Abstract
BACKGROUND AND PURPOSE:
CTA is becoming the frontline modality to reveal aneurysms in patients with SAH. However, in about 20% of SAH patients no aneurysm is found. In these cases, intra-arterial DSA is still performed. Our aim was to evaluate whether negative findings on CTA can reliably exclude aneurysms in patients with acute SAH.
MATERIALS AND METHODS:
We conducted a retrospective analysis of all negative findings on CTAs performed from 2005 to 2009 in patients with spontaneous SAH. Findings were compared with DSA. CTAs were performed with a 64-section multidetector row CT scanner.
RESULTS:
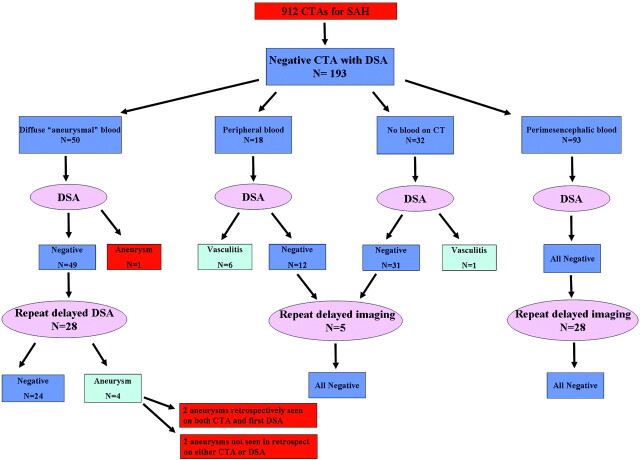
One hundred ninety-three patients with SAH and negative findings on CTA who underwent subsequent DSA were identified. The distribution of blood on unenhanced CT was the following: PMH in 93 patients, diffuse aneurysmal pattern in 50, no blood on CT (xanthochromic LP) in 32, and peripheral sulcal distribution in 18. All patients with PMH had negative findings on DSA. One patient with no blood on CT had vasculitis on DSA. Six of 18 (33%) patients with peripheral blood had vasculitis on DSA. Three of these were also diagnosed by CTA. All except 1 patient with diffuse aneurysmal blood had negative findings on DSA. One patient was diagnosed with an aneurysm on DSA (1/50, 0.5%). Repeat delayed DSA performed in 28 of these patients revealed a small aneurysm in 4 (14%). Five patients had a complication of DSA (2.6%); 1 was a clinical stroke (0.5%).
CONCLUSIONS:
In patients with SAH, negative CTA findings are reliable in ruling out aneurysms in the PMH pattern or no blood on CT. DSA is indicated in the diffuse aneurysmal pattern of SAH, and repeat delayed DSA is required if the initial DSA findings are negative. When the blood is peripheral, CTA should be scrutinized for vasculitis and DSA is recommended for confirmation.
Imaging of head and neck vessels with CTA has evolved rapidly since the introduction of helical CT with the ability to scan quickly and capture the arterial phase of enhancement. Multisection CT scanners such as the 64-section scanner enable isotropic imaging, which provides high-resolution 3D reconstructions and further shortened acquisition time.1
Recently Agid et al2 evaluated the efficacy of 64-section CTA in detecting the cause of acute SAH and its ability to display the vascular anatomy for choosing the appropriate treatment. It was shown that CTA is useful in deciding whether to coil or clip an aneurysm. It has been proposed that multidetector CTA is an accurate and sufficient tool for detecting and characterizing aneurysms in the setting of acute SAH.2–4 At both institutions participating in the current study, CTA is the first-line imaging technique for patients presenting with SAH, eliminating the necessity for DSA in most patients.5,6
A significant percentage (15%–20%) of patients presenting with SAH have no aneurysm or other underlying vascular abnormality detected on their initial vascular imaging.7 Our current practice for all patients with negative CTA findings is to perform a DSA. If the DSA findings are negative and there is a diffuse aneurysmal bleed, a repeat delayed DSA is typically performed 5–7 days later to detect a previously occult aneurysm.
The purpose of this study was to evaluate the reliability of negative findings on CTA in excluding an underlying aneurysm as the cause of SAH, with the secondary aim to avoid performing DSA in these patients.
Materials and Methods
Two large neurosurgical and neurointerventional tertiary referral centers participated in the study, which was approved by our local institutional ethics review boards. The results of all consecutive CTAs performed for the indication of spontaneous SAH from February 2005 until March 2009 were retrospectively evaluated. All patients who presented with an acute SAH and in whom the initial CTA findings were negative for intradural cerebral aneurysms were included in the study. Patients with a history of trauma or with hemorrhage confined to the ventricles or with frank intraventricular hemorrhage were excluded. Patients without DSA were also excluded from this study.
The results of the CTA, defined as the interpretation by the neuroradiologist before DSA, were compared with the findings on the subsequent DSA. All CTA interpretations were independent of DSA findings and were dictated before performing DSA.
The patients were divided into 4 groups according to the distribution of subarachnoid blood on the unenhanced CT of the brain at presentation: 1) PMH, 2) diffuse aneurysmal pattern, 3) positive LP (xanthochromic LP) with no blood on CT, and 4) peripheral sulcal pattern (with absence of basal cistern blood). The reliability of CTA was assessed for each of these patterns. Examples of the patterns of bleeding are illustrated in Fig 1. In group 1, PMH was defined as blood confined to the cisterns around the midbrain in the prepontine, interpeduncular, and ambient cisterns. Slight thin extension into the suprasellar cistern, the most proximal aspect of the Sylvian fissures, and proximal anterior interhemispheric fissure was still regarded as PMH, but not extension into the distal parts of these fissures and not into the ventricle.8 The presence of thick blood above the perimesencephalic cisterns was also not included as PMH.8 Whenever a case was ambiguous, it was regarded as group 2, diffuse aneurysmal pattern.
Fig 1.
