Abstract
BACKGROUND AND PURPOSE:
Lobular capillary hemangioma is a benign capillary proliferation of unknown etiology. To our knowledge, no comprehensive review of imaging findings of LCHNC has been presented. Thus, we investigated characteristic CT features of LCHNC.
MATERIALS AND METHODS:
This retrospective study included 6 patients (2 men and 4 women; age range, 30–65 years; mean age, 49.2 years) with histologically proved LCHNC. We evaluated the size, site of origin, attenuation on NECT, degree and pattern of enhancement, and bony changes.
RESULTS:
The LCHNC lesion was 13.0–45.0 mm (average, 25.0 mm) in diameter. These lesions arose from the inferior turbinate in 5 (83.3%) patients and the anterior nasal septum in 1 (16.7%). Compared with the masticator muscles, the LCHNC lesion was hypoattenuating in 2 (33.3%) and isoattenuating on NECT in 4 (66.7%) patients. In 5 (83.3%) patients, the LCHNC lesion consisted of 2 distinct areas on CECT: a lobular intensely enhancing mass and an iso- or hypoattenuating cap of variable thickness around the intensely enhancing mass. Bony changes included erosion in 3 (50.0%) and displacement in 2 (33.3%) patients.
CONCLUSIONS:
CT features of LCHNC consist of an intensely enhancing mass and an iso- or hypoattenuating cap on CECT. The inferior turbinate seems to be a common site of origin, and bony changes are not uncommon features of LCHNC. CT is useful not only in identifying the site of origin and assessing the extent but also in suggesting the nature of LCHNC.
Lobular capillary hemangioma, formerly known as pyogenic granuloma, is a benign capillary proliferation with a microscopically distinct lobular architecture that affects the skin and mucous membranes of the oral and, rarely, of the nasal cavities.1,2 It occurs in all ages but commonly in women of the third to fifth decades.3–6 Mucosal LCHNC is found most often arising from the anterior nasal septum (the Little area or Kiesselbach plexus), followed by the turbinates. Other sites include the maxillary sinus and the roof of the nasal cavity.1,7,8 The Little area, also called the Kiesselbach area, is a region in the anteroinferior part of the nasal septum, where 4 arteries (the sphenopalatine, greater palatine, anterior ethmoidal, and superior labial arteries) anastomose to form a vascular plexus called the Kiesselbach plexus.9 It is the site of 90% of the cases of epistaxis. Traumatic (habitual nose picking or nasal packing) and hormonal (pregnancy or oral contraceptives) factors have been implicated as the etiology of these tumors.2,10–12 Although several case reports and clinical studies1,3,4,13–15 have mentioned imaging findings of LCHNC, to our knowledge, no comprehensive review of CT imaging features has been presented.
In this study, we investigated characteristic CT features of LCHNC.
Materials and Methods
Patients
This retrospective study included 6 patients (2 men and 4 women; age range, 30–65 years; mean age, 49.2 years) with histologically proved LCHNC between November 2005 and October 2008, who had undergone preoperative CT and subsequently endoscopic surgery. Approval was obtained from the institutional review board to carry out this retrospective study.
CT Imaging and Image Interpretation
Multidetector-row CT was performed by using a Sensation 16 or Sensation 64 scanner (Siemens, Erlangen, Germany). NECT and CECT images of soft-tissue algorithms were obtained in all patients. CECT images were obtained immediately after intravenous administration of 100 mL of contrast media (iopromide, Ultravist 350; Schering, Seoul, Korea) at a rate of 1.8 mL per second. It took approximately 7.8 seconds to obtain the CECT images. No delayed imaging was performed in any of the 6 patients. Images were displayed in axial and coronal planes with section thickness of 2.0–3.0 mm. We evaluated the following CT features of the LCHNC lesions: the size, site of origin, attenuation on NECT, degree and pattern of enhancement on CECT, and bony changes. The attenuation value of the mass compared with the masticator muscles was categorized as hypoattenuating, isoattenuating, or hyperattenuating. The degree of enhancement of the mass was visually assessed. Findings at MR imaging (n = 2) performed with a 3T scanner (Signa VHi; GE Healthcare, Milwaukee, Wisconsin) and angiography (n = 3) were also investigated. Two experienced radiologists (S.K.L. and H.W.C.) interpreted the CT, MR imaging, and angiographic images. The final decisions regarding the findings were reached by consensus.
