Abstract
BACKGROUND AND PURPOSE:
The pathophysiology of IIH remains unknown. TS stenoses have been observed in a high proportion of these patients. Stent placement to remove this potential obstruction to venous outflow has been proposed as a treatment option for patients with IIH refractory to medical treatment.
MATERIALS AND METHODS:
The clinical presentation, treatment, and outcome of patients with refractory IIH evaluated for venous sinus stent placement at a tertiary care center was retrospectively reviewed.
RESULTS:
Thirteen female patients with IIH were evaluated for sinovenous stent placement. Moderate sinus stenoses with normal intrasinus pressures were found in 3 patients and therefore stent placement was not performed. Ten patients had elevated intrasinus pressures (pressure gradient across stenosis, 11–50 mm Hg), which decreased following unilateral TS stent placement. Headaches improved or resolved in all stented patients. Papilledema resolved completely or almost completely in 8 patients and significantly improved in 2 patients. One patient developed optic atrophy. There were no major periprocedural complications.
CONCLUSIONS:
In this small case series, restoring the patency of stenotic venous sinuses with a stent in patients with refractory IIH resulted in symptomatic improvement in all treated patients. The safety and efficacy of this procedure should be evaluated in a randomized controlled study to determine its role within the armamentarium of therapeutic options for patients with IIH.
Idiopathic intracranial hypertension is a syndrome of raised intracranial pressure without an identifiable etiology most prevalent in, but not restricted to, young overweight females.1–4 Patients typically present with headaches, visual disturbances, and pulsatile tinnitus and are found to have papilledema on clinical examination. The major morbidity associated with IIH is insidious visual loss, which may be severe in up to 25% of patients.5,6 Up to 3% of patients develop a fulminant or malignant course with rapidly progressive visual loss.7–9 Treatments focus on normalization of intracranial pressure. Initial therapy usually consists of acetazolamide in conjunction with dietary counseling for weight loss. If visual impairment or headaches persist or progress despite maximally tolerated acetazolamide, alternate strategies are employed, including addition of furosemide, topiramate, or short-term corticosteroid therapy, serial lumbar punctures to remove CSF or CSF diversion procedures such as ventriculo- or lumboperitoneal shunt surgery, optic nerve sheath decompression, or subtemporal decompression. There are no evidence-based guidelines in the management of IIH, and randomized studies supporting treatment efficacy are lacking.10
The pathophysiology of raised intracranial pressure in IIH remains unclear.1–4 Various mechanisms have been proposed, including excess CSF production, impaired CSF absorption, or increased cerebral venous pressure. More recently it has been recognized that a high proportion of patients with IIH have uni- or bilateral TS stenosis (Fig 1).11,12 There is significant debate, however, about whether these sinovenous stenoses play a role in the pathophysiology of this condition or are simply a consequence of raised intracranial pressure.13–18 Restoring the patency of the stenotic venous sinus with stent placement can normalize intracranial pressure and significantly improve or render some patients asymptomatic.14,19–27 We report the clinical presentation and outcome of a series of patients with IIH considered for TS stent placement.
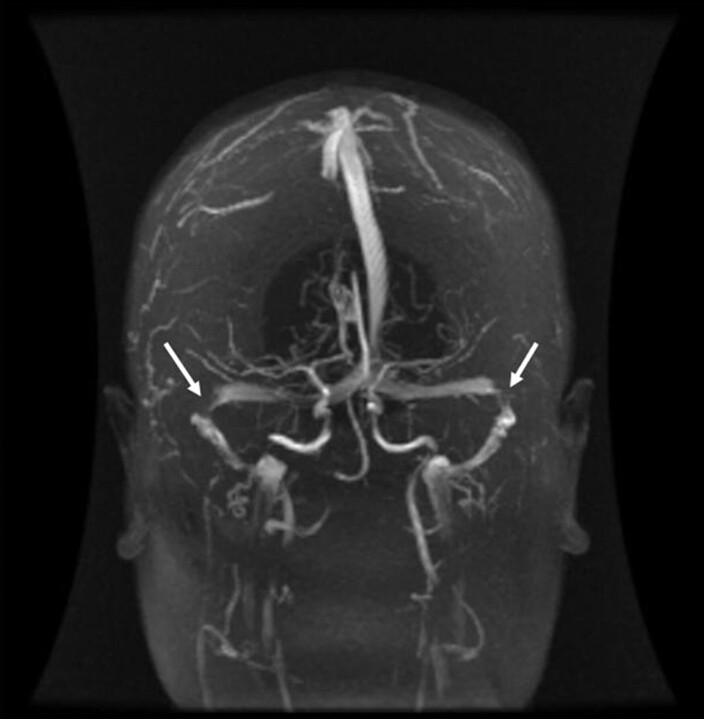
Fig 1.
Time-of-flight MR venogram of patient 9. Arrows indicate location of bilateral severe TS stenosis.