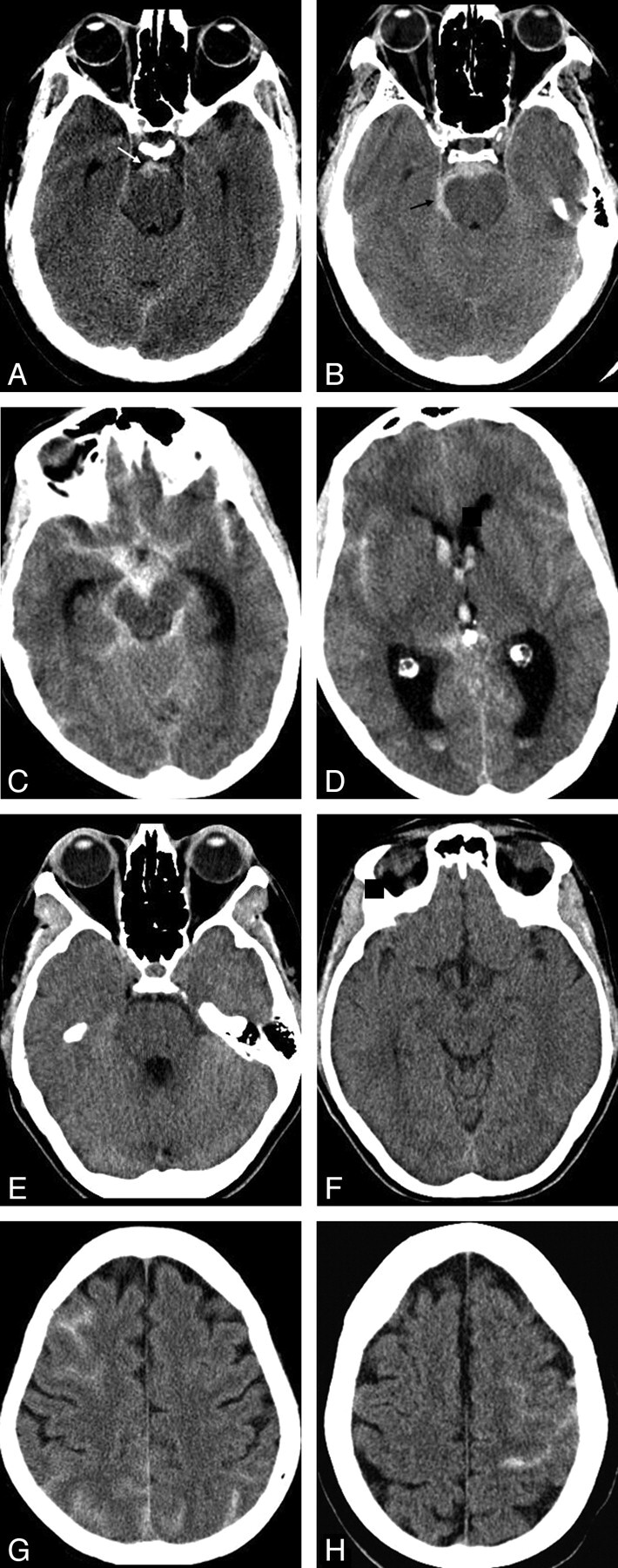
Four groups of patients with a negative CTA findings according to the distribution of subarachnoid blood on the initial plain CT of the brain. A and B, Group 1: typical perimesencephalic distribution of blood as seen in 2 of our patients. Blood is confined to the prepontine and interpeduncular cisterns (white arrow, A). Extension into the right ambient cistern (black arrow, B) which is still accepted as PMH.9 C and D, Group 2: two CT images from 1 patient with a diffuse aneurysmal pattern of blood distribution. Blood is diffusely distributed throughout the basal cisterns and extends distally into the Sylvian fissures and ventricles with mild hydrocephalus. E and F, Two CT images of a patient from group 3 who presented with thunderclap headaches and a positive LP but no subarachnoid blood noted on CT. G and H, Two CT images from 2 patients in group 4, with blood located peripherally in the sulci of the brain and no blood in the basal cisterns.
CT Angiography
All CTA examinations were performed by using a 64-section multidetector CT scanner.
CTA Protocol at Hospital 1 (97 cases).
The CT scanner used was an Aquilion 64 (Toshiba Medical Systems, Tokyo, Japan). Scanning was performed from the aortic arch to the vertex. Intravenous contrast (iodixanol, Visipaque 270; GE Healthcare, Mississauga, Canada) was administered by using a mechanical injector (Stellant CT injector; Medrad, Indianola, Pennsylvania). The injection rate was 4 mL/s, for a total volume of 60 mL followed by 20 mL of normal saline. Adequate timing of the CTA acquisition was achieved according to a Sure Start (Toshiba Medical Systems) technique. Radiation parameters were 300 mA; 120 kV; matrix, 512 × 512; FOV, 28–32 cm; section thickness, 0.5 mm; pitch, 1.0; and an isotropic voxel size of 0.5 mm. Acquisition time was 11–16 seconds. Image processing consisted of standardized axial, coronal, and sagittal multiplanar volume reformatted MIP images (8-mm thickness, 2-mm reconstruction interval) and 3D volume-rendered reconstructions (performed on Advantage Windows Workstation, Version 4.2 or 4.3; GE Healthcare, Milwaukee, Wisconsin).
CTA Protocol at Hospital 2 (96 cases).