Results
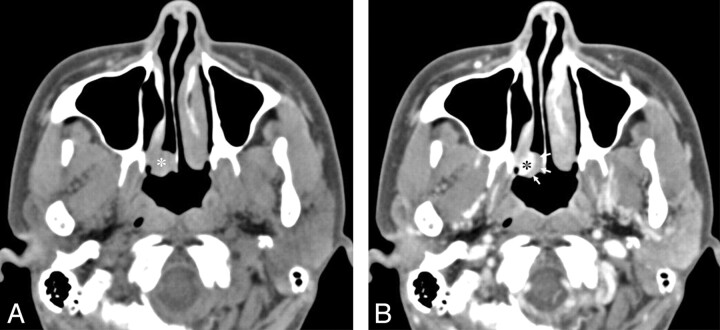
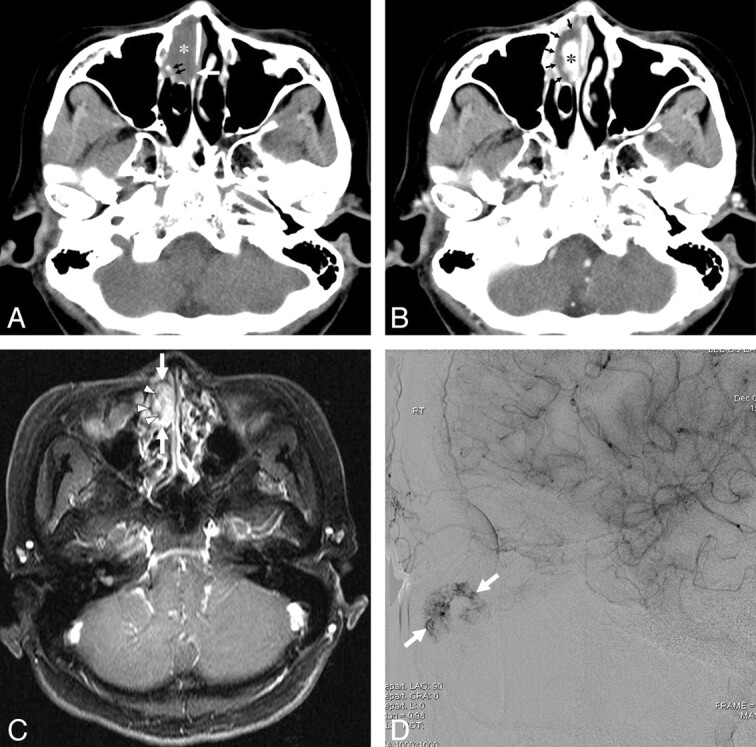
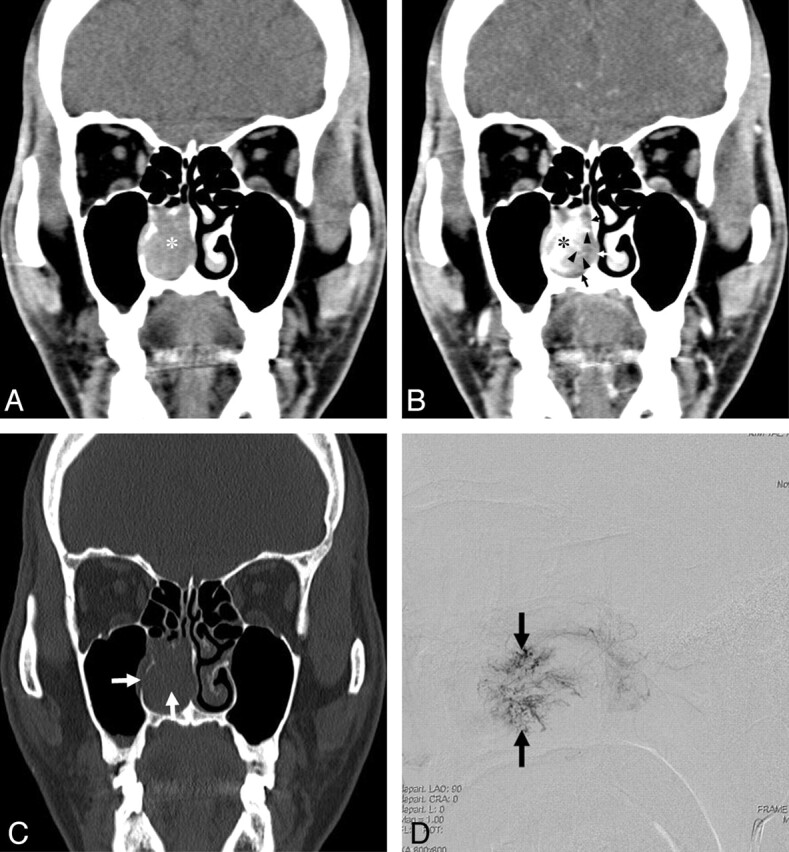
Pregnancy was identified as a possible cause of LCHNC in 1 patient (16.7%) (patient 2 in Table 1). The LCHNC lesion did not regress spontaneously after delivery in this patient. Thus, she underwent endoscopic surgery. A history of nasal trauma as a predisposing factor was not identified in any of the 6 patients. All patients presented with nasal obstruction and epistaxis. Demographic data and CT features are provided in Table 1. The LCHNC lesions ranged from 13.0 to 45.0 mm with an average diameter of 25.0 mm. They arose from the anterior nasal septum in 1 (16.7%) (Fig 1) and the inferior turbinate in 5 (83.3%) patients (Figs 2–4) and predominantly involved the anterior (Fig 1), middle (Figs 2 and 3), and posterior (Fig 4) portions of a nasal cavity in 2 (33.3%) patients each. Compared with the masticator muscles, the LCHNC lesion was hypoattenuating (Fig 1) in 2 (33.3%) and isoattenuating (Figs 2–4) in 4 (66.7%) patients on NECT. In 5 (83.3%) of 6 patients, the LCHNC lesion consisted of 2 distinct areas on CECT: a lobular intensely enhancing mass and an iso- or hypoattenuating cap of variable thickness