Abstract
BACKGROUND AND PURPOSE:
The efficacy of the endovascular treatment in providing stable occlusion of intracranial aneurysms is still controversial and should be precisely analyzed. A first step is to carefully study immediate anatomical results. CLARITY (Clinical and Anatomical Results in the Treatment of Ruptured Intracranial Aneurysms) is a prospective multicenter consecutive series including patients treated by coiling for ruptured aneurysms. Immediate anatomic results are presented.
MATERIALS AND METHODS:
Postoperative anatomic results were evaluated by DSA by the treating physician and anonymously and independently by 2 experienced neuroradiologists by using the 3-point Montreal Scale. Patients were divided into 2 groups: patients treated with GDC and those treated with Matrix detachable coils.
RESULTS:
A total of 773 patients (461 women, 312 men; 19–80 years of age; mean, 51.2 ± 13.2 years) with 773 ruptured aneurysms were included in the study. The rate of occlusion as determined by the treating physician was designated complete for 586 aneurysms (75.8%), neck remnant for 145 aneurysms (18.8%), and aneurysm remnant for 42 aneurysms (5.4%). The same evaluation as reported by the 2 independent reviewers was complete occlusion for 366 aneurysms (47.4%), neck remnant for 324 aneurysms (41.9%), and aneurysm remnant for 83 aneurysms (10.7%). Postoperative anatomic results were significantly linked to age but not to the technique of endovascular treatment or aneurysm characteristics (location, size, dome-to neck ratio). Results were not significantly different between the GDC and Matrix group.
CONCLUSIONS:
Endovascular treatment of ruptured intracranial aneurysms resulted in a high rate of satisfactory occlusion (complete occlusion and neck remnant in 89.3%). Patient age was the only factor associated with the rate of occlusion. The rate of aneurysm occlusion differed insignificantly between GDC and Matrix coils.
CLARITY is a prospective multicenter series con-ducted in France from November 2006 to September 2008 to evaluate the clinical and anatomic results after endovascular treatment of ruptured intracranial aneurysms by using GDC (Boston Scientific Neurovascular, Fremont, California) or Matrix detachable coils (Boston Scientific Neurovascular) (2 groups).
Endovascular treatment of ruptured aneurysms has been widely used after the results of the ISAT study, but some controversy remains regarding the efficacy of this treatment in obtaining stable anatomic results.1 For this reason, the precise analysis of immediate, midterm, and long-term anatomic results is mandatory. The present article focuses on immediate postoperative anatomic results.
Materials and Methods
Protocol
CLARITY is a prospective multicenter consecutive series that was conducted in 20 French centers. Inclusion criteria were consecutive patients, 18–80 years of age, with aneurysms <15 mm in maximal diameter and a diagnosed rupture having occurred fewer than 7 days before. Exclusion criteria included dissecting or fusiform aneurysms, aneurysms associated with a brain arteriovenous malformation, aneurysms already treated by a clip or coils, and patients previously treated for another aneurysm. The initial CLARITY series (conducted between November 3, 2006, and June 29, 2007) involved patients treated with GDC coils (CLARITY-GDC). In the second CLARITY series (conducted between April 23, 2007, and September 5, 2008), patients were treated with Matrix detachable coils (CLARITY Matrix).
Immediate Postoperative Imaging
Immediate postoperative anatomic evaluation was obtained at the end of the endovascular treatment by using DSA. Anatomic evaluation was performed with nonsubtracted and subtracted images in frontal, lateral, and working views. 3D images were not required. Anonymous images were collected through the Web-based data base that was also used for clinical data collection by Kika Medical, Nancy, France.
Image Analysis
We used the Modified Montreal Scale, which classifies the degree of aneurysmal occlusion into 3 groups: complete occlusion, neck remnant, and aneurysm remnant.2 Postoperative anatomic results were first evaluated by the performing physician. Anatomic results were then anonymously and independently reviewed by 2 experienced neuroradiologists (F.R. and R.A.) who were blinded to all clinical information. Discrepancies were resolved by consensus. Another scale (the CLARITY scale) was used by the core lab but will be presented and analyzed in another article.
Statistical Analysis
Data management and statistical analyses were independently conducted by Kika Medical to determine patient demographics, aneurysm characteristics, anatomic results according to the Montreal and CLARITY scales and to analyze anatomic results in relation to the demographic and anatomic parameters. Mean and frequency comparisons were performed with the Student t test and the χ2 test or the Fisher exact test, respectively. Differences were considered significant at P = .05. Statistical analysis was performed with SPSS, Version 15.0 (SPSS, Chicago, Illinois).