Materials and Methods
Patients
This study was approved by the Lawson Health Research Institute Clinical Research Impact and Ethics Committee. Thirteen patients with IIH referred to the interventional neuroradiology service at the London Health Sciences Centre for consideration of TS stent placement between 2001 and 2008 were retrospectively identified (on-line Table 1). Patients were referred if they had persistent or progressive optic neuropathy and/or intractable headaches despite maximally tolerated medical therapy as determined by a neuro-ophthalmologist (D.N. or A.P.) and/or neurologist. All patients fulfilled standard criteria for IIH.4,28,29
The patients were all female ranging from 16 to 65 years of age (median age, 34 years) with a mean body mass index of 35.9 kg/m2 (range, 27.2–47.4 kg/m2). All patients suffered from headaches, most had visual symptoms (10/13), and pulsatile tinnitus was present in 3 out of 13 patients. Symptom duration before the procedure ranged from 2 weeks to >15 years. Two patients (patients 7 and 12) presented with a rapidly progressive course and were considered for stent placement within 1 month of symptom onset. All but 1 patient (patient 6) had active papilledema, with moderate or marked swelling noted in 9 patients (69%). Visual fields were significantly affected in 4 patients (31%). A recent lumbar puncture documented elevated CSF pressure in all patients ranging from 25 to 50 cm of water.
MR imaging of the brain and time-of-flight venography were performed to exclude a mass lesion, hydrocephalus, or venous sinus thrombosis and to assess the TSs for stenoses. The severity of sinovenous stenoses was assessed by a neuroradiologist (D.P.) and graded according to the venous conduit score of Farb et al.11 Briefly, the grade was defined by the highest degree of stenosis on axial source images or maximum intensity projection images encountered from the torcula to the distal sigmoid sinus and given a corresponding number from 0 to 4. A score of 0 represented a discontinuity (gap) or aplastic segment; 1 represented hypoplasia or severe stenosis (>75% narrowing relative to the diameter of the lumen of the distal superior sagittal sinus); 2, moderate narrowing (50%–75%); 3, mild narrowing (25%–50%); and a score of 4 represented no or very mild narrowing (<25%). Unilateral moderate to severe TS stenosis was observed in 4 patients, and bilateral stenoses were seen in 9 patients. One patient had a TS stenosis and a contralateral severe stenosis at the sigmoid-internal jugular junction (patient 10).
Patients received maximally tolerated acetazolamide with daily doses ranging from 0.5 to 2 g. One patient (patient 2) received a course of steroids and another patient (patient 4) was concurrently taking topiramate. None of these patients had yet been treated with optic nerve sheath fenestration, shunt surgery, or subtemporal decompression. Three patients were receiving long-term anticoagulation due to a history of thrombophilia and deep venous thrombosis. Two of these patients had antiphospholipid antibodies (patients 2 and 12) and 1 harbored the factor V Leiden mutation (patient 1).
Manometry and Stent Placement
All patients were assessed by anesthesia for suitability to undergo general anesthesia. Enteric-coated acetylsalisylic acid 81 mg daily and clopidogrel 75 mg daily were prescribed for 5 days before the stent placement procedure. The 3 patients with a history of thrombophilia were anticoagulated before the procedure and received single agent antiplatelet therapy. The procedure was performed under general anesthesia with systemic anticoagulation by using intravenous heparin administered to maintain an activated clotting time of more than twice the normal level.
Femoral venous access was obtained and a guiding catheter positioned into the right or left jugular bulb. A high-flow microcatheter was navigated into the superior sagittal sinus with the support of a microwire. Venography was performed by selective contrast injections in the TSs through the microcatheter to confirm the presence and location of stenoses. Using a pressure transducer attached to the microcatheter referenced to 0 at the level of the midaxillary line, intrasinus pressure measurements (manometry) were obtained.
TS stent placement was performed only when a TS stenosis was associated with an abrupt pressure gradient of at least 10 mm Hg. This threshold pressure gradient was chosen because it significantly exceeds the normal gradual drop in intrasinus pressure of up to 5–6 mm Hg from the superior sagittal sinus to the internal jugular bulb.30,31 In patients with bilateral stenoses, the stenosis associated with the most significant gradient was selected for stent placement. After deployment of a balloon-expandable or self-expanding stent across the stenosis, sized to the normal adjacent sinus diameter, venography and manometry were repeated when possible. For illustration purposes, venograms obtained during the stent placement procedure for patient 9 are shown in Figs 2 and 3.
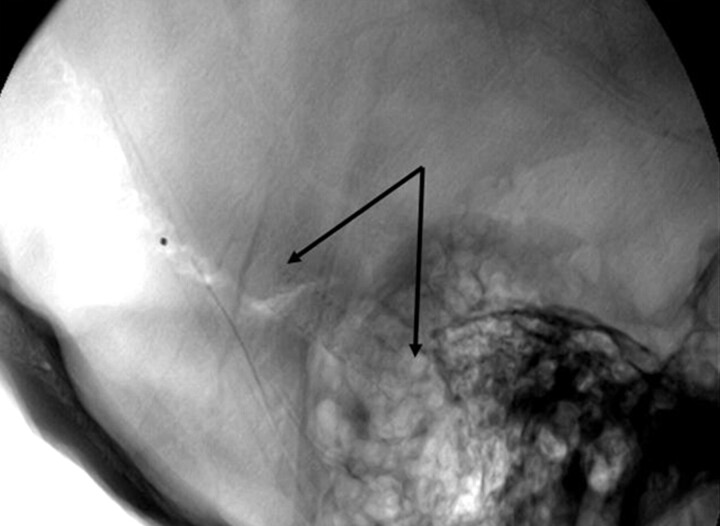
Fig 2.