around the intensely enhancing mass (Figs 1, 2, and 4). Of these, 3 (60.0%) cases showed linear and/or spotty enhancement within the cap (Fig 2). In the remaining patient (16.7%), the LCHNC lesion consisted of a lobular intensely enhancing mass and a hypoattenuating area containing linear and spotty enhancing foci (Fig 3). Bony changes included erosion in 3 (50.0%) patients and displacement in 2 (33.3%). Bony erosion occurred at the inferior turbinate and medial wall of the maxillary sinus in 2 (33.3%) patients each and at the nasal septum in 1 (16.7%). Bony displacement included lateral displacement of the middle turbinate and medial wall of the maxillary sinus in 1 (16.7%) patient each.
Table 1:
Data of 6 patients with LCHNCa
| Patient No./Age (yr)/Sex | Size (mm) | Location | NECT | CECT |
Bony Changes |
||
|---|---|---|---|---|---|---|---|
| Intensely Enhancing Mass | Iso or Hypo Cap | Erosion | Displacement | ||||
| 1/56/F | 22 | NS | Hypo | + | + | NS | MT |
| 2/30/F | 25 | IT | Hypo | + | + | – | – |
| 3/65/F | 19 | IT | Iso | + | + | – | – |
| 4/53/M | 28 | IT | Iso | + | + | IT, MS | – |
| 5/56/M | 45 | IT | Iso | + | – | IT, MS | MS |
| 6/35/F | 13 | IT | Iso | + | + | – | – |
+ indicates present; –, absent.
Fig 1.