Results
Patient Population, Aneurysm Characteristics, and Modalities of Treatment
The initial population in the CLARITY-GDC series was 405 patients. Endovascular treatment failed in 3 patients, and they were subsequently excluded from the analysis. Another patient had no immediate postoperative DSA control due to the breakdown of the angiographic system. The initial population in the CLARITY-Matrix series was 377 patients. Endovascular treatment failed in 2 patients, and they were excluded from the analysis. In 3 patients, immediate postoperative DSA control was not available or readable. Therefore, analysis of immediate postoperative anatomic results was conducted in a population of 773 patients (401 treated with GDC coils and 372 treated with Matrix coils) including 461 women (59.6%) and 312 men (40.4%), 19–80 years of age (mean, 51.2 ± 13.2 years). Age was <65 years in 650 patients (84.1%) and ≥65 years in 123 patients (15.9%). The World Federation of Neurosurgical Societies grade at admission was 1 in 358 patients (46.3%), 2 in 167 patients (21.6%), 3 in 27 patients (3.5%), 4 in 116 patients (15.0%), and 5 in 105 patients (13.6%). There was no significant difference between the GDC and Matrix groups for demographic characteristics, except for sex (women: 56.1% in the GDC group and 63.4% in the Matrix group; P = .038).
Aneurysm location included the ICA in 210 patients (27.2%), the ACA/AcomA in 391 patients (50.6%), the MCA in 105 patients (13.6%), and the VB in 67 patients (8.7%). Most aneurysms (448 aneurysms, 58.0%) measured ≤6 mm. Dome-to-neck ratio was ≤1.5 in 322 aneurysms (41.7%) and >1.5 in 451 cases (58.3%). There was no significant difference in the anatomic characteristics between the GDC and Matrix groups.
Endovascular coiling without the use of an adjunctive device was performed in 601 aneurysms (77.8%). The balloon remodeling technique was used in 158 (20.4%), and intracranial stent placement was performed in 14 (1.8%). Stent placement was more frequently used in the Matrix group compared with the GDC group (respectively 3.2% and 0.5%, P = .014). The difference is probably the result of the Matrix cases being performed later than the platinum cases, at a time when stent use was more widespread.
Anatomic Evaluation Comparisons
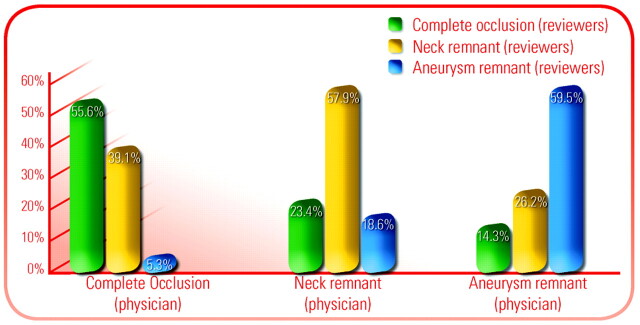
Occlusion rates as reported by the performing physician were complete in 586 aneurysms (75.8%), neck remnant in 145 aneurysms (18.8%), and aneurysm remnant in 42 aneurysms (5.4%). Occlusion rates as determined by the 2 independent reviewers (consensus) were complete in 366 aneurysms (47.4%), neck remnant in 324 aneurysms (41.9%), and aneurysm remnant in 83 aneurysms (10.7%). The interobserver agreement between the physician and the reviewers (Montreal Scale) was low (κ = 0.395) and is shown in Fig 1. The interobserver agreement between the 2 reviewers of the core lab was good (κ = 0.905).
Fig 1.
Graph shows the comparison of anatomic results as evaluated by the treating physician and the core lab.
Analysis of Immediate Anatomic Results in Correlation with Demographic, Technical, and Anatomic Factors
Anatomic results are shown in correlation with age (Table 1), technique of endovascular treatment (Table 2), aneurysm location (Table 3), aneurysm size (Table 4), and dome-to-neck ratio (Table 5). Only age was significantly linked to the quality of postoperative aneurysm occlusion.
Table 1:
Anatomic results (Montreal Scale, reviewers) in relation to patient's agea
| <65 years |
≥65 years |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete occlusion | 318 | 48.9 | 48 | 39.0 |
| Neck remnant | 270 | 41.5 | 54 | 43.9 |
| Aneurysm remnant | 62 | 9.5 | 21 | 17.1 |
| Total | 650 | 100.0 | 123 | 100.0 |
P= .021.