The CT scanner used was LightSpeed VCT (GE Healthcare). Scanning was performed from below the posterior arch of C1 to above the corpus callosum; all investigations included the intradural part of the vertebral arteries to the pericallosal arteries with a minimum 12 cm of scanning. Intravenous contrast (iomeprol, Iomeron 400; Bracco, Milan, Italy) was administered by using a power dual injector (Stellant D CT Injector; Medrad). The injection rate was 4 mL/s, for a total volume of 60 mL followed by a saline chaser of 80 mL. Adequate timing of the CTA acquisition was achieved according to a SmartPrep technique (GE Healthcare). Data acquisition was performed according to the following protocol: spiral mode; 0.4-second sections; collimation, 32 × 0.625 mm; pitch, 0.516:1; section thickness, 0.625 mm; reconstruction interval, 0.315 mm. Acquisition parameters were 600 mA and 100 kV, a head filter with a display FOV of 15 cm, and a standard reconstruction algorithm. The images were reformatted in axial, sagittal, and coronal 4-mm-thick MIPs with 50% overlap as well as 3D volume-rendered reconstructions using an Advantage Workstation, Version 4.3 or 4.4, with the software Volume Viewer (GE Healthcare).
CTAs were evaluated for the cause of SAH, including the presence or absence of an intradural cerebral arterial aneurysm. Each CTA was interpreted by an experienced neuroradiologist before performing the DSA.
Diagnostic Cerebral Angiograms (DSA)
DSA was performed in most patients (168/193, 87%) on the same day or within 24 hours following CTA. In the remainder of patients (25/193, 13%), DSA was performed between 24 hours and 5 days after CTA (mean, 1.25 days). Reasons for delay varied and included need for patient stabilization or transfer from other hospital campuses.
DSA Protocol at Hospital 1 (97 cases).
DSA was performed by using a dedicated biplane neuroangiography unit (LUA/LCN, GE Medical Systems, Buc, France) and included 4- to 6-vessel studies with standard Towne and lateral views as well as rotational spin angiograms with 3D reconstructions. A standard 4F or 5F Berenstein diagnostic catheter (AngioDynamics, Queensbury, New York) was used with the help of an angled 0.35-inch guidewire (Terumo; Meditech, Watertown, Massachusetts). The contrast media used for injections was nonionic (iodixanol, Visipaque 320; GE Healthcare). Injections were performed with a power injector (Mark V ProVis; Medrad). Standard injection rates and volumes were as follows: 4–5 mL/s for 8–10 mL for the ICAs, 3–4 mL/s for 7–8 mL/s for the vertebral arteries, and 2–3 mL/s for 5–8 mL for the external carotid arteries.
DSA Protocol at Hospital 2 (96 cases).
DSA was performed by using a dedicated biplane neuroangiography unit (Allura Xper FD20/20; Philips Medical Systems, Best, the Netherlands) and comprised 4- to 6-vessel studies with standard Towne and lateral views as well as rotational spin angiography with 3D reconstructions. A standard 4F Berenstein diagnostic catheter (Merit Medical Systems, South Jordan, Utah) was used with the help of an angled 0.35-inch guidewire. The contrast media used was nonionic (iodixanol, Visipaque 270; GE Healthcare). Injections were performed with a power injector (Mark V ProVis; Medrad). Standard injection volumes and rates were as follows: 6 mL/s for 9 mL for the ICAs, 4 mL/s for 8 mL for the vertebral arteries, and 2–3 mL/s for 5–8 mL for the external carotid arteries.
Statistical Analysis
Descriptive statistics (ie, the number and percentage of cases correctly classified by CTA) were calculated to summarize the degree of agreement between CTA and DSA for each of the outcome measures. Sensitivity, specificity, PPV, and NPV were also calculated for outcomes in which both positive and negative events were found to occur (ie, vasculitis).
Results
From a total of 912 CTAs performed for SAH in the study period, we identified 202 patients with negative findings on CTA. One hundred ninety-three had undergone DSA and were thus included in the study. (Nine patients did not receive DSA. Four of these were patients with perimesencephalic pattern of hemorrhage in whom the referring neurosurgeon elected to accept the CTA results. Five patients presented with diffuse SAH in grave unstable clinical grade in whom treatment was not considered). One hundred seventeen patients were males, and 76 were females between the ages 16 and 82 (average, 51.6 years; median, 52 years). The results are summarized in Fig 2. Ninety-three patients presented with PMH (58 men, 35 women; ages, 27–82 years); 50, with diffuse aneurysmal blood pattern (33 men, 17 women; ages 19–79 years); 32, with no blood on CT but a positive LP (20 males, 12 females; ages, 16–75 years); and 18, with peripheral sulcal blood (6 men, 12 women; ages 27–80 years).
Fig 2.
Diagram represents the results of DSA in all patients included in this study, according to patterns of blood distribution on the initial CT.
Examples of the 4 patterns of bleeding are illustrated in Fig 1.
Clinical Presentation
All of the patients with a perimesencephalic bleed and all patients with no blood on CT and a positive LP (groups 1 and 3) presented in excellent clinical grade with a GCS score of 15. All except 2 of the patients with a peripheral sulcal bleed (group 4) presented with a GCS score of 15; 2 patients presented with a GCS score of 14. The patients who presented with diffuse aneurysmal bleed (group 2) had GCS scores ranging from 4 to 15.
Comparison of CTA with DSA
Group 1: Perimesencephalic Bleed.
All 93 patients had negative findings on DSA confirming the negative results of the CTA. Twenty-eight (28/93, 30.1%) of these patients had follow-up imaging. Fourteen underwent repeat delayed DSA; 6, repeat delayed CTA; 6, MRA; and 2, repeat CTA and DSA. Most of these studies were performed 1 week after presentation (n = 17); some were performed 1–3 months later (n = 11). Findings of all follow-up studies were negative. Findings on 1 CTA in these 93 patients were suspected of representing vasculitis; however, DSA findings were normal and negative for vasculitis.
Group 2: Diffuse Aneurysmal Bleed.