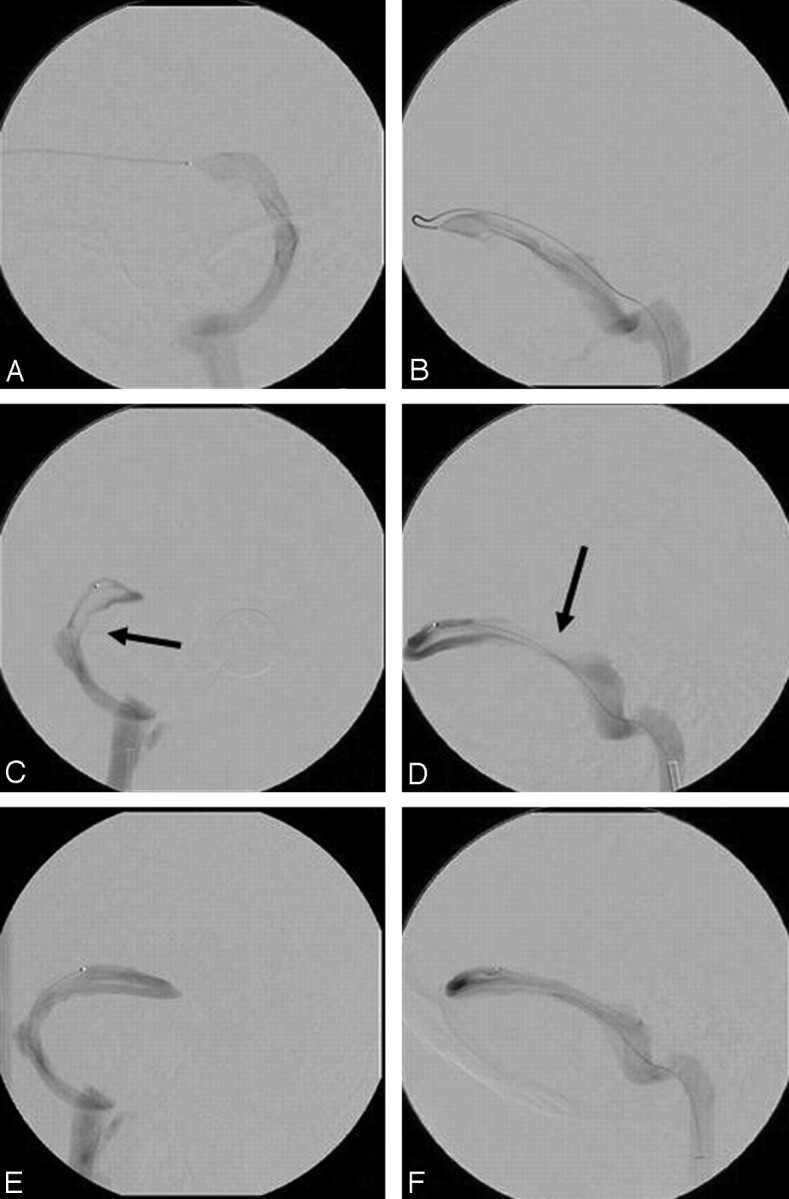
Venography, manometry, and right TS stent placement of patient 9. A, C, and E, Anteroposterior projection; B, D, and F, lateral projection. A and B represent venograms of the left TS, demonstrating no significant stenosis. Intrasinus manometry demonstrated a very gradual decline in pressure from 24 mmHg at the torcula to 13 mmHg at the sigmoid sinus. C and D represent venograms of the right TS with arrows indicating the stenosis. Intrasinus manometry demonstrated an abrupt pressure drop in the lateral TS at the stenosis from 25 to 14 mm Hg. E and F represent venography after stent placement of the right TS stenosis. Manometry no longer demonstrated a significant pressure drop with pressure of 17 to 15 mm Hg above and below the stent.
Fig 3.
Lateral skull x-ray of patient 9. Arrows outline the stent in the right TS.
Patients were carefully monitored overnight and discharged home the following day. Patients were maintained on dual antiplatelet therapy for 3 months after stent placement followed by clopidogrel discontinuation. Oral anticoagulation was maintained in the 3 patients with known thrombophilia. Follow-up venography to confirm stent patency was not performed routinely.
