy of the TNM-staging of laryngeal cancer. We, therefore, examined the potential benefit of these images to determine if the additional radiation and time to perform the examination were justified. The purpose of this study was to determine if the dedicated larynx protocol imaging improved the accuracy of TNM-staging and increased radiologists' confidence in assessing TNM-staging compared with the standard neck CT alone.
Materials and Methods
Patient Selection
The institutional review board at our institution approved the review of patients' medical records and CT findings. The radiology information system was searched for patients who had undergone the laryngeal CT protocol for a diagnosis of laryngeal squamous cell carcinoma from June 1, 2004, to May 31, 2006, for a period of 24 months. Patients who had previous radiation therapy or previous surgery were excluded. Patients who could not receive IV contrast due to a history of allergy or renal failure were also excluded. Patients who underwent the laryngeal CT protocol but never had biopsy-proved laryngeal cancer were also excluded, as were patients younger than 18 years of age. Twenty-seven patients met our criteria. There were 9 patients with glottic cancer, 16 with supraglottic cancer, 1 with subglottic cancer, and 1 who was initially thought to have laryngeal primary cancer but was later diagnosed with hypopharyngeal cancer.
Image Acquisition
CT examination was performed by using a 64-channel multidetector CT scanner (volume CT, LightSpeed; GE Healthcare, Milwaukee, Wisconsin). The laryngeal CT protocol at our institution included standard neck CT and a dedicated larynx scan (Figs 1 and 2).
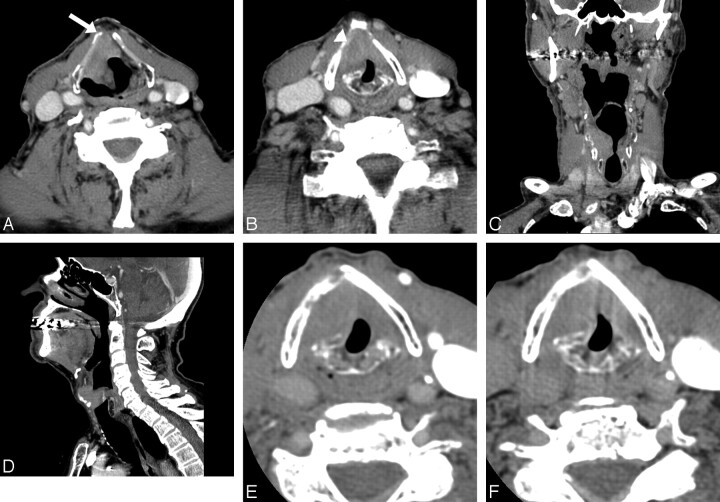
Fig 1.
An 89-year-old man with supraglottic laryngeal cancer. A and B, The standard neck CT images at the supraglottic (A) and glottic (B) levels show a large soft-tissue mass involving the pre-epiglottic space (arrow) and right aryepiglottic fold, extending to the right true vocal cord (arrowhead). C and D, Coronal (C) and sagittal (D) reformatted images of the standard neck CT scan show transglottic extension of the tumor without subglottic extension. E and F, Dedicated laryngeal CT images with straw-blowing (E) and breath-holding (F) at the level of true vocal cord show no change in the location of vocal cord, indicating the fixed vocal cord. Clinically, vocal cord mobility is impaired but not fixed. This is staged as T3 for pre-epiglottic extension.
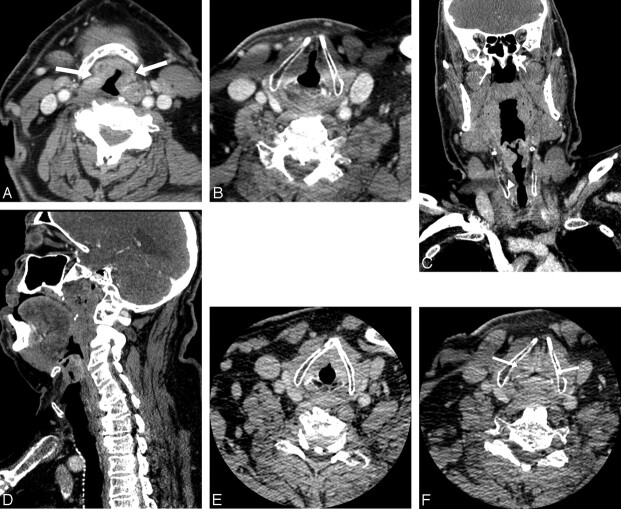
Fig 2.