A 56-year-old woman with a lobular capillary hemangioma arising from the nasal septum. A, An axial NECT image shows a homogeneous hypoattenuating mass (asterisk) in the right anterior nasal cavity. The nasal septum is eroded (arrow), and the right middle turbinate is displaced laterally (small arrows). B, An axial CECT image reveals a lobular intensely enhancing mass (asterisk) arising from the nasal septum and a hypoattenuating cap (arrows). C, A Gd-enhanced SE T1WI (TR/TE, 700/19 ms) demonstrates only a lobular intensely enhancing mass (arrow), resulting from fill-in of Gd in the hypoattenuating cap of the CECT image. Note the tubular high-velocity SI voids (arrowheads) within the mass. D, A lateral view of the delayed phase of a carotid angiogram demonstrates a lobular capillary blush of the mass (arrows).
Fig 2.
A 53-year-old man with a lobular capillary hemangioma arising from the right inferior turbinate. A, A coronal NECT image shows a homogeneous isoattenuating mass (asterisk) in the right nasal cavity. B, A coronal CECT image reveals a lobular intensely enhancing mass (asterisk) arising from the right inferior turbinate and an isoattenuating cap (arrows) containing linear and spotty enhancing foci (arrowheads). C, A coronal NECT image of a bone algorithm demonstrates erosion of the medial wall of the right maxillary sinus and bony destruction of the right inferior turbinate (arrows). D, A lateral view of the delayed phase of a microcatheter angiogram of the right sphenopalatine artery demonstrates a lobular capillary blush of the mass (arrows).
Fig 4.
A 35-year-old woman with a lobular capillary hemangioma arising from the posterior tip of the right inferior turbinate. A, An axial NECT image shows a homogeneous isoattenuating mass (asterisk) arising from the posterior tip of the right inferior turbinate near the posterior choana. B, An axial CECT image demonstrates an intensely enhancing mass (asterisk) and an isoattenuating cap (arrows).
Fig 3.
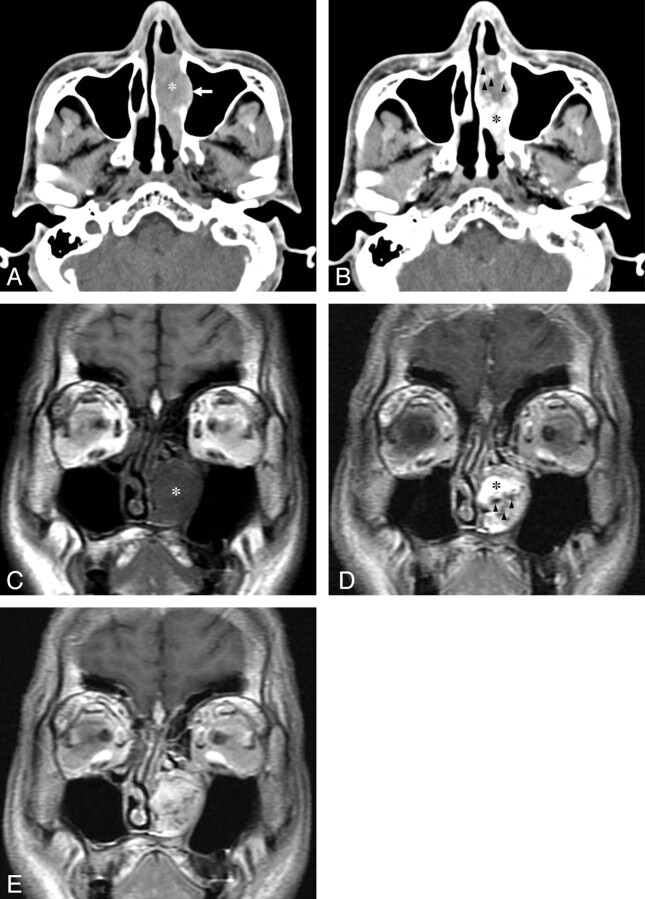
A 56-year-old man with a lobular capillary hemangioma arising from the left inferior turbinate. A, An axial NECT image shows a homogeneous isoattenuating mass arising from the destroyed left inferior turbinate (asterisk) with lateral displacement and erosion of the medial wall of the left maxillary sinus (arrow). B, An axial CECT image reveals a lobular intensely enhancing mass (asterisk) and a hypoattenuating area containing spotty enhancing foci (arrowheads). C, A SE T1WI (TR/TE, 600/11 ms) shows a hypointense LCHNC lesion (asterisk) compared with the masticator muscle. D, A dynamic Gd-enhanced SE T1WI (TR/TE, 350/11 ms) obtained at 1 minute after intravenous administration of Gd demonstrates a lobular intensely enhancing mass (asterisk) and a less intensely enhancing area containing tubular high-velocity SI voids (arrowheads). E, A dynamic Gd-enhanced SE T1WI (TR/TE, 350/11 ms) obtained at 4 minutes after intravenous administration of Gd reveals more homogeneous enhancement of the entire LCHNC lesion.