Ophthalmologic Assessment
Detailed ophthalmologic examinations were performed before and after stent placement. Papilledema was graded as not present, acute active, chronic active, or chronic gliotic and severity was graded according to the Frisén scale.32 Visual fields were assessed by manual kinetic Goldman perimetry and graded according to the scale of Shah et al.6 In brief, grade 0 represented no visual field defect. Grade 1 represented a nerve fiber layer type visual field defect with a normal 12e isopter encompassing the blind spot and the central visual field. Grade 2 represented constriction of the 12e isopter (without absence) that did not encompass the blind spot and central area completely, with total visual field >20°. Grade 3 represented absence of the 12e isopter in the central area with total central visual field >20°, and grade 4 a total visual field <20°.
Results
Transfemoral access to the intracranial sinuses for pressure measurements was successful in 12 of 13 patients (92%). In 1 patient (patient 13), the internal jugular veins could not be cannulated by using this approach and therefore a direct puncture of the right internal jugular vein was performed during the same procedure. A significant pressure gradient (>10 mm Hg) across stenoses in the TS was observed in 10 of 13 patients (Table 1). TS stent placement was performed in these 10 patients.
Table 1:
Outcome
| Pressure Gradient across Stenosis |
Transverse Sinus Stented (Left, Right, None) | Duration of Follow-Up (mo) | Time to Significant Improvement of Symptoms (mo) | Headachea | Papilledemaa: Frisén (0–5) | Visual Acuitya |
|||
|---|---|---|---|---|---|---|---|---|---|
| Before (mm Hg) | After (mm Hg) | Left | Right | ||||||
| 1 | >10 | Not recorded | Right | 32 | 1.5 | Improved | 1 | 20/30 | 20/40 |
| 2 | >10 | Not recorded | Left | 60 | 1.5 | Improved | 1 | 20/20 | 20/20 |
| 3 | 5 | n/a | None | 18 | 4 | Improved | 1 | 20/20 | 20/20 |
| 4 | 30 | 5 | Right | 19 | 0.5 | Improved | 0 | 20/25 | 20/20 |
| 5 | 50 | 23 | Right | 12 | Immediate | Improved | 1 | 20/20 | 20/20 |
| 6 | 16 | 5 | Right | 5 | Immediate | Improved | 1 | 20/25 | 20/25 |
| 7 | 36 | 12 | Right | 7 | Immediate | Resolved | 0 | 20/25 | 20/20 |
| 8 | 28 | 14 | Right | 4 | Immediate | Resolved | 1 | 20/20 | 20/20 |
| 9 | 11 | 2 | Right | 13 | 0.5 | Improved | 0 | 20/20 | 20/20 |
| 10 | 40 | 19 | Right | 6 | 1.5 | Improved | 0 (atrophy) | 20/25 | 20/150 |
| 11 | 5 | n/a | None | 3 | 3 | Improved | 0 | 20/25 | 20/20 |
| 12 | 15 | 10 | Left | 43 | 0.5 | Improved | 0 | 20/25 | 20/25 |
| 13 | 8 | n/a | None | 8 | Immediateb | Improvedb | 1b | 20/40 | 20/40 |
Headache, papilledema, and visual acuity assessed at 6 mo or at the time of longest follow-up.
Symptoms and papilledema improved on 2 g daily diamox, and headache further improved after ventriculoperitoneal shunt placement.
Nine of 10 TS stenoses were successfully stented via a femoral approach. In 1 patient (patient 12), a stent could not be advanced beyond the sigmoid-jugular junction due to vessel tortuosity. This patient had presented with fulminant IIH and rapidly progressive visual loss. A lumbar drain was used as a temporizing measure to control intracranial pressure. Stent placement was then performed via a left temporo-occipital craniectomy to access the TS by using image guidance and transfemoral cerebral venography to accurately position the burr hole and stent. Pressure gradients across the stent decreased in all patients where intrasinus pressures were measured poststenting (Table 1).
At 6 months or at longest follow-up, stented patients reported their headaches as either significantly improved (8/10) or completely resolved (2/10). Four patients had significant relief of their symptoms immediately after TS stent placement was performed. The remainder (6/10 patients) described a more gradual improvement in symptoms over 2–6 weeks. Papilledema resolved completely or almost completely in 8 patients and was noted to be significantly improved in 2 patients. One patient developed optic atrophy (patient 10). Visual acuity normalized or significantly improved in the 4 patients with impairment before stent placement (patients 1, 7, 10, and 12), though 1 patient had persistent significant visual loss associated with optic atrophy (patient 10). Interestingly, despite intrasinus pressures remaining elevated in 4 patients after stent placement (patients 5, 7, 8, and 10), 3 of these patients reported almost immediate relief of symptoms (patients 5, 7, and 8) and the fourth reported significant early improvement.
There were no major periprocedural complications. Transient contrast extravasation was observed in 1 patient (patient 13) without development of significant intracranial hemorrhage and without clinical consequence. Two patients complained of transient mild headache ipsilateral to the stented sinus. There were no clinically obvious occurrences of stent thrombosis, though follow-up venography was not routinely performed.