A 72-year-old man with supraglottic laryngeal cancer. A, The standard neck CT images during quiet breathing at the supraglottic level show a mass involving the epiglottis and aryepiglottic fold bilaterally (arrows), extending slightly to the pre-epiglottic space. B, Axial image at the level of glottis shows normal abduction of the true vocal cord at the paramedial location. C and D, Coronal (C) and sagittal (D) reformatted images of the standard neck CT scan show an infiltrative tumor involving the supraglottic structures without transglottic extension. The true vocal cord appears normal (arrowhead). E and F, Dedicated laryngeal CT images with straw-blowing (E) and breath-holding (F) at the level of the true vocal cord show normal adduction of the vocal cord bilaterally during breath-holding (arrows) (F). Clinically, the vocal cord is mobile bilaterally. This is staged as T3 for pre-epiglottic extension.
Our standard neck CT images were reformatted by using 0.625-mm axial images with a 2.5-mm reconstruction following administration of 75 mL of IV contrast material with a 60-second delay. The neck CT images were obtained from the base of the skull to the arch of the aorta while the patient performed quiet breathing. The scanning parameters included 120–140 kV(p), tube current of 180–200 mA, 0.5-second scanning time, and a 512 × 512 pixel matrix. The FOV was 16–18 cm. Coronal and sagittal reformatted images of the entire neck were also obtained. Images were reviewed with soft-tissue window and bone window settings.
Following an additional 75 mL of IV contrast material, the larynx scans were obtained through the area from the hyoid bone to the bottom of the cricoid cartilage, with 0.625-mm axial images reconstructed to 1.25-mm images. The laryngeal CT was repeated once during breath-holding and once during straw-blowing. Quiet breathing from the standard neck CT allowed scanning of the vocal cords during abduction. The angle of the axial images was set parallel to the true vocal cords. The FOV was from 10 to 12 cm. During breath-holding, the cords adduct to midline. Blowing through a small-caliber straw distended the piriform sinuses with air, improving visualization of these areas.
Chart Review
Retrospective medical chart review was performed to obtain information regarding clinical examination, including the visible extent of the tumor, the palpable extent of the tumor, involvement of the vocal cords, mobility of the vocal cords, and the primary site of the tumor. Histopathology reports were reviewed to confirm the diagnosis of squamous cell carcinoma. Among 27 patients, 17 underwent surgery: 7 with a supraglottic primary carcinoma, 9 with glottic carcinoma, and 1 with subglottic carcinoma. Ten patients underwent radiation and/or chemotherapy. For cases in which surgery was performed, the operative and pathology reports were reviewed to define the extent of tumor invasion. The combined information in each patient's medical record had been evaluated in the otolaryngology clinic, and TNM-staging was performed for each patient. This assigned clinical TNM-stage was used as a reference standard. For patients who did not undergo surgery, there was less information on which to base a TNM-stage. Therefore as an internal check, the accuracy in the subset of patients who had surgery was compared with the accuracy of the group as a whole to determine if there was any significant difference.
CT Reading
Two radiologists with experience in head and neck radiology read the scans of the study subjects independently. The readers were blinded to the subjects' medical records and thereby blinded to patients' TNM-stages. We asked the radiologists to read the standard neck CT scans and to record, on a standardized data abstraction form, specific structures into which the tumors had invaded. The readers were then asked to assign a TNM-stage based on the criteria for head and neck cancers in the AJCC Cancer Staging Manual.3 Last, the readers were asked to rate their level of confidence in the TNM-staging for each scan read, on a 5-point scale (1 = least confident and 5 = most confident.) Three to 6 weeks later, the radiologists were then asked to read the scans again, this time including the larynx protocol images with the standard neck CT. They were again asked to record invaded structures, assign a TNM-stage, and rate their confidence level.