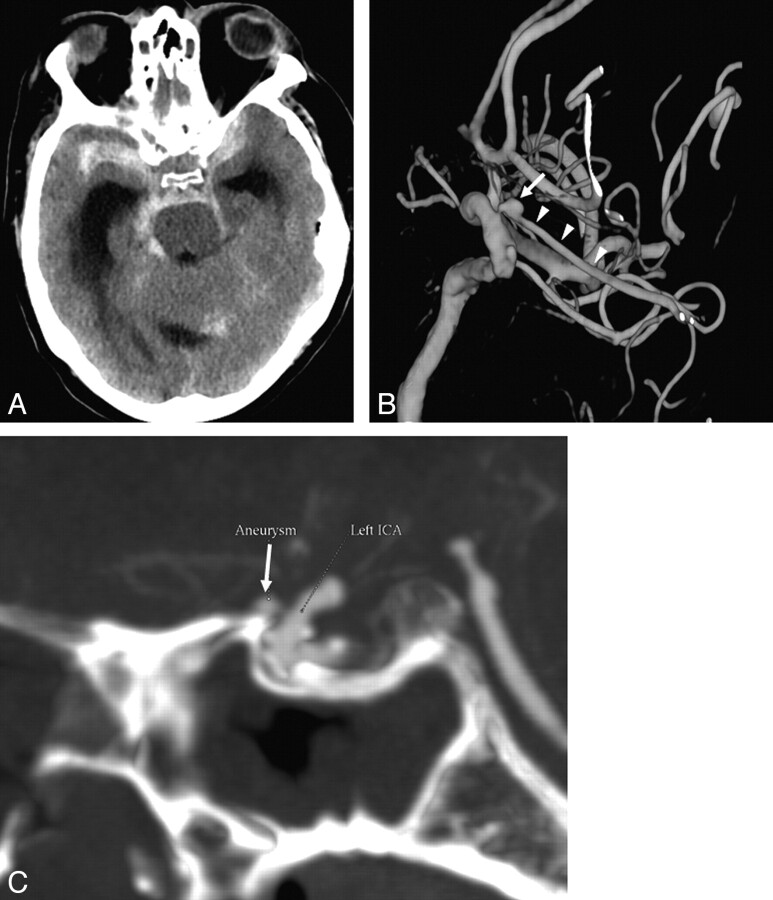
Forty-nine of these patients had negative findings on the first DSA. One DSA revealed an aneurysm (1/50, 2%). This aneurysm measured 2.9 mm and was located on the ophthalmic artery, 2 mm from its origin. This aneurysm was proved during surgery to be the source of hemorrhage. Retrospectively, this aneurysm could be seen on the CTA as well (Fig 3).
Fig 3.
False-negative CTA findings in a 58-year-old man who presented with SAH and a GCS score of 4. A, Axial nonenhanced CT at presentation demonstrates a diffuse aneurysmal pattern of SAH and hydrocephalus. CTA did not reveal an aneurysm; however, an ophthalmic aneurysm measuring 2.9 mm in diameter was demonstrated on DSA. B, 3D reconstructions of DSA of the left ICA shows the aneurysm (arrow) located proximally on the ophthalmic artery (arrowheads). C, Retrospective analysis of the CTA revealed the ophthalmic artery aneurysm as seen on the sagittal MIP reconstruction (arrow). A second small extradural cavernous carotid aneurysm is noted as well (B), which is of no clinical significance.
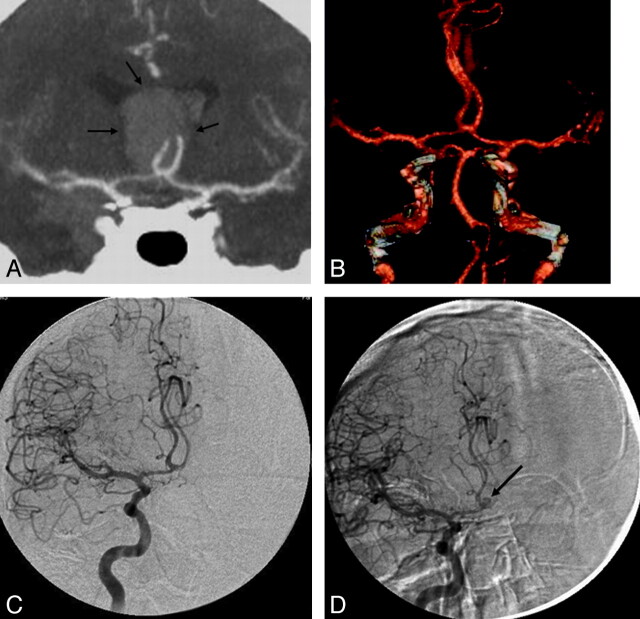
Repeat delayed DSA was performed in 28 patients, revealing a small aneurysm in 4 (4/28, 14%). These included a 3-mm distal posterior inferior cerebellar artery aneurysm and a 1.4-mm M1 segment aneurysm, which could be retrospectively seen on both the CTA and the first DSA. A 1.4-mm basilar tip aneurysm and a 3-mm AcomA aneurysm could not be identified retrospectively on the CTA or the first DSA (Figs 4 and 5). The patient with the AcomA aneurysm underwent a repeat delayed CTA in addition to DSA that showed the aneurysm; however, the patient with the basilar tip aneurysm did not undergo a repeat delayed CTA.
Fig 4.