Compared with the masticator muscle, the LCHNC lesion was hypointense on SE T1WI (TR/TE, 600–700/11–19 ms) (Fig 3C) and hyperintense with tubular high-velocity signal-intensity voids on FSE T2WI (TR/TE, 4000–4200/103.8–105.3 ms; echo-train length, 16–18) in 2 patients who underwent MR imaging (patients 1 and 5). Imaging in 1 patient (patient 1) demonstrated only an intensely enhancing mass with tubular high-velocity SI voids on Gd-enhanced SE T1WI (TR/TE, 700/19 ms) (Fig 1C). Imaging in the other patient (patient 5) demonstrated 2 different components within the mass on dynamic Gd-enhanced SE T1WI (TR/TE, 350/11 ms): an intensely enhancing area and a less intensely enhancing area containing tubular high-velocity signal-intensity voids at 1 minute (Fig 3D) with gradual fill-in of Gd at 2, 3, and 4 minutes (Fig 3E) after intravenous administration of Gd. In all 3 patients who underwent angiography (1 carotid, 1 sphenopalatine, and 1 internal maxillary arteriography each), the delayed phase demonstrated a lobular capillary blush of the mass (Figs 1D and 2D).
Discussion
The term “pyogenic granuloma” is a misnomer because clear histologic and microbiologic features indicating an infectious origin are lacking. In addition, the term “granuloma” would allude to the presence of granulation tissue in the lesion, which is not typical of a lobular capillary hemangioma. Lobular capillary hemangioma and reactive granuloma are thought to be distinct entities. Nevertheless, lobular capillary hemangioma and reactive granuloma share several characteristics, including acute inflammation, an endothelial cell prominence, and an edematous and fibrous environment.6,16 Although the differentiation can be challenging, reactive granuloma lacks the classic lobular growth pattern of the capillary proliferation that characterizes lobular capillary hemangioma. The histologic features of lobular capillary hemangioma are quite similar to those of rapidly involuting infantile hemangioma in that both lesions are composed of lobules of capillaries and surrounding fibrous tissue17 but are different from those of venous vascular malformation (also known as “cavernous hemangioma”), which are composed of dilated blood-filled spaces lined by flattened endothelium.
Puxeddu et al,4 in their clinical study comprising the largest number of patients with LCHNC to date, have identified previous nasal trauma and pregnancy as a possible cause in only 15% and 5% of patients with LCHNC, respectively. In a study comprising 128 children with lobular capillary hemangioma,16 76.9% of lesions were found in the head and neck, and 76.7% had no history of trauma or predisposing factors. Congenital lobular capillary hemangioma in the oral mucosa also has been documented previously.18 Given these clinical and histologic features, lobular capillary hemangioma seems to be a benign neoplasm rather than a reactive process secondary to infection or trauma.6
The LCHNC lesion usually appears at endoscopy as a single red-to-purple, hypervascularized mass with a predilection for the anterior nasal fossa, particularly the anterior nasal septum. In our study, the LCHNC lesion arose from inferior turbinate in 5 (83.3%) patients and the nasal septum in 1 (16.7%) patient and predominantly involved the anterior, middle, and posterior portions of a nasal cavity in 2 (33.3%) patients each. The distribution of LCHNC in our study was quite different from that in the literature, including a clinical study comprising 40 patients with LCHNC.4 The difference in the distribution of LCHNC between our study and that in the literature may not attain clinical significance because of the small number of patients included in our study. Nevertheless, the inferior turbinate seems to be a common site of the origin of LCHNC. A few cases of LCHNC in pregnant women have been reported.2,19,20 Some of the lesions have been known to regress after parturition.21 In our study, pregnancy was identified as a possible cause of LCHNC in 1 patient (16.7%). The LCHNC lesion did not regress spontaneously after delivery in this patient. Thus, she underwent endoscopic excision.