Data Analysis
Each TNM-stage from the blinded readers was compared with the final TNM-stage for each patient for the neck and laryngeal CT scans. The accuracy was defined as the correct number of TNM stages (T1, T2, T3, and T4) between CT staging and clinicopathologic staging divided by the total number of patients. Sublevel staging was considered the same level (ie, T4a and T4b were both considered T4). We averaged the percentages of accuracy between the readers. In addition, we recorded the cases in which the radiologist overstaged and understaged the tumors. We also examined the dichotomous staging accuracy of early (T1 and T2) versus advanced (T3 and T4) tumors.
Radiologists' levels of confidence for staging being accurate were measured for both CT techniques. A McNemar test was used to determine the statistical significance between the neck CT and the dedicated laryngeal CT protocol.
Results
Diagnostic Accuracy: Standard Neck CT versus Larynx CT
The accuracies (defined as the correct rate between CT staging and clinicopathologic staging) for the 2 readers were 0.61 and 0.59 for the standard neck CT and 0.59 and 0.48 for the larynx protocol (standard neck CT in combination with the larynx-specific CT scans). Despite the additional information that was available from the laryngeal CT protocol, the accuracy of staging slightly decreased for both readers, compared with readings of the standard neck CT. The McNemar test showed no statistically significant difference in either reader. Subgroup analysis based on the primary site showed that the larynx protocol led to a 23% increase in correct staging for glottic cancers. However, the staging with laryngeal CT decreased the accuracy of staging for supraglottic laryngeal cancer by 31% for reader B (Table 1).
Table 1:
Accuracy of the radiologists' interpretationsa
| Reader A |
Reader B |
Averaged |
||||
|---|---|---|---|---|---|---|
| Standard Neck CT | Larynx Protocol | Standard Neck CT | Larynx Protocol | Standard Neck CT | Larynx Protocol | |
| Accuracy (total), n = 27 | 0.63 (17) | 0.59 (16) | 0.59 (16) | 0.48 (13) | 0.61 | 0.54 |
| Supraglottis, n = 16 | 0.56 (9) | 0.41 (7) | 0.75 (12) | 0.44 (7) | 0.65 | 0.44 |
| Glottis, n = 9 | 0.67 (6) | 0.89 (8) | 0.33 (3) | 0.56 (5) | 0.50 | 0.73 |
Accuracy is expressed as a fraction of the number of cases that were assigned a TNM-stage that matched the accepted TNM-stage for that case.. A total for each reader is shown, as is a breakdown for the cases of primary supraglottic tumors and glottic tumors. The total number of patients with subglottic and hypopharyngeal cancers (1 of each) were included in the overall analysis but were excluded in the subgroup analysis because of the small number of such cases.
Examination of only those cases in which patients underwent surgery showed an accuracy very similar to the total data (Table 2). This subset analysis suggests no significant difference in accuracy of staging between those patients who underwent surgery and those who did not. Therefore, cases both with surgical pathologic confirmation and those with only clinical and radiographic staging were included.
Table 2:
Accuracy for patients who had surgerya
| Surgical Pathology | Reader A |
Reader B |
Averaged |
|||
|---|---|---|---|---|---|---|
| Standard Neck CT | Larynx Protocol | Standard Neck CT | Larynx Protocol | Standard Neck CT | Larynx Protocol | |
| Accuracy (total), n = 17 | 0.65 (11) | 0.71 (12) | 0.35 (6) | 0.35 (6) | 0.50 | 0.53 |
| Supraglottis, n = 7 | 0.57 (4) | 0.57 (4) | 0.71 (5) | 0.14 (1) | 0.64 | 0.36 |
| Glottis, n = 9 | 0.67 (6) | 0.89 (8) | 0.00 (0) | 0.44 (4) | 0.34 | 0.67 |
The subset of patients who had surgery and thus had surgical pathology information was analyzed separately. As in Table 1, the accuracy is expressed as a total and individually for supraglottic cases and glottic cases.
Confidence Level
The confidence levels of each reader were found to be similar for each type of scan. The confidence level was lower in the larynx protocol for reader A (4.4 for the standard neck CT and 4.0 for the larynx protocol) and remained unchanged for reader B (3.8) (Table 3).