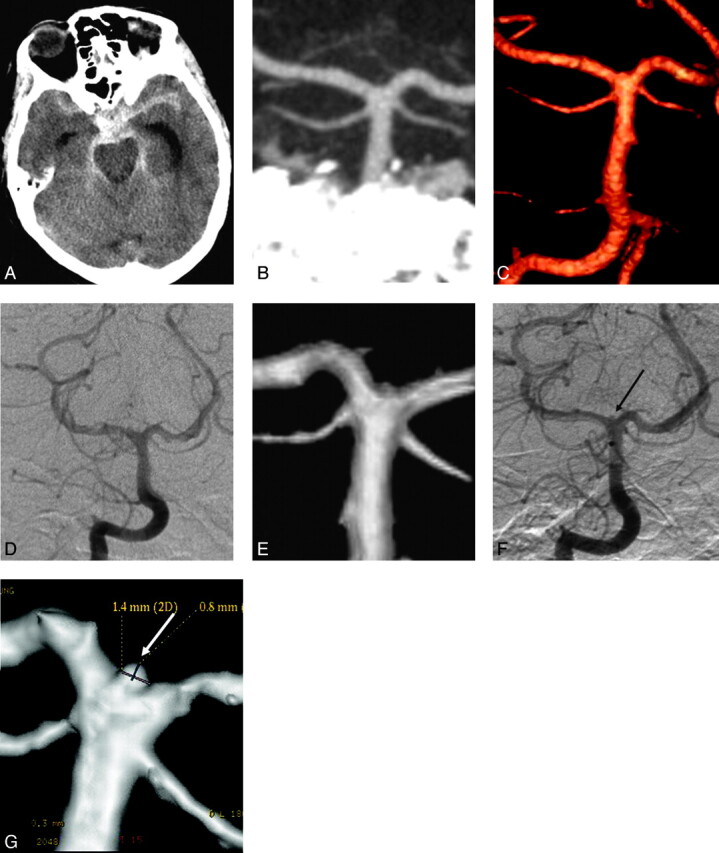
A 64-year-old woman who presented with SAH. A, Plain axial CT of the brain at presentation demonstrates a diffuse aneurysmal pattern of blood. B and C, CTA images of the posterior intracranial circulation, including a coronal MIP reconstruction (B) and a 3D reconstruction (C), show an intact basilar tip with no evidence of an aneurysm. The remainder of the CTA is also unremarkable. D and E, A conventional catheter angiogram (DSA) of the right vertebral artery from the same day in an anteroposterior projection (D) and a 3D reconstruction (E) confirm the results of CTA. F and G, A repeat delayed DSA 1 week later reveals a 1.4-mm aneurysm at the basilar tip on both the anteroposterior and 3D views (arrows). This aneurysm was likely partially thrombosed at the acute stage. Note a tiny “bump” at the basilar tip on the 3D reconstructions of both the CTA and first DSA (C and E). In retrospect, this is the location of the aneurysm; however, there was not enough evidence for it to be called an aneurysm on the original scans.
Fig 5.
A 53-year-old man presented with a diffuse aneurysmal pattern of SAH. A and B, A thick clot at the anterior interhemispheric fissure raised suspicion of an ICA aneurysm responsible for the hemorrhage (arrows, A). However, CTA at presentation did not reveal an aneurysm, as seen on the coronal MIP (A) and 3D reconstructions (B). C, DSA is also negative for aneurysms. D, A repeat delayed DSA performed 1 week later reveals a 3-mm aneurysm at the ICA (arrow), which was likely thrombosed at presentation.
The false-negative rate of the CTA for the patients with the diffuse aneurysmal pattern was thus 6% (3/50). This included the aneurysm seen on the first DSA and 2 aneurysms missed on both CTA and the first DSA.
Twenty-one (21/49) patients did not have repeat delayed DSA. Seventeen of these patients were at an institution where repeat delayed DSA is not routinely practiced. Four elderly patients underwent repeat delayed CTA instead of DSA due to tortuous vascular anatomy and technical difficulties during the original DSA. All 4 scans were negative for aneurysms. No early rebleeds were encountered in any of these patients.
Three patients underwent a third DSA, the findings of which were still negative. Six of the patients who had delayed DSA also underwent repeat CTA. Four of these were performed at the same time as the delayed DSA, and 2 were performed 2 months and 1 week after the delayed DSA. All of these scans were negative for aneurysms.
In total, 7 patients with diffuse aneurysmal blood with repeat delayed DSA also underwent repeat delayed CTA. Findings in 2 of these patients were positive for an aneurysm on repeat DSA (AcomA and M1 segment). Both aneurysms were seen on the repeat CTA as well.
Group 3: No Blood on CT and Positive LP.
All 32 patients had a DSA negative for aneurysms. One patient (1/32, 3.1%) was diagnosed by DSA with vasculitis. The arterial irregularities could be seen retrospectively on CTA.
Most of these patients did not have follow-up imaging. Three (3/32) patients had follow-up MRA, 1 patient at 2 weeks, 1 at 3 months; 1 had 4 repeat MRAs ≤5 years posthemorrhage. Findings of all follow-up studies were negative.
Group 4: Peripheral Sulcal Bleed.
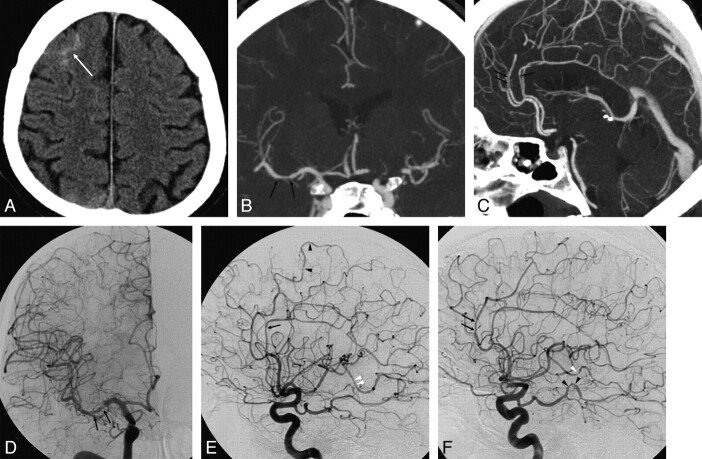
All 18 patients had a DSA negative for aneurysms. Six of these patients (6/18, 33.3%) were diagnosed with vasculitis by DSA. Three of these vasculitis cases were diagnosed by CTA and confirmed by DSA. Three patients with vasculitis were only diagnosed on the DSA, but it could be retrospectively seen on the CTA (Fig 6). Two of the patients with negative DSA findings (2/12) underwent delayed DSA (at 2 weeks and 3 months after presentation), both with negative findings.
Fig 6.