In the 3 patients where no significant pressure gradient (<10 mm Hg) was measured in the TSs (patients 3, 11, and 13), stent placement was not performed. One patient (patient 3) symptomatically improved on medical therapy alone. The second patient (patient 11) has remained stable but with persistent moderate-grade headaches and low-grade papilledema at 3 month follow-up. Ventriculoperitoneal shunt surgery was performed in the third patient (patient 13), which resulted in immediate improvement of headache and gradual improvement in papilledema during follow-up.
Discussion
Distal TS stenoses are observed in a high proportion of patients with IIH.11,12 Using 3D gadolinium-enhanced MR venography, bilateral sinovenous stenoses were seen in 27 of 29 patients with IIH but in only 4 of 59 controls.11 In another study, absence of segmental flow (flow gaps) in both TSs was seen in 13 of 20 patients with IIH but not observed in 40 healthy headache-free controls by using phase-contrast and time-of-flight MR venography.12 Consistent with these findings, direct intrasinus manometry has demonstrated elevated pressures in the superior sagittal and proximal TSs of patients with IIH, with a significant drop in venous pressures in the distal TS.30,31 Taken together, these data suggest that TS stenoses may play a role in the pathophysiology of IIH in some patients by causing partial venous outflow obstruction. It is well known that the clinical presentation of other conditions that cause cerebral venous outflow obstruction, such as venous sinus thrombosis, can mimic IIH.
The etiology of lateral sinus stenoses in patients with IIH remains uncertain. Considerable congenital asymmetry between the TSs has been found in anatomic studies. The right lateral sinus is larger or dominant in up to 73% of cases, and partial or total agenesis of portions of a TS are observed in up to 23% of cases.33 The cortical vein of L'Abbé drains into the distal third of the TS, in addition to several smaller tributaries from the inferolateral temporal lobe, occipital lobe, and cerebellum.34 Arachnoid granulations are frequently seen in the lateral TS associated with these venous entry sites,35 and if large may be a cause of venous outflow obstruction.36 Large transversely oriented septations or fenestrations were present in 10% of TSs evaluated in 1 postmortem study and are postulated to be a cause of venographic cryptic stenosis.37 Incomplete recanalization after thrombosis of a TS may occur causing filling defects and sinus narrowing due to residual chronic organized (fibrosed) thrombus.38,39 There are also case reports of focal fatty deposits21 and heterotopic brain or small brain hernia21,40 causing TS obstruction.
Other recent studies have suggested that the sinovenous stenoses seen in IIH may be manifestations of raised intracranial pressure. The Monro-Kellie hypothesis postulates that changes in CSF are in part compensated by changes in intracranial blood volume reflected primarily in the venous system.41 Raised intracranial pressure may therefore cause a collapse or extrinsic compression of the sinus walls. In agreement with this hypothesis, several authors have reported reversibility of lateral sinus stenoses by CSF removal or diversion procedures in patients with IIH.12,14–18 Using a lateral C1–2 puncture to remove 20–25 mL of CSF, King et al15 demonstrated resolution of venous hypertension measured by intrasinus manometry in patients with IIH. Normalization of venous sinus caliber on MR venography after lumbar puncture17 or subsequent to ventriculo- or lumboperitoneal shunt surgery13,14,16 has also been observed in selected patients with IIH. However, in a study by Bono et al,42 TS stenoses persisted after normalization of CSF pressure with acetazolamide and weight loss in 14 patients with IIH, suggesting either that some patients have fixed obstructions or that large acute shifts in CSF volume by lumbar puncture or CSF diversion procedures are required to produce changes in the caliber of sinuses.
The current case series adds to a small but growing body of evidence14,19–27 suggesting that selected patients with IIH may benefit from TS stent placement. Higgins et al19 reported the first patient with refractory IIH and TS stenosis treated with a stent. They described a 30-year-old woman with a 22-month history of headache, visual disturbance, and papilledema and an MR venogram demonstrating bilateral TS stenosis. Direct cerebral venography and manometry confirmed the presence of the stenoses with raised pressures proximal to the obstructions. Dilation of 1 of the sinuses with a stent reduced the pressure gradient with dramatic symptomatic improvement. To date, 40 patients with IIH treated with sinovenous stent placement have been reported in the literature (on-line Table 2 and Table 2). Most of the patients were female (36/40), ranging in age from 15–65 years old. Symptom duration ranged from 2 weeks to 15 years. Clinical follow-up ranged from 4 to 60 months. After stent placement (Table 2), 33 of 40 (82.5%) patients reported their headaches as resolved or significantly improved and papilledema improved or resolved in 30 of 33 (91%) patients with active papilledema at presentation. Eight patients with active papilledema developed chronic optic nerve changes at follow-up. Periprocedural complications were infrequently reported. The most commonly reported adverse event was transient headache ipsilateral to the stent (>50% of patients). Transient unsteadiness and hearing loss were each reported in 1 patient.20 Two patients in this series required thrombolysis for in-stent thrombosis.20 The preprocedural antiplatelet regimen was not clearly described in this cohort, and lack of pretreatment may have contributed to thrombus development. There have been no reported cases to date of in-stent stenosis, though numbers are small and follow-up limited. Two patients had new stenoses develop proximal to the stent.14,21 One of these patients was treated with a ventriculoperitoneal shunt due to persistent symptoms.21