Table 3:
Radiologists' self-reported levels of confidencea
| Confidence | Standard Neck CT | Neck CT with Larynx Protocol |
|---|---|---|
| Reader A | 4.4 ± 0.69 | 4.0 ± 0.54 |
| Reader B | 3.8 ± 1.0 | 3.8 ± 1.0 |
The average confidence level for the standard CT and the standard CT with larynx protocol for each reader is shown. Range includes least confident (1) to most confident (5).
Over- and Understaging of Tumors
Overall analysis of the data showed that the rate at which both readers over- and understaged tumors changed very little between the standard neck CT and the larynx protocol (Table 4). There were only 2 cases of sublevel discrepancy in our study. The first case was a T4a supraglottic cancer. Reader A designated the standard neck CT as T4b but correctly staged it as T4a on the laryngeal CT protocol. The second case was a T4a glottic cancer. Reader A staged it as T4b on the standard neck CT but redesignated this case as a T3 after evaluating the laryngeal CT protocol. Most interesting, reader A had a tendency to overstage supraglottic cancer, and reader B had a tendency to understage glottic cancer, regardless of imaging techniques.
Table 4:
Percentage of cases over- and understageda
| Reader A |
Reader B |
|||
|---|---|---|---|---|
| Standard Neck CT | Larynx Protocol | Standard Neck CT | Larynx Protocol | |
| Overstage (total), n = 27 | 0.30 (8) | 0.33 (9) | 0.07 (2) | 0.04 (1) |
| Understage (total), n = 27 | 0.07 (2) | 0.07 (2) | 0.33 (9) | 0.48 (13) |
The number of over- and understaged cases are expressed here as a fraction of the total number of cases for each reader. Reader A tends to overstage and reader B tends to understage cancer, regardless of imaging technique.
High-versus-Low-Stage Tumors
The readers' ability to differentiate a lesion with an advanced TNM-stage (T3 and T4) from that of an early TNM-stage (T1 and T2) by using the standard neck CT versus the laryngeal CT protocol was assessed. The accuracy of differentiating advanced from early staging increased slightly for reader A (88% for neck CT and 92% for laryngeal CT) and decreased slightly for reader B (86% for neck CT and 68% for laryngeal CT protocol) (Table 5).
Table 5:
Accuracy for identification of advanced vs. early stagesa
| Standard Neck CT | Larynx CT Protocol | |
|---|---|---|
| Reader A, n = 25 | 0.88 (22) | 0.92 (23) |
| Supraglottic, n = 16 | 0.81 (13) | 0.88 (14) |
| Glottic, n = 9 | 1.0 (9) | 1.0 (9) |
| Reader B, n = 25 | 0.84 (21) | 0.68 (17) |
| Supraglottic, n = 16 | 0.81 (13) | 0.63 (10) |
| Glottic, n = 9 | 0.89 (8) | 0.78 (7) |
| Average, n = 25 | 0.86 | 0.80 |
The accuracy of our radiologists in distinguishing a high-stage lesion (T3 or T4) from a low-stage lesion (T1 or T2) for supraglottic and glottic tumors is shown. The accuracy is given as a fraction of the total number of cases that were correctly assigned a high or low stage.
Discussion
The overall accuracy of TNM-staging for laryngeal cancer did not improve with additional laryngeal CT. However subset analysis showed that the accuracy of TNM-staging was improved for glottic cancer, in which vocal cord mobility is a key factor for its staging. The staging accuracy decreased, however, for supraglottic laryngeal cancer. If the vocal cords did not appear involved on neck CT and did not adduct on larynx breath-holding, it was uncertain if this indicated vocal cord fixation or noncompliance by the patient. The discrepancy between 2 sets of imaging studies might have adversely affected the radiologist's confidence.