A 48-year-old man who presented with severe sudden headache. A, Axial nonenhanced CT at presentation demonstrates peripheral sulcal blood (arrow) with no hemorrhage at the basal cisterns and no history of trauma. B and C, Coronal (B) and sagittal (C) MIPs of the CTA reveal no aneurysm. D−F, Anteroposterior and lateral projections of a catheter angiogram of the right ICA (D and E) and a lateral projection of the left ICA angiogram (F) demonstrate multiple irregularities and narrowings in medium-sized vessels, including the M1 segment of the right MCA (arrows in D), A3 branches of the ACAs (arrows in E and F), P3 branches of the left PCA (black arrowheads in F), and M3 branches of the right and left MCA (white arrowheads in E and F). These findings on DSA suggest underlying vasculitis as the cause of hemorrhage. Retrospective analysis of coronal and sagittal MIP reconstructions of the CTA reveals reciprocal irregularities and narrowings at the M1 segment of the right MCA and at the A3 branches of the ACAs (arrows in B and C).
General Statistics
Exclusion of Aneurysms by Negative CTA Findings.
All of the missed aneurysms were in patients with diffuse aneurysmal patterns of blood. Five of these patients were eventually diagnosed with an aneurysm (5/50, 10%). Of these, 1 aneurysm was a true false-negative (1/50, 2%) because it was missed on CTA and discovered on the first DSA. Two aneurysms that were missed on both CTA and the first DSA and diagnosed on repeat DSA could be seen in retrospect on both original modalities. These 2 aneurysms were missed on CTA and could be added to the false-negative rate of CTA, making it 6% (3/50).
Diagnosis of Vasculitis on CTA
This study included 7 patients with suggested vasculitis on DSA (7/193, 3.6%). Three of these (3/7, 43%) were diagnosed on CTA, and 4 (4/7, 57%) were only seen retrospectively. One case of vasculitis supposedly diagnosed on CTA was proved to be negative on the DSA. The sensitivity of CTA for the diagnosis of vasculitis was 0.43 (95% CI: 0.12, 0.80), the specificity was 0.99 (95% CI: 0.97, 1.00), the PPV was 0.75 (95% CI: 0.22, 0.99), and the NPV was 0.98 (95% CI: 0.94, 0.99). Most of the vasculitis cases were in patients who had peripheral distribution of blood (6/7, 85.7%).
The diagnosis of cerebral vasculitis suggested on DSA was supported in 4 of the 7 patients clinically and by lab results. These 4 patients were treated with steroids, and 3 of them also received immunosuppressive treatment for primary cerebral angiitis. None of the patients with vasculitis underwent a brain biopsy.
Complications of DSA
Five patients had complications from the DSA (5/193, 2.6%): One patient (0.5%) had memory loss from a thalamic stroke, 1 had an asymptomatic arterial occlusion of the anterior inferior cerebellar artery, and 3 had asymptomatic arterial dissections.
Discussion
CTA is helpful in triaging patients with ruptured aneurysms to either endovascular coiling or surgical clipping.2–4 However, when CTA findings are negative, DSA is still performed at most institutions due to the concern that an intradural cerebral aneurysm has been missed. In patients with a perimesencephalic distribution of blood, an aneurysm in the posterior circulation must be excluded. Some authors have doubted the ability of CTA to reveal small posterior circulation aneurysms due to bony artifacts.9 However, several studies have shown that CTA is sensitive in detecting small aneurysms, including those in the posterior circulation.2,3,10–12
Patients with a DSA negative SAH are not a homogeneous group. Rinkel et al7 divided these patients according to the pattern of blood distribution as it appears on the initial nonenhanced CT scan of the brain.7 According to Rinkel et al, 2/3 of these patients have a perimesencephalic pattern of hemorrhage. In PMH, the blood is centered in the perimesencephalic cisterns, mostly in the prepontine and interpeduncular cisterns. PMH can extend into the ambient cisterns and sometimes into the suprasellar cistern and even into the most proximal aspect of the Sylvian fissures and anterior interhemispheric fissure, but not into the distal parts of these fissures and not into the ventricle.8 Patients with a PMH present in good clinical grade (Hunt and Hess scale 1 or World Federation of Neurological Surgeons Scale 1) with headaches only, no neurologic deficits, no loss of consciousness, and have a good prognosis.13
The second group of patients are those with a diffuse “aneurysmal pattern” of blood on CT. As opposed to those with PMH, these patients have a potential risk of rebleeding, likely due to an unstable clot within an occult aneurysm. Even without a proved rebleed, this group of patients have more significant symptoms at onset, a rough in-hospital clinical course, and a relatively poor prognosis compared with those with a PMH.14 The underlying cause of hemorrhage in patients with a diffuse aneurysmal pattern of blood and negative repeat vascular imaging is still unclear. Possible causes include an extremely small aneurysm that cannot be detected by DSA, a dissection not depicted on DSA, or rupture of an atherosclerotic vessel wall.14–17 The last group that Rinkel et al7 mentioned had no blood on CT but a positive LP. This group is rare and often similar clinically to patients with PMH with a low rebleed risk.7 In our study, we added a fourth group to the classification of Rinkel et al on the basis of distribution of blood. Peripheral sulcal SAH without blood in the basal cisterns represents a distinct group of patients with nontraumatic SAH. In this study, this pattern was suggestive of vasculitis (33%) that could be detected on CTA (50% prospectively, 50% retrospectively).
According to Rinkel et al,7 the cause of PMH is likely not aneurysmal but may be venous and the patients have no risk of rebleed.18 Thus, Rinkel et al have stated that patients with PMH do not need a repeat DSA after initial negative findings on DSA.18 They also suggested that DSA is not needed at all in patients with PMH who had negative CTA findings.12 To reinforce this point, Ruigrok et al19 used a mathematic model to evaluate the utility of 4 different strategies for excluding aneurysms in patients with a PMH pattern of SAH. The 4 options studied were the following: no further investigation, DSA only, CTA only, or CTA followed by DSA. They based their calculations on previously published literature (4% prevalence of vertebrobasilar aneurysms in PMH, 97% sensitivity and specificity of CTA for detecting aneurysms, 99.5% sensitivity and 100% specificity of DSA for detecting aneurysms, 2.5% complication rate of DSA) and concluded that “CTA only” was the best option for the work-up of these patients. In addition, they concluded that only when a DSA complication rate of <0.2% could be achieved was “DSA only” a preferred strategy. This study was theoretic and has not been adopted by many institutions.