Table 2:
Outcome of studies of IIH patients treated by transverse sinus stenting
| Referencea | Stenting |
Pressure Gradient |
Follow-Up Duration (mo) | Headache |
Papilledemab |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Before (mm Hg) | After (mm Hg) | No Change | Resolved or Improved | Worse | No Change | Resolved or Improved | Atrophy | ||
| Higgins et al 200320 | 10 | 2 | 8–37 | 2–15 | 7–26 | 5 | 7 | 0 | 2 | 4 | 3 |
| Owler et al 200321 | 4 | 0 | 12–25 | 0–1 | 5–12 | 0 | 4 | 0 | 0 | 4 | 2 |
| Ogungbo et al 200322 | 1 | 0 | 25 | ns | 6 | 0 | 1 | 0 | 0 | 1 | 0 |
| Rajpal et al 200523 | 1 | 0 | 25 | ns | 6 | 0 | 1 | 0 | 0 | 1 | 0 |
| Rohr et al 200714 | 1 | 0 | 39 | 1 | 0.25 | 0 | 1 | 0 | 0 | 0 | 0 |
| Donnet et al 200826 | 9 | 1 | 14–34 | ns | 6–36 | 2 | 8 | 0 | 0 | 10 | 2 |
| Paquet et al 200827 | 1 | 0 | 15 | ns | ns | 0 | 1 | 0 | 0 | 1 | 0 |
| This study | 10 | 0 | 11–50 | 2–23 | 4–60 | 0 | 10 | 0 | 0 | 9 | 1 |
| Totals | 37 | 3 | 8–50 | 0–23 | 0.25–60 | 7 | 33 | 0 | 2 | 30 | 8 |
Higgins et al 2002,19 Metellus et al 2005,24 and Metellus et al 200725 were excluded due to uncertainty as to whether patients were reported in subsequent publications.
Rohr et al 200714: outcome of papilledema after stenting not specified. Higgins et al 200320: outcome of papilledema after stenting not specified for 1 patient, 4 patients did not have papilledema at presentation, and 3 patients had optic atrophy at presentation. Present study: 1 patient did not have papilledema at the time of stenting.
Three patients in the present case series were not found to have significantly elevated pressures in the TS. Time-of-flight MR venography was used to image the cerebral veins in these patients. It is possible that the severity of the stenoses was overestimated due to the insensitivity of this technique to in-plane flow or due to its sensitivity to slow flow or turbulence.39 Gadolinium-enhanced MR venography, which is not susceptible to in-plane saturation effects, may have resulted in more accurate estimation of sinus caliber11,39 and improved patient selection for possible stent placement. Alternatively, these patients may have lacked a significant pressure gradient because the visualized stenosis may not be contributing to the pathophysiology of their symptoms, perhaps due to sufficient venous collateral drainage.
An increased incidence of prothrombotic factors or genetic thrombophilia has been recently described in patients with IIH.43–48 This association has led to the hypothesis that microthrombus formation in arachnoid villi may impair CSF outflow and thereby contribute to the pathogenesis of IIH.44,47,48 There is little evidence, however, to support this hypothesis. It has also been suggested that cranial venous outflow obstruction may occur due to unrecognized nonocclusive thrombus or due to incomplete recanalization of a previously occluded sinus.38,39,46 In the present series, headaches and papilledema improved in all 3 patients with a history of thrombophilia subsequent to restoration of the diameter of the stenotic TS with a stent.
We acknowledge several limitations of this small retrospective case series. First, the clinical course of IIH is quite variable. It can be a subacute and remitting disorder or follow a more protracted course with delayed worsening occurring even years after a period of stability.6 Thus, although the response of this small group of patients to TS stent placement is encouraging, a true assessment of the efficacy and durability of any treatment for IIH would require a larger randomized controlled study. Second, weight fluctuations were not systematically recorded during follow-up. Weight loss on its own may result in clinical improvement in a proportion of patients with IIH. Third, the long-term risk of in-stent stenosis or delayed thrombosis of stents within dural venous sinuses in primarily young female patients is unknown.
Although most patients with IIH improve with conservative therapy, an estimated 18%–22% of patients are considered refractory with visual impairment and/or headaches persisting or progressing despite maximally tolerated treatment with acetazolamide, furosemide, and dietary counseling for weight loss.49,50 Surgical procedures, such as optic nerve sheath fenestration, ventriculo- or lumboperitoneal shunt surgery, and subtemporal decompression are usually considered for such patients to prevent visual loss and treat intractable headaches. These procedures are not universally effective, however. Failure rates as high as 30% have been reported and complications are not infrequent.50–53 Venous sinus stent placement may be an alternative treatment option for patients with IIH refractory to medical therapy. The safety and efficacy of this procedure, however, should be evaluated in a larger randomized controlled study before it is applied more widely.
