SUMMARY:
Bevacizumab is a humanized monoclonal antibody that was the first FDA approved therapy designed to inhibit angiogenesis. This paper will review the mechanism of action and clinical role of this antiangiogenic agent.
Bevacizumab (Avastin, Genentech/Roche, South San Francisco, California) was the first US Food and Drug Administration−approved therapy designed to inhibit angiogenesis.1,2 In 2009, bevacizumab was approved for recurrent glioblastoma, and its use in early tumors is undergoing clinical trials. Before that, it had been used for treatment of various metastases. In 1989, a team of scientists isolated the human VEGF proteins, which are believed to be some of the most potent causes of angiogenesis. The oxygen and nutrient requirements of rapidly proliferating tumor cells are thought to cause the release of a hypoxia inducible factor, which in turn leads to the production of VEGF. These proteins are involved in increasing vascular permeability, inducing angiogenesis and vasculogenesis, promoting endothelial cell growth and migration, and precluding apoptosis. Because sustained angiogenesis is a hallmark of many cancers, arresting it is critical.1–4
Proposed Mechanism of Action
Bevacizumab is a humanized monoclonal antibody (initially it came from the mouse) that targets VEGF-A, an isoform of VEGF that stimulates endothelial cell proliferation and subsequent migration.2 Bevacizumab specifically binds to the VEGF-A protein, thereby inhibiting the process of angiogenesis (Fig 1). Studies have shown that anti-VEGF agents result in regression of existing microvessels, normalization of surviving mature vasculature, and inhibition of vessel growth and neovascularization. Maintaining the VEGF ligand inhibition may prevent tumor growth and may result in tumor shrinkage with time.1–4
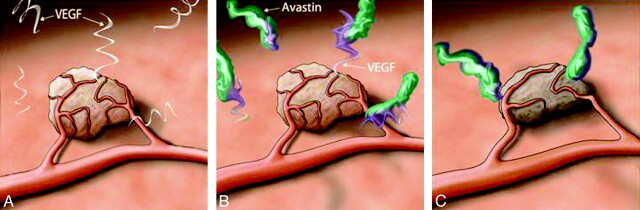
Fig 1.
Schematic Illustration of the mechanism of bevacizumab. A, There is a hypervascular tumor surrounded with VEGF protein. B, The bevacizumab compound binds to the free VEGF and reduces the concentration of the free VEGF. C, The reduction of available VEGF results in diminished blood supply to the tumor and tumor shrinkage.
Clinical Indications
Bevacizumab has been approved for the following clinical situations: metastatic colorectal cancer, nonsquamous cell lung cancer, metastatic breast cancer, metastatic renal cell cancer, prostate cancer, and glioblastoma (Fig 2).5–12
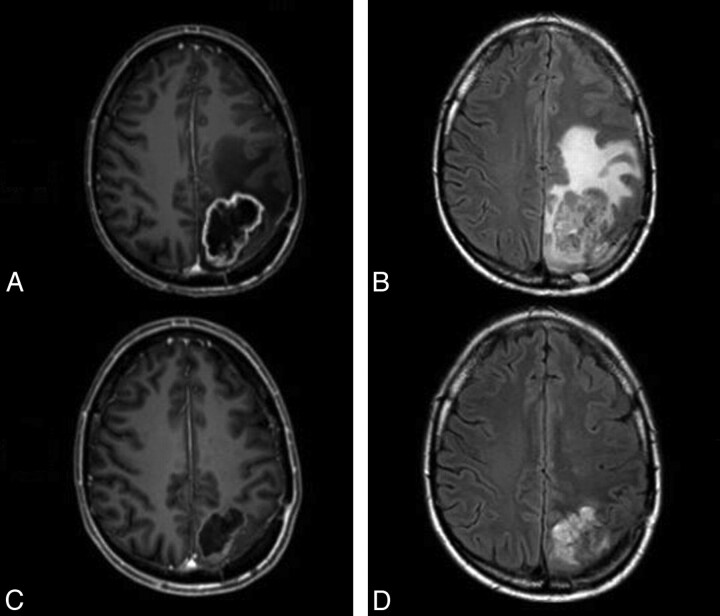
Fig 2.
Imaging findings associated with successful treatment with bevacizumab. A and B, Postcontrast T1-weighted (A) and fluid-attenuated inversion recovery sequences (B) show a ring-enhancing mass associated with vasogenic edema and mass effect. C and D, Following successful treatment, there is a reduction in the size of the mass and enhancement (C) and a substantial reduction in vasogenic edema (D).
Administration and Effects
Bevacizumab is a prescription-only drug administered intravenously. Its half-life is 20 days, and its metabolism route is not clear. Neurologic-related side effects include hypertension, which may lead to posterior reversible encephalopathy syndrome; hemorrhage; and nasal septum perforation.3
Economic Issues
Bevacizumab is very expensive and may not be covered by insurance. In countries with public health systems such as Canada and the United Kingdom, insurance coverage is limited. Sales of Avastin totaled 2.7 billion US dollars in 2007.4 Treatment cost per patient may be up to 100,000.00 US dollars per year, though this cost may be smaller in patients with recurrent glioblastoma due to their limited survival period.
Clinical Issues
Many of the issues described in the previous section arise because bevacizumab does not cure the underlying tumor but only extends the life span.
Abbreviations
- FDA
U.S. Food and Drug Administration
- VEGF
vascular endothelial growth factor
References
- 1. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581–611 [DOI] [PubMed] [Google Scholar]
- 2. Genentech, Inc. United States Securities and Exchange Commission. http://www.sec.gov/Archives/edgar/data/318771/000031877109000003/form10-k_2008.htm. Accessed October 6, 2009
- 3. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27 [DOI] [PubMed] [Google Scholar]
- 4. Bevacizumab. Wikipedia. http://en.wikipedia.org/wiki/Bevacizumab. Accessed October, 6, 2009
- 5. Daniele G, Marciano R, Tortora G. The role of bevacizumab in breast cancer. Eur J Cancer 2008;6(suppl):26–29 [Google Scholar]
- 6. Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol 2004;14:123–30 [DOI] [PubMed] [Google Scholar]
- 7. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997;57:4593–99 [PubMed] [Google Scholar]
- 8. Yuan F, Chen Y, Dellian M, et al.. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A 1996;93:14765–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671–80 [PubMed] [Google Scholar]
- 11. Borgström P, Hillan KJ, Sriramarao P, et al. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res 1996;56:4032–39 [PubMed] [Google Scholar]
- 12. Borgström P, Bourdon MA, Hillan KJ, et al. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate 1998;35:1–10 [DOI] [PubMed] [Google Scholar]