Table 2:
Anatomic results (Montreal Scale, reviewers) in relation to the treatment techniquea
| Coiling |
Remodeling |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete occlusion | 282 | 46.9 | 79 | 50.0 |
| Neck remnant | 250 | 41.6 | 71 | 44.9 |
| Aneurysm remnant | 69 | 11.5 | 8 | 5.1 |
| Total | 601 | 100.0 | 158 | 100.0 |
The 14 patients treated by stenting were excluded from analysis. P = .059.
Table 3:
Anatomic results (Montreal Scale, reviewers) in relation to aneurysm locationa
| ACA/AcomA |
ICA |
MCA |
VB |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Complete occlusion | 190 | 48.6 | 87 | 41.4 | 48 | 45.7 | 41 | 61.2 |
| Neck remnant | 163 | 41.7 | 100 | 47.6 | 41 | 39.1 | 20 | 29.9 |
| Aneurysm remnant | 38 | 9.7 | 23 | 11.0 | 16 | 15.2 | 6 | 9.0 |
| Total | 391 | 100.0 | 210 | 100.0 | 105 | 100.0 | 67 | 100.0 |
P = .083.
Table 4:
Anatomic results (Montreal Scale, reviewers) in relation to aneurysm sizea
| ≤6 mm |
>6 mm |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete occlusion | 220 | 49.1 | 146 | 44.9 |
| Neck remnant | 179 | 40.0 | 145 | 44.6 |
| Aneurysm remnant | 49 | 10.9 | 34 | 10.5 |
| Total | 448 | 100.0 | 325 | 100.0 |
P = .425.
Table 5:
Anatomic results (Montreal Scale, reviewers) in relation to dome-to-neck ratioa
| ≤1.5 |
>1.5 |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete occlusion | 153 | 47.5 | 213 | 47.2 |
| Neck remnant | 128 | 39.8 | 196 | 43.5 |
| Aneurysm remnant | 41 | 12.7 | 42 | 9.3 |
| Total | 322 | 100.0 | 451 | 100.0 |
P =.264.
Comparison of Immediate Anatomic Results in GDC and Matrix groups
Anatomic results in both GDC and Matrix groups are shown in Table 6 and are not significantly different.
Table 6:
Anatomic results (Montreal Scale, reviewers) in GDC and Matrix groupsa
| GDC |
Matrix |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete occlusion | 197 | 49.1 | 169 | 45.4 |
| Neck remnant | 155 | 38.7 | 169 | 45.4 |
| Aneurysm remnant | 49 | 12.2 | 34 | 9.1 |
| Total | 401 | 100.0 | 372 | 100.0 |
P = .112.
Discussion
The ISAT showed that in patients with ruptured aneurysms suitable for neurosurgical clipping and endovascular coiling, endovascular coiling was more likely to result in independent survival at 1 year (absolute risk reduction of 7.4%).1 However, as outlined in the ISAT publication, a crucial issue for endovascular coiling is the uncertainty about the long-term durability of aneurysm occlusion and protection from further aneurysm rupture.
The CARAT study was conducted in a cohort of 1010 patients treated with coil embolization or surgical clipping at 9 hospitals in 8 high-volume centers in the United States and showed that the risk of rerupture tended to be greater after coil embolization compared with surgical clipping.3 The degree of aneurysm occlusion was strongly associated with the risk of rerupture (cumulative risk: 1.1% for complete occlusion, 2.9% for 91%–99% occlusion, 5.9% for 70%–90% occlusion, and 17.6% for occlusion <70%). In fact, as was demonstrated in both ISAT and CARAT, the degree of occlusion was different in the coiling and clipping groups.1,3 In ISAT, angiographic occlusion was complete in 66% of aneurysms treated by coiling and in 82% of aneurysms treated by clipping; subtotal in 26% and 12%, respectively; and incomplete in 8% and 6% respectively. Similar results were reported in the CARAT study: complete occlusion in 38.6% of the coiling group and 91.5% in the clipping group; a small residual neck in 43.4% and 6.4%, respectively; a residual neck in 13.9% and 1.4%, respectively; and a partial occlusion in 4.1% and 0.7%, respectively. As demonstrated in ISAT and CARAT, the difference in aneurysm occlusion in the coiling and clipping groups is partially explained by the fact that postoperative evaluation is not always performed by using DSA after surgery.