We were able to find only 1 article in the literature dealing with purely negative CTA findings in patients with SAH.20 Kershenovich et al20 retrospectively evaluated 30 patients with a perimesencephalic SAH pattern and a negative CTA finding. DSA findings were negative in all of these patients. They concluded that CTA alone is a conclusive diagnostic tool for ruling out aneurysms in patients presenting with the classic perimesencephalic SAH pattern and thus can replace DSA.
Westerlaan et al3 reported 60 CTAs with negative findings of 224 performed for SAH. Of these patients, 30 had PMH, 17 patients had diffuse non-PMH blood distribution, and 13 had no blood on CT. Compared with the results of DSA, all of the findings of patients with PMH were true-negative, as were those of all of the 13 patients with no blood on the initial CT. In the diffuse non-PMH group of patients, 5 (5/17, 29%) were eventually found to harbor an aneurysm. This included 1 aneurysm seen only on DSA (false-negative for CTA) and 4 patients with rebleeds after initial negative findings on CTA and DSA. Three of these 4 patients had aneurysms diagnosed on repeat delayed vascular imaging (CTA or DSA) that were not seen on either CTA or on the first DSA. Two of these 4 aneurysms were discovered on repeat delayed CTA, 1 aneurysm was discovered on repeat DSA, and 1 patient died before repeat imaging. These findings are in keeping with the recommendations of Rinkel et al7 because no false-negative findings on CTAs were encountered in either PMH or no blood on CT. It also supports the need for repeat vascular imaging for the diffuse aneurysmal SAH group of patients.3 Our study reinforces these finding and supports the recommendations of Rinkel's group.12,19 Our 93 patients with a PMH and our 32 patients with no blood on CT had CTAs and DSAs that were negative for aneurysms.
In our patients with the diffuse aneurysmal pattern of SAH, 5 of the 50 were eventually diagnosed with an aneurysm (10%). Only 1 of these aneurysms was a true false-negative on CTA compared with DSA (Fig 6). Four aneurysms were diagnosed only on repeat DSA. Two of these 4 were actually present on both initial DSA and CTA but were overlooked. Thus interpreting either CTA or DSA is prone to human error. The 2 remaining aneurysms could only be diagnosed on delayed repeat DSA (2/28, 7.1%). This finding supports the need for repeat delayed vascular imaging and immediate DSA after the initial negative findings on CTA. In patients with SAH, the role of repeat delayed imaging after initial normal results has been debated.14,21–24 A meta-analysis found that 22 aneurysms were revealed on repeat angiography in 145 patients with SAH after initial negative results.25
A weakness of our study is that only 28 of 50 DSA-negative patients with diffuse blood underwent repeat angiography. This is because we included patients from 2 separate institutions and repeat delayed DSA is only routinely practiced in 1 of the hospitals. Thus, potentially more partially thrombosed aneurysms were missed in the remaining patients. However, none of these patients have rebled. Another potential flaw in our study was that the CTAs were performed by using different protocols in the 2 participating hospitals. However, this could be a strength of the study because the results may be applicable to a variety of CTA protocols.
In our study, vasculitis was diagnosed by DSA in 6 patients with peripheral sulcal distribution of blood and in 1 patient with no blood on CT. The diagnosis was made by CTA before performing DSA in 3 of these patients. Careful retrospective analysis of the CTAs of the 4 remaining patients revealed irregular arteries. Vasculitis should be considered in patients with peripheral blood or negative CT findings. One patient with PMH was suspected of having vasculitis on CTA, but this proved to be false-positive by DSA. Thus, whenever vasculitis is suspected, DSA is still indicated to verify suspicion of this diagnosis. None of the 7 patients with vasculitis underwent a brain biopsy and thus were not proved to actually represent primary cerebral angiitis. The diagnosis of cerebral vasculitis on DSA was supported by clinical findings and by lab results in 4 of the 7 cases and was treated accordingly. A diagnosis of cerebral vasculitis on the grounds of DSA findings alone has been shown to be nonspecific.26 Patients with clinical and angiographic features of vasculitis are commonly found to have other pathologic diagnoses such as intracranial atherosclerosis, infections, drug abuse, or reversible cerebral segmental vasoconstriction.26
Our complication rate (overall, 2.6%; symptomatic, 0.5%) from DSA was similar to that in reports in the literature.5,6 One of our patients with PMH had a clinically significant stroke after DSA, and 4 other patients had asymptomatic complications that warranted imaging follow-up. This complication rate is higher than 0.2% and thus supports the proposal of Ruigrok et al19 that “CTA only” is the preferred strategy for imaging these patients.
In summary, CTA should be the first-line investigation of nontraumatic SAH. A negative CTA finding is reliable to rule out aneurysms in the face of SAH when there is a perimesencephalic pattern of blood or when no blood is evident on CT. On the basis of our findings, DSA is not needed in these 2 groups. In a diffuse aneurysmal pattern of SAH, DSA is indicated and repeat delayed DSA is warranted if the initial DSA finding is negative. When the blood is peripherally located, CTA should be carefully scrutinized for vasculitis and DSA is recommended for confirmation. This suggested approach is demonstrated in Fig 7.
Fig 7.
Suggested imaging approach to patients presenting with acute nontraumatic SAH based on the current study.