The accuracy of TNM-staging for laryngeal cancer was assigned solely on the basis of CT without any clinical information in this research study. This is not the standard of practice because cancer staging should be done with a combination of imaging and laryngoscopic findings. Mucosal involvement of laryngeal cancer and vocal cord mobility are best addressed by laryngoscopy. Cross-sectional imaging, such as CT or MR imaging, is used to determine deep tissue extension of primary tumor and nodal staging.1–4 In our preliminary study, there was no benefit in demonstrating vocal cord mobility by using CT. Current 64-channel multidetector CT scanning technology with isovoxel sagittal and coronal reformatted images, the standard neck CT with 1- to 2-mm reformatted images, appears to suffice for staging of laryngeal cancer.
The accuracy of laryngeal cancer staging by using a combination of clinical examination and CT has been reported to be between 73% and 88% compared with 55% and 64% by clinical examination alone.5
Agada et al6 reported the accuracy of CT staging compared with pathologic specimens, demonstrating that 45% of patients were overstaged and 10% were understaged by CT. Other studies have examined the potential benefits of smaller sections, by using coronal/sagittal and 3D reconstructions and having patients do a variety of breathing maneuvers, including quiet breathing, breath-holding, and modified Valsalva maneuvers.7 Results in these studies varied, but most found that reconstructions are helpful and that breathing maneuvers improve imaging quality. Keberle et al8 found that coronal reformats led to a better visualization of tumor extension in 33% of patients and influenced the treatment decisions in 19% of patients.8 Lell et al9 found that CT imaging with the patient phonating correctly identified all of the patients in their study with vocal cord fixation and that E phonation was better than the Valsalva maneuver. Another study by Stadler et al,10 comparing nonfunctional CT (during quiet breathing) and functional CT (including Valsalva and E-phonation maneuver), reported that functional CT was more accurate than nonfunctional CT for TNM-staging of laryngeal and hypopharyngeal cancers. Keberle et al7 also noted that image sections obtained at 1.25 mm were no more beneficial than those obtained at 3 mm.
The drawbacks to the laryngeal CT protocol in our study include additional radiation exposure and time requirements. A new report released by the National Council on Radiation Protection and Measurements of Bethesda, Maryland, indicated that the US population's exposure to ionizing radiation from medical procedures grew more than 7-fold between the early 1980s and 2006—an increase largely attributable to the rapid growth in the use of CT.11 As radiologists, we should be conscientious in setting CT protocol for the maximal diagnostic benefits with minimum radiation dose. Another drawback is added time to complete the study, because the dedicated laryngeal CT requires changing the angle of the CT gantry, setting up the upper and lower limits of scanning range, instructing patients regarding the breathing maneuver, and changing the FOV. All of these steps take approximately 5 minutes, added to the time required for the radiologists to interpret the additional images.
Challenges of dedicated larynx protocol are inherent in scanning during specific maneuvers requiring patient compliance. When both true vocal cords showed no adduction in the images acquired during breath-holding, it was unclear whether the cords were fixed or whether the patient did not follow the instructions. Some patients with large tumors might have had difficulty holding their breath. The assessment of vocal cord mobility could be done more efficiently and reliably with a clinical mirror examination or endoscopy than with CT. Even though vocal cord mobility is one of the important TNM-staging criteria by the AJCC classification,12 involvement of other anatomic structures often determines TNM-staging, such as pre-epiglottic space, cartilaginous destruction, or extralaryngeal spread. These are the areas in which cross-sectional imaging plays a major role in identifying the tumor spread, which may not be readily accessed by laryngoscope or mirror examinations.13–15 Coronal and sagittal reformatted images provide an accurate and reliable assessment of craniocaudal extension of disease, which is often limited by the axial image acquisition only, even with additional maneuvers. In the era of isovoxel multidetector CT technology, the staging of laryngeal cancer should be performed with contrast-enhanced neck CT with thin-section coronal and sagittal reformation.
One of the limitations of this study is that not all patients underwent surgical resection. The standard references were not based on pathologic staging for all patients. Given the widespread organ-preservation treatment, if we had limited subjects to only those who underwent a surgical resection, the subjects in this study would have been limited to those with early-stage laryngeal cancer. The subgroup analysis for those patients who underwent surgery revealed comparable results, with overall accuracy including nonsurgical patients.