Imaging features of LCHNC have been reported in the literature as a part of clinical study or as single case reports (Table 2).1,3,4,13–15 Previously reported CT features of LCHNC can be summarized as a soft-tissue mass without bony destruction or invasion into the paranasal sinuses.1,3,13 Histologically, the LCHNC lesion consists of 2 areas: a lobular and a superficial ulcerative area. The lobular area is characterized by capillary proliferation with a microscopically distinct lobular architecture. The superficial portion of the lesion may undergo secondary nonspecific changes, including stromal edema, capillary dilation, inflammation, and a granulation tissue reaction.6,22
Table 2:
Summary of CT and/or MR imaging features of LCHNC in the literature
| Authors | No. of Cases |
CT Features | MR Imaging Features | Bony Changes |
||
|---|---|---|---|---|---|---|
| CT | MRI | Erosion | Displacement | |||
| Puxeddu et al4 | 6 | 4 | Soft-tissue mass with intense CE (6/6) | High SI on T2WI, low SI on T1WI with intense CE (4/4) | NM | NM |
| Katori and Tsukuda14 | 1 | 1 | Soft-tissue mass | Isointense to muscle on T1WI, hyperintense with numerous small signal voids on T2WI | NM | NM |
| Iwata et al23 | 3 | 3 | A well-circumscribed mass (3/3) | High SI on T2WI with CE (3/3) | – | NM |
| Miller et al1 | 1 | 0 | Soft-tissue mass | – | – | NM |
| Ozcan et al3 | 1 | 0 | Soft-tissue mass | – | – | NM |
| Kurtaran et al13 | 1 | 0 | Soft-tissue mass | – | – | NM |
–, indicates not present.
In our study, the findings of the LCHNC lesion on CECT consisted of an intensely enhancing mass and an iso- or hypoattenuating cap of variable thickness around the intensely enhancing mass in 5 (83.3%) of 6 patients. Of these, 3 (60.0%) cases showed linear and/or spotty enhancement within the cap. The LCHNC lesion in the remaining (16.7%) patient showed a hypoattenuating area containing linear or spotty enhancing foci, in addition to an intensely enhancing mass. We retrospectively speculated that the intensely enhancing mass corresponded histologically to a lobular area in all 6 patients; and the iso- or hypoattenuating cap of variable thickness in 3 patients and the hypoattenuating area in 1 patient, which contained linear and/or spotty enhancing foci, might correspond to the superficial ulcerative areas, respectively, though radiologic-histologic correlation has not been made in each case. The iso- or hypoattenuating cap of variable thickness that did not contain linear or spotty enhancing foci may be either a superficial ulcerative area or an inflammatory secretion, as in 2 patients in our study (patients 1 and 6). In 2 patients who underwent Gd-enhanced SE T1WI (TR/TE, 700/19 ms) (patient 1) and dynamic Gd-enhanced SE T1WI (TR/TE, 350/11 ms) at 1, 2, 3, and 4 minutes after intravenous administration of Gd (patient 5), the hypoattenuating cap and hypoattenuating area detected on CECT were intensely enhanced and gradually enhanced until 4-minute delayed imaging, respectively.
The discrepancy of findings between CECT and Gd-enhanced SE T1WI might be due to the relatively long scanning time of MR imaging, during which time, gradual fill-in of Gd in the hypoattenuating cap and area might occur. Accordingly, the hypoattenuating cap, which did not contain linear and/or spotty enhancing foci on CECT in 1 patient (patient 1), might correspond to a superficial ulcerative area rather than to an inflammatory secretion.