Abbreviations
- ACA
anterior cerebral artery
- AcomA
anterior communicating artery
- CI
confidence interval
- CTA
CT angiography
- DAVF
dural arteriovenous fistula
- DSA
digital subtraction angiography
- GCS
Glasgow Coma Scale
- ICA
internal carotid artery
- LP
lumbar puncture
- MIP
maximum intensity projection
- MCA
middle cerebral artery
- MRA
MR angiography
- NPV
negative predictive value
- PMH
perimesencephalic hemorrhage
- PPV
positive predictive value
- SAH
subarachnoid hemorrhage
Footnotes
Paper previously presented at: 10th Conference of the World Federation of Interventional and Therapeutic Neuroradiolgy, June 29–July 3, 2009; Montreal, Quebec, Canada.
References
- 1. Goddard AJ, Tan G, Becker J. Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: current status. Clin Radiol 2005; 60: 1221–36 [DOI] [PubMed] [Google Scholar]
- 2. Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology 2006; 48: 787–94 [DOI] [PubMed] [Google Scholar]
- 3. Westerlaan HE, Gravendeel J, Fiore D, et al. Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology 2007; 49: 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lubicz B, Levivier M, François O, et al. Sixty-four-row multisection CT angiography for detection and evaluation of ruptured intracranial aneurysms: interobserver and intertechnique reproducibility. AJNR Am J Neuroradiol 2007; 28: 1949–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003; 227: 522–28 [DOI] [PubMed] [Google Scholar]
- 6. Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta analysis. Stroke 1999; 30: 317–20 [DOI] [PubMed] [Google Scholar]
- 7. Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm: a review of the causes. Stroke 1993; 24: 1403–09 [DOI] [PubMed] [Google Scholar]
- 8. Rinkel GJ, Wijdicks EF, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol 1991; 12: 829–34 [PMC free article] [PubMed] [Google Scholar]
- 9. Karamessini MT, Kagadis GC, Petsas T, et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms: comparison with intra-arterial DSA and the surgical findings. Eur J Radiol 2004; 49: 212–23 [DOI] [PubMed] [Google Scholar]
- 10. Villablanca JP, Achiriolaie A, Hooshi P, et al. Aneurysms of the posterior circulation: detection and treatment planning using volume-rendered three-dimensional helical computerized tomography angiography. J Neurosurg 2005; 103: 1018–29 [DOI] [PubMed] [Google Scholar]
- 11. Villablanca JP, Jahan R, Hooshi P, et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. AJNR Am J Neuroradiol 2002; 23: 1187–98 [PMC free article] [PubMed] [Google Scholar]
- 12. Velthuis BK, Rinkel GJ, Ramos LM, et al. Perimesencephalic hemorrhage: exclusion of vertebrobasilar aneurysms with CT angiography. Stroke 1999; 30: 1103–09 [DOI] [PubMed] [Google Scholar]
- 13. Brilstra EH, Hop JW, Rinkel GJ. Quality of life after perimesencephalic haemorrhage. J Neurol Neurosurg Psychiatry 1997; 63: 382–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruigrok YM, Rinkel GJ, Van Gijn J. CT patterns and long-term outcome in patients with an aneurysmal type of subarachnoid hemorrhage and repeatedly negative angiograms. Cerebrovasc Dis 2002: 14: 221–27 [DOI] [PubMed] [Google Scholar]
- 15. Spallone A, Ferrante L, Palatinsky E, et al. Subarachnoid haemorrhage of unknown origin. Acta Neurochir (Wien) 1986; 80: 12–17 [DOI] [PubMed] [Google Scholar]
- 16. Sakai N, Yamada H, Ando T, et al. Prevention of rebleeding after operation for subarachnoid hemorrhage of unknown cause. Neurosurgery 1985; 17: 942–46 [DOI] [PubMed] [Google Scholar]
- 17. Tatter SB, Crowell RM, Ogilvy CS. Aneurysmal and microaneurysmal “angiogram-negative” subarachnoid hemorrhage. Neurosurgery. 1995; 37: 48–55 [DOI] [PubMed] [Google Scholar]
- 18. van der Schaaf IC, Velthuis BK, Gouw A, et al. Venous drainage in perimesencephalic hemorrhage. Stroke 2004; 35: 1614–18 [DOI] [PubMed] [Google Scholar]
- 19. Ruigrok YM, Rinkel GJ, Buskens E, et al. Perimesencephalic hemorrhage and CT angiography: a decision analysis. Stroke 2000; 31: 2976–83 [DOI] [PubMed] [Google Scholar]
- 20. Kershenovich A, Rappaport ZH, Maimon S. Brain computed tomography angiographic scans as the sole diagnostic examination for excluding aneurysms in patients with perimesencephalic subarachnoid hemorrhage. Neurosurgery 2006; 59: 798–801 [DOI] [PubMed] [Google Scholar]
- 21. Gilbert JW, Lee C, Young B. Repeat cerebral pan-angiography in subarachnoid hemorrhage of unknown etiology. Surg Neurol 1990; 33: 19–21 [DOI] [PubMed] [Google Scholar]
- 22. du Mesnil de Rochemont R, Heindel W, Wesselmann C, et al. Nontraumatic subarachnoid hemorrhage: value of repeat angiography. Radiology 1997; 202: 798–800 [DOI] [PubMed] [Google Scholar]
- 23. Urbach H, Zentner J, Solymosi L. The need for repeat angiography in subarachnoid haemorrhage. Neuroradiology 1998; 40: 6–10 [DOI] [PubMed] [Google Scholar]
- 24. Topcuoglu MA, Ogilvy CS, Carter BS, et al. Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg 2003; 98: 1235–40 [DOI] [PubMed] [Google Scholar]
- 25. Rinkel GJ, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm. In: Yanagihara T, Piepgras DG, Atkinson JLD. eds. Subarachnoid Hemorrhage: Medical and Surgical Management. New York: M. Dekker; 1998: 139–58 [Google Scholar]
- 26. Kadkhodayan Y, Alreshaid A, Moran CJ, et al. Primary angiitis of the central nervous system at conventional angiography. Radiology 2004; 233: 878–82 [DOI] [PubMed] [Google Scholar]