Conclusions
The larynx protocol CT may have some diagnostic benefit for glottic tumors; however, the additional images did not improve overall staging accuracy or the reader's confidence level in this study. Considering the additional radiation exposure to the patient and the additional time required for the larynx protocol, a standard contrast-enhanced neck CT with multiplanar reformation and a clinical examination by an otolaryngologist suffice for the staging of laryngeal cancer.
Acknowledgments
We thank Larry F. Hughes, PhD, from the Division of Otolaryngology Head and Neck Surgery, Southern Illinois University, for his assistance with the statistical analysis.
Abbreviations
- AJCC
American Joint Committee on Cancer
- IV
intravenous
- kV(p)
kilovolt (peak)
- NCI
National Cancer Institute
- TNM-staging
tumor-node-metastasis staging
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 31–June 5, 2008; New Orleans, LA.
References
- 1. Curtin HD. Imaging of the larynx: current concepts. Radiology 1989;173:1–11 [DOI] [PubMed] [Google Scholar]
- 2. Mancuso AA, Hanafee WN. A comparative evaluation of computed tomography and laryngography. Radiology 1979;133:131–38 [DOI] [PubMed] [Google Scholar]
- 3. Greene FL. AJCC Cancer Staging Manual, 6th ed. Springer; 2002. [Google Scholar]
- 4. Mancuso AA, Hanafee WN, Juillard GJ, et al. The role of computed tomography in the management of cancer of the larynx. Radiology 1977;124:243–44 [DOI] [PubMed] [Google Scholar]
- 5. Keberle M, Kenn W, Hahn D. Current concepts in imaging of laryngeal and hypopharyngeal cancer. Eur Radiol 2002;12:1672–83 [DOI] [PubMed] [Google Scholar]
- 6. Agada FO, Nix PA, Salvage D, et al. Computerised tomography vs. pathological staging of laryngeal cancer: a 6-year completed audit cycle. Int J Clin Pract 2004;58:714–16 [DOI] [PubMed] [Google Scholar]
- 7. Wang SG, Seo CJ, Chon KM, et al. Clinical usefulness of 3-dimensional computed tomography laryngography in laryngeal and hypopharyngeal cancer. Am J Otolaryngol 2005;26:314–23 [DOI] [PubMed] [Google Scholar]
- 8. Keberle M, Sandstede J, Hoppe F, et al. Diagnostic impact of multiplanar reformations in multi-slice CT of laryngeal and hypopharyngeal carcinomas [in German]. Rofo 2003;175:1079–85 [DOI] [PubMed] [Google Scholar]
- 9. Lell MM, Greess H, Hothorn T, et al. Multiplanar functional imaging of the larynx and hypopharynx with multislice spiral CT. Eur Radiol 2004;14:2198–205 [DOI] [PubMed] [Google Scholar]
- 10. Stadler A, Kontrus M, Kornfehl J, et al. Tumor staging of laryngeal and hypopharyngeal carcinomas with functional spiral CT: comparison with nonfunctional CT, histopathology, and microlaryngoscopy. J Comput Assist Tomogr 2002;26:279–84 [DOI] [PubMed] [Google Scholar]
- 11. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States. National Council on Radiation Protection and Measurements; 2009. Available at: http://www.ncrppublications.org/Reports/160 [DOI] [PubMed] [Google Scholar]
- 12. Greene F, Compton C, Fritz A. AJCC Cancer Staging Atlas. Berlin: Springer-Verlag; 2006. [Google Scholar]
- 13. Becker M, Zbaren P, Laeng H, et al. Neoplastic invasion of the laryngeal cartilage: comparison of MR imaging and CT with histopathologic correlation. Radiology 1995;194:661–69 [DOI] [PubMed] [Google Scholar]
- 14. Curtin HD. Importance of imaging demonstration of neoplastic invasion of laryngeal cartilage. Radiology 1995;194:643–44 [DOI] [PubMed] [Google Scholar]
- 15. Castelijns JA, Gerritsen GJ, Kaiser MC, et al. Invasion of laryngeal cartilage by cancer: comparison of CT and MR imaging. Radiology 1988;167:199–206 [DOI] [PubMed] [Google Scholar]