CT is superior to any other imaging modality in the evaluation of delicate bony architecture of the nasal cavities and paranasal sinuses. Cases with bony erosion rarely have been reported in the literature.4 Puxeddu et al4 reported a case of LCHNC involving the middle turbinate with erosion of the medial wall of the maxillary sinus. In our study, bony erosion (50.0%) and displacement (33.3%) were more common than those reported in the literature.1,3,13 Bony erosion predominantly involved the site of the origin of the LCHNC lesion.
MR imaging findings of LCHNC have been sporadically reported in the literature.4,14,23 Katori and Tsukuda14 reported a case of LCHNC that was isointense to muscle on T1WI and hyperintense with numerous small flow voids on T2WI. Puxeddu et al4 noted that the LCHNC lesion appeared as a soft-tissue mass with low SI on T1WI and high SI on T2WI and intense enhancement after intravenous administration of Gd. The MR imaging features of our patients (patients 1 and 5) were not significantly different from those in the literature (Table 2). The similarity of histologic features to rapidly involuting infantile hemangioma17 and high-velocity SI voids on FSE T2WI and Gd-enhanced SE T1WI in 2 patients in our study suggest that LCHNC is a high-flow lesion.
A differential diagnosis of a hypervascular mass of the nasal cavity in patients with nasal obstruction and/or epistaxis might include juvenile angiofibroma, angiomatous polyp, hemangioma, hemangiopericytoma, paraganglioma, angiosarcoma, and hypervascular metastases, particularly from kidney, thyroid, lung, or breast. None of these lesions have been reported to have an iso- or hypoattenuating cap of variable thickness around the intensely enhancing lobular mass in the literature. Accordingly, an intensely enhancing lobular mass and an iso- or hypoattenuating cap themselves are sufficient for suggesting the diagnosis of LCHNC, though a tailored prospective assessment with a large number of cases is anticipated.
Conclusions
LCHNC is a rare lesion of unknown etiology, which should be considered in the differential diagnosis of rapidly enlarging vascular lesions within the nasal cavity. CT features of an LCHNC lesion consist of an intensely enhancing mass and an iso- or hypoattenuating cap of variable thickness around it on CECT. The inferior turbinate seems to be a common site of origin, and bony changes are not uncommon features of LCHNC. CT is useful not only in identifying the site of origin and assessing the extent but also in suggesting the nature of the LCHNC.
Abbreviations
- CE
contrast enhancement
- CECT
contrast-enhanced CT
- FSE
fast spin-echo
- Gd
gadolinium
- Hypo
hypoattenuating
- Iso
isoattenuating
- IT
inferior turbinate
- LCHNC
lobular capillary hemangioma of the nasal cavity
- MS
medial wall of maxillary sinus
- MT
middle turbinate
- NECT
nonenhanced CT
- NM
not mentioned
- NS
nasal septum
- SE
spin-echo
- SI
signal intensity
- T1WI
T1-weighted image
- T2WI
T2-weighted image
References
- 1. Miller FR, D'Agostino MA, Schlack K. Lobular capillary hemangioma of the nasal cavity. Otolaryngol Head Neck Surg 1999; 120: 783–84 [DOI] [PubMed] [Google Scholar]
- 2. Jones JE, Nguyen A, Tabaee A. Pyogenic granuloma (pregnancy tumor) of the nasal cavity: a case report. J Reprod Med 2000; 45: 749–53 [PubMed] [Google Scholar]
- 3. Ozcan C, Apa DD, Görür K. Pediatric lobular capillary hemangioma of the nasal cavity. Eur Arch Otorhinolaryngol 2004; 261: 449–51 [DOI] [PubMed] [Google Scholar]