Therefore, the quality of aneurysm occlusion after endovascular treatment must be precisely evaluated by using the appropriate scale and an independent core lab.4 Most published series reporting anatomic results of endovascular treatment of ruptured and/or unruptured aneurysms do not include independent evaluation. In ISAT, the rate of occlusion was assessed by the local investigator,1 and in CARAT, aneurysmal occlusion was abstracted from procedural reports.3 Similarly in another large series published by Gallas et al,5 anatomic results were evaluated by the performing physician. In a recent series published by Holmin et al,6 which assessed long-term clinical follow-up in relation to morphologic treatment results, how the anatomic analysis was performed is not clearly indicated.
More recent published reports have shown that self-reported evaluations by the performing physician vary significantly from those performed by an independent core lab (or blinded reader), with a tendency toward better anatomic results reported by the treating physician. For example, in a series dealing with the endovascular treatment of intracranial aneurysms with Matrix coils, analysis performed by the treating neuroradiologist reported complete occlusion in 61.5% of aneurysms, neck remnant in 33.2% of aneurysms, and aneurysm remnant in 5.3% of aneurysms, whereas results from the independent core lab were complete occlusion in 44.0%, neck remnant in 25.0%, and aneurysm remnant in 31.0% of aneurysms.7
Anatomic results in the CLARITY series were evaluated by an independent core lab, which included 2 experienced neuroradiologists with a combined treatment experience of >10 years, to ensure precise analysis. Analysis was conducted by using the Montreal Scale, which is currently used in most endovascular published reports. A new scale was also used by the core lab (the CLARITY scale) but will be presented in another article.
As previously shown, the anatomic results in CLARITY are different when evaluated by the treating physician and the independent neuroradiologists.7 The rate of complete occlusion was reported higher by the treating neuroradiologist (75.8%) than by the core lab (47.3%). On the other hand, neck and aneurysm remnants (41.9% and 10.7%, respectively) were more frequently depicted by the core lab than by the treating neuroradiologist (18.8% and 5.4%, respectively). In fact, results are very heterogeneous with a low interobserver agreement between the performing physician and the core lab (κ = 0.395). For example, what was defined as a complete occlusion by the core lab was sometimes classified as a neck remnant or aneurysm remnant by the treating neuroradiologist (Fig 1). The interobserver agreement by the 2 independent neuroradiologists of the core lab was good (κ = 0.905).
The comparison of our series with other previously published large series is difficult due to the methodologic heterogeneity previously outlined (Table 7).1,5,8–11 Including our series, the rate of postoperative complete occlusion was between 49.1% and 72.6%. More important, aneurysm remnants were reported to be between 1.9% and 12.2%. The lower rate of complete occlusions and the higher rate of aneurysm remnants observed in our series are likely due to the methodology we used. Effectively, the evaluations reported by the treating physicians in our series (complete occlusion, 75.1%; neck remnant, 18.2%; aneurysm remnant, 6.7%) are very close to those reported in other large series in which anatomic evaluations were also self-reported. According to CARAT (see below), the risk of rebleeding is primarily a risk for aneurysm remnants (aneurysm occlusion <90%). Thus, accumulation of all complete occlusion and neck remnants resulted in an overall high rate of satisfactory occlusion (89.3% in CLARITY and 92.2%–98.1% in other series, Table 7). Comparison with surgical series is much more difficult because the postoperative evaluation is rarely performed by using DSA but visually during neurosurgery as was the case in ISAT and CARAT.
Table 7:
Anatomic outcome in the largest series dealing with the endovascular treatment of ruptured intracranial aneurysms
In the analysis of factors that may affect anatomic results, only age was significant (P = .021), as was previously reported in ATENA.4 In patients older than 65 years, the rate of complete occlusion was lower compared with that in younger patients (respectively 39.0% and 48.9%). On the other hand, aneurysm remnants were more frequent in patients older than 65 years (17.1%) compared with younger patients (9.5%); this outcome is likely due to less aggressive treatment in older patients to reduce the risk of complications. Anatomic results were not significantly affected by anatomic factors (aneurysm location, aneurysm size, dome-to-neck ratio). In ATENA, the quality of aneurysmal occlusion was linked to 2 anatomic factors: aneurysm size and dome-to-neck ratio. Finally, results were slightly better in those aneurysms in which the balloon remodelling technique was used compared with aneurysms treated by coiling alone (complete occlusion achieved in 50.0% and 46.9% of aneurysms, respectively; aneurysm remnants in 5.1% and 11.5% of aneurysms, respectively). Anatomic results were not significantly different according to the type of coils used (GDC or Matrix). The number of patients in this series may be too small to depict some clinical or morphologic factors that can affect immediate postoperative anatomic results.