Conclusions
In this small case series, restoring the patency of stenotic TSs with a stent in patients with refractory IIH resulted in resolution or significant improvement in headache and papilledema in all treated patients. The safety and efficacy of this procedure, however, should be evaluated in a larger randomized study before it is applied more widely.
Supplementary Material
Acknowledgments
Drs. Andrew Leung, Donald Lee, Irene Gulka, and Stephen Lownie were involved in the venous sinus stent placement procedures at the London Health Sciences Centre.
Abbreviations
- BMI
body mass index
- IIH
idiopathic intracranial hypertension
- LP
lumbar puncture
- ns
not significant
- ONSF
optic nerve sheath fenestration
- OP
opening pressure
- STD
subtemporal decompression
- TS
transverse sinus
- TVOs
transient visual obscurations
Footnotes
Indicates article with supplemental on-line tables.
References
- 1. Acheson JF. Idiopathic intracranial hypertension and visual function. British Med Bull 2006; 79: 233–44 [DOI] [PubMed] [Google Scholar]
- 2. Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol 2006; 5: 433–42 [DOI] [PubMed] [Google Scholar]
- 3. Friedman DI. Pseudotumor cerebri. Neurol Clin N Am 2004; 22: 99–131 [DOI] [PubMed] [Google Scholar]
- 4. Digre KB, Corbett JJ. Idiopathic intracranial hypertension: a reappraisal. Neurologist 2001; 7: 2–67 [Google Scholar]
- 5. Corbett JJ, Savino PJ, Thompson HS. Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol 1982; 39: 461–74 [DOI] [PubMed] [Google Scholar]
- 6. Shah VA, Kardon RH, Lee AG, et al. Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience. Neurology 2008; 70: 634–40 [DOI] [PubMed] [Google Scholar]
- 7. Thambisetty M, Lavin PJ, Newman NJ, et al. Fulminant idiopathic intracranial hypertension. Neurology 2007; 68: 229–32 [DOI] [PubMed] [Google Scholar]
- 8. Liu GT, Volpe NJ, Schatz NJ, et al. Severe sudden visual loss caused by pseudotumor cerebri and lumboperitoneal shunt failure. Am J Ophthalmol 1996; 122: 129–31 [DOI] [PubMed] [Google Scholar]
- 9. Kidron D, Pomeranz S. Malignant pseudotumor cerebri: report of two cases. J Neurosurg 1989; 71: 443–45 [DOI] [PubMed] [Google Scholar]
- 10. Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev 2005: CD003434. [DOI] [PubMed] [Google Scholar]
- 11. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003; 60: 1418–24 [DOI] [PubMed] [Google Scholar]
- 12. Higgins JNP, Gillard JH, Owler BK, et al. MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Psychiatry 2004; 75: 621–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JNP, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004; 62: 1907–08 [DOI] [PubMed] [Google Scholar]
- 14. Rohr A, Dorner L, Stingele R, et al. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2007; 28: 656–59 [PMC free article] [PubMed] [Google Scholar]
- 15. King JO, Mitchell PJ, Thomson KR, et al. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002; 58: 26–30 [DOI] [PubMed] [Google Scholar]
- 16. Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004; 62: 1445–46 [DOI] [PubMed] [Google Scholar]
- 17. De Simone R, Marano E, Fiorillo C, et al. Sudden re-opening of collapsed transverse sinus and longstanding clinical remission after a single lumbar puncture in a case of idiopathic intracranial hypertension: pathogenic implications. Neurol Sci 2005; 25: 342–44 [DOI] [PubMed] [Google Scholar]
- 18. McGonigal A, Bone I, Teasdale E. Resolution of transverse sinus stenosis in idiopathic intracranial hypertension after L-P shunt. Neurology 2004; 62: 514–15 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JN, Owler BK, Cousins C, et al. Venous sinus stenting for refractory benign intracranial hypertension. Lancet 2002; 359: 228–30 [DOI] [PubMed] [Google Scholar]
- 20. Higgins JNP, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003; 74: 1662–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 2003; 98: 1045–55 [DOI] [PubMed] [Google Scholar]
- 22. Ogungbo B, Roy D, Gholkar A, et al. Endovascular stenting of the transverse sinus in a patient presenting with benign intracranial hypertension. Br J Neurosurg 2003; 17: 565–68 [DOI] [PubMed] [Google Scholar]
- 23. Rajpal S, Niemann DB, Turk AS. Transverse venous sinus stent placement as treatment for benign intracranial hypertension in a young male: case report and review of the literature. J Neurosurg 2005; 102: 342–46 [DOI] [PubMed] [Google Scholar]
- 24. Metellus P, Levrier O, Fuentes S, et al. Endovascular treatment of benign intracranial hypertension by stent placement in the transverse sinus: therapeutic and pathophysiological considerations illustrated by a case report [in French]. Neurochirurgie 2005; 51: 113–20 [DOI] [PubMed] [Google Scholar]