- 4. Puxeddu R, Berlucchi M, Ledda GP, et al. Lobular capillary hemangioma of the nasal cavity: a retrospective study on 40 patients. Am J Rhinol 2006; 20: 480–84 [DOI] [PubMed] [Google Scholar]
- 5. el-Sayed Y, al-Serhani A. Lobular capillary haemangioma (pyogenic granuloma) of the nose. J Laryngol Otol 1997; 111: 941–45 [DOI] [PubMed] [Google Scholar]
- 6. Mills SE, Cooper PH, Fechner RE. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma—a study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol 1980; 4: 470–79 [PubMed] [Google Scholar]
- 7. Kapadia SB, Heffner DK. Pitfalls in the histopathologic diagnosis of pyogenic granuloma. Eur Arch Otorhinolaryngol 1992; 249: 195–200 [DOI] [PubMed] [Google Scholar]
- 8. Raboso E, Rosell A, Plaza G, et al. Haemangioma of the maxillary sinus. J Laryngol Otol 1997; 111: 638–40 [DOI] [PubMed] [Google Scholar]
- 9. Doyle DE. Anterior epistaxis: a new nasal tampon for fast, effective control. Laryngoscope 1986; 96: 279–81 [DOI] [PubMed] [Google Scholar]
- 10. Bhattacharyya N, Wenokur RK, Goodman ML. Endoscopic excision of a giant pyogenic granuloma of the nasal cavity caused by nasal packing. Rhinology 1997; 35: 44–45 [PubMed] [Google Scholar]
- 11. Sheen TS, Ko JY, Hsu YH. Pyogenic granuloma: an uncommon complication of nasal packing. Am J Rhinol 1997; 11: 225–27 [DOI] [PubMed] [Google Scholar]
- 12. Kapella M, Panosetti E, Rombaux P, et al. Lobular capillary haemangioma of the nasal cavity: observation of three specific cases. Acta Otorhinolaryngol Belg 2001; 55: 241–46 [PubMed] [Google Scholar]
- 13. Kurtaran H, Uraldi C, Ark N, et al. Lobular capillary hemangioma of the middle turbinate. Acta Otolaryngol 2006; 126: 442–44 [DOI] [PubMed] [Google Scholar]
- 14. Katori H, Tsukuda M. Lobular capillary hemangioma of the nasal cavity in child. Auris Nasus Larynx 2005; 32: 185–88. Epub 2005 Mar 23 [DOI] [PubMed] [Google Scholar]
- 15. Lee HM, Lee SH, Hwang SJ. A giant pyogenic granuloma in the nasal cavity caused by nasal packing. Eur Arch Otorhinolaryngol 2002; 259: 231–33 [DOI] [PubMed] [Google Scholar]
- 16. Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol 2004; 21: 10–13 [DOI] [PubMed] [Google Scholar]
- 17. Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol 2003; 6: 495–510 [DOI] [PubMed] [Google Scholar]
- 18. Willies-Jacobo LJ, Isaacs H, Stein MT. Pyogenic granuloma presenting as a congenital epulis. Arch Pediatr Adolesc Med 2000; 154: 603–05 [DOI] [PubMed] [Google Scholar]
- 19. Lance E, Schatz C, Nach R, et al. Pyogenic granuloma gravidarum of the nasal fossa: CT features. J Comput Assist Tomogr 1992; 16: 663–64 [PubMed] [Google Scholar]
- 20. Choudhary S, MacKinnon CA, Morrissey GP, et al. A case of giant nasal pyogenic granuloma gravidarum. J Craniofac Surg 2005; 16: 319–21 [DOI] [PubMed] [Google Scholar]
- 21. Yuan K, Lin MT. The roles of vascular endothelial growth factor and angiopoietin-2 in the regression of pregnancy pyogenic granuloma. Oral Dis 2004; 10: 179–85 [DOI] [PubMed] [Google Scholar]
- 22. Toida M, Hasegawa T, Watanabe F, et al. Lobular capillary hemangioma of the oral mucosa: clinicopathological study of 43 cases with a special reference to immunohistochemical characterization of the vascular elements. Pathol Int 2003; 53: 1–7 [DOI] [PubMed] [Google Scholar]
- 23. Iwata N, Hattori K, Nakagawa T, et al. Hemangioma of the nasal cavity: a clinicopathologic study. Auris Nasus Larynx 2002; 29: 335–39 [DOI] [PubMed] [Google Scholar]