Abbreviations
- ACA
anterior cerebral artery
- AcomA
anterior communicating artery
- ATENA
Analysis of Treatment by Endovascular Approach of Nonruptured Aneurysms
- CARAT
Cerebral Aneurysm Rerupture After Treatment
- CLARITY
Clinical and Anatomical Results in the Treatment of Ruptured Intracranial Aneurysms
- CH
Centre Hospitalier
- CHU
Centre Hospitalier Universitaire
- DSA
digital subtraction angiography
- GDC
Guglielmi detachable coils
- ICA
internal carotid artery
- ISAT
International Subarachnoid Aneurysm Trial
- MCA
middle cerebral artery
- VB
vertebrobasilar system
Appendix
Participating Centers and Investigators:
CHU Larrey, Angers, France; Anne Pasco
CHU Jean Minjoz, Besançon, France; Jean-François Bonneville
CHU Pellegrin, Bordeaux, France; Xavier Barreau, Jérôme Berge
CHU de la Côte de Nacre, Caen, France; Patrick Courthéoux, Suzana Saleme
CHU Gabriel Montpied, Clermont Ferrand, France; Emmanuel Chabert, Jean Gabrillargues
CHU Roger Salengro, Lille, France; Xavier Leclerc, Jean-Pierre Pruvo, Christian Taschner
CHU La Timone, Marseille, France; Olivier Lévrier
CHU Gui de Chauliac, Montpellier, France; Alain Bonafé
CHU G et R Laënnec, Nantes, France; Hubert Desal, Axel de Kersaint-Gilly
CHU La Pitié Salpêtrière, Paris, France; Alessandra Biondi, Fabrice Bonneville, Betty Jean, Nader Sourour
CH Fondation Rothschild, Paris, France; Jacques Moret, Charbel Mounayer, Michel Piotin, Laurent Spelle, Raphaël Blanc
CH Sainte-Anne, Paris, France; Sylvie Gordon-Hardy, Jean-François Meder, Denis Trystram
CHU de la Milétrie, Poitiers, France; Jacques Drouineau
CHU Maison Blanche, Reims, France; Sophie Gallas, Laurent Pierot
CHU Bellevue, Saint-Etienne, France; Fabrice-Guy Barral, Luis Manera
CHU Hautepierre, Strasbourg, France; Rémy Beaujeux, Fazel Boujan
CH Sainte-Anne, Toulon, France; Charles Arteaga
CHU Purpan, Toulouse, France; Christophe Cognard, Anne-Christine Januel, Philippe Tal
CHU Bretonneau, Tours, France; Denis Herbreteau, Richard Bibi
CHU Saint-Roch, Nice, France; Yves Chau, Jacques Sedat
Footnotes
This work was supported by Boston Scientific France.
References
- 1. Molyneux AJ, Kerr RS, Yu LM, et al. , for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 2. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 3. Johnston SC, Dowd CF, Higashida RT, et al. , for the CARAT investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysm: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke 2008;39:120–25 [DOI] [PubMed] [Google Scholar]
- 4. Pierot L, Spelle L, Vitry F, ATENA investigators. Immediate anatomical results after the endovascular treatment of unruptured intracranial aneurysms: analysis of the ATENA series. AJNR Am J Neuroradiol 2010;31:140–44 Epub 2009 Sep 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallas S, Pasco A, Cottier JP, et al. A multicenter study of ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2005;26:1723–31 [PMC free article] [PubMed] [Google Scholar]
- 6. Holmin S, Krings T, Ozanne A, et al. Intradural saccular aneurysms treated by Guglielmi detachable bare coils at a single institution between 1993 and 2005. Stroke 2008;39:2288–97 [DOI] [PubMed] [Google Scholar]
- 7. Pierot L, Leclerc X, Bonafé A, et al. , for the French Matrix Registry Investigators. Endovascular treatment of intracranial aneurysms with Matrix detachable coils: midterm anatomical follow-up from a prospective multicenter registry. AJNR Am J Neuroradiol 2008;29:57–61. Epub 2007 Oct 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 9. Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coils embolization of cerebral aneurysms: 11 years' experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 10. Niimi Y, Song J, Madrid M, et al. Endosaccular treatment of intracranial aneurysms using Matrix coils: early experience and midterm follow-up. Stroke 2006;37:1028–32 [DOI] [PubMed] [Google Scholar]
- 11. Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]