- 25. Metellus P, Levrier O, Fuentes S, et al. Endovascular treatment of benign intracranial hypertension: analysis of eight consecutive patients [in French]. Neurochirurgie 2007; 53: 10–17 [DOI] [PubMed] [Google Scholar]
- 26. Donnet A, Metellus P, Levrier O, et al. Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology 2008; 70: 641–47 [DOI] [PubMed] [Google Scholar]
- 27. Paquet C, Poupardin M, Boissonnot M, et al. Efficacy of unilateral stenting in idiopathic intracranial hypertension with bilateral venous sinus stenosis: a case report. Eur Neurol 2008; 60: 47–48 [DOI] [PubMed] [Google Scholar]
- 28. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002; 59: 1492–95 [DOI] [PubMed] [Google Scholar]
- 29. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24 (suppl 1): 9–160 [DOI] [PubMed] [Google Scholar]
- 30. Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982; 45: 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King JO, Mitchell PJ, Thomson KR, et al. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology 1995; 45: 2224–28 [DOI] [PubMed] [Google Scholar]
- 32. Karahalios DG, Rekate HL, Khayata MH, et al. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996; 46: 198–202 [DOI] [PubMed] [Google Scholar]
- 33. Zouaoui A. Cerebral venous sinuses: anatomical variants or thrombosis? Acta Anat (Basel) 1988; 133: 318–24 [DOI] [PubMed] [Google Scholar]
- 34. Rhoton AL, Jr. The cerebral veins. Neurosurgery 2002; 51 (suppl 1): 159–205 [PubMed] [Google Scholar]
- 35. Leach JL, Jones BV, Tomsick TA, et al. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol 1996; 17: 1523–32 [PMC free article] [PubMed] [Google Scholar]
- 36. Arjona A, Delgado F, Fernandez-Romero E. Intracranial hypertension secondary to giant arachnoid granulation. J Neurol Neurosurg Psychiatry 2003; 74: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subramaniam RM, Tress BM, King JO, Eizenberg N, et al. Transverse sinus septum: a new aetiology of intracranial hypertension? Australas Radiol 2004; 48: 114–16 [DOI] [PubMed] [Google Scholar]
- 38. Leach JL, Fortuna RB, Jones BV, et al. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 2006; 26 (suppl 1): S19–S41 [DOI] [PubMed] [Google Scholar]
- 39. Agid R, Shelef I, Scott JN, et al. Imaging of the intracranial venous system. Neurologist 2008; 14: 12–22 [DOI] [PubMed] [Google Scholar]
- 40. Kollar C, Johnston I, Parker G, et al. Dural arteriovenous fistula in association with heterotopic brain nodule in transverse sinus. AJNR Am J Neuroradiol 1998; 19: 1126–28 [PMC free article] [PubMed] [Google Scholar]
- 41. Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001; 56: 1746–48 [DOI] [PubMed] [Google Scholar]
- 42. Bono F, Giliberto C, Mastrandrea C, et al. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology 2005; 65: 1090–93 [DOI] [PubMed] [Google Scholar]
- 43. Sussman J, Leach M, Greaves M, et al. Potentially prothrombotic abnormalities of coagulation in benign intracranial hypertension. J Neurol Neurosurg Psychiatry 1997; 62: 229–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Backhouse O, Metcalfe T, Goulding P, et al. Factor V Leiden mutation in association with idiopathic intracranial hypertension. Br J Ophthalmol 1998; 82: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dogulu CF, Kansu T, Leung MY, et al. Evidence for genetic susceptibility to thrombosis in idiopathic intracranial hypertension. Thromb Res 2003; 111: 389–95 [DOI] [PubMed] [Google Scholar]
- 46. Dunkley S, Johnston I. Thrombophilia as a common predisposing factor in pseudotumor cerebri. Blood 2004; 103: 1972–73 [DOI] [PubMed] [Google Scholar]
- 47. Glueck CJ, Goldenberg N, Golnik K, et al. Idiopathic intracranial hypertension: associations with thrombophilia and hypofibrinolysis in men. Clin Appl Thromb Hemost 2005; 11: 441–48 [DOI] [PubMed] [Google Scholar]
- 48. Glueck CJ, Aregawi D, Goldenberg N, et al. Idiopathic intracranial hypertension: polycystic ovary syndrome and thrombophilia. J Lab Clin Med 2005; 145: 72–82 [DOI] [PubMed] [Google Scholar]
- 49. Burgett RA, Purvin VA, Kawasaki A. Lumboperitoneal shunting for pseudotumor cerebri. Neurology 1997; 49: 734–39 [DOI] [PubMed] [Google Scholar]
- 50. Garton HJL. Cerebrospinal fluid diversion procedures. J Neuro-Ophthalmol 2004; 24: 146–55 [DOI] [PubMed] [Google Scholar]
- 51. Feldon SE. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus 2007; 23: E6. [DOI] [PubMed] [Google Scholar]
- 52. Brazis PW. Clinical review: the surgical treatment of idiopathic pseudotumour cerebri (idiopathic intracranial hypertension). Cephalgia 2008; 28: 1361–73 [DOI] [PubMed] [Google Scholar]
- 53. Spoor TC, McHenry JG. Long-term effectiveness of optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol 1993; 111: 632–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.