SUMMARY:
Cetuximab, a recombinant chimeric monoclonal antibody, has been successfully used in the treatment of the head and neck and colorectal cancers. We present a review of its mechanism of action, indications, side effects and economic issues, accompanied by a clinical example from our institution.
Cetuximab (Erbitux), a recombinant human/mouse chimeric monoclonal antibody, has been approved by the FDA for use in patients with locally advanced SCCHN and in combination with irinotecan for the treatment of metastatic colorectal cancer.1–5 Recent published studies positively evaluated cetuximab as a new option in the first-line treatment of advanced non-small cell lung cancer, but this has not yet been approved by the FDA.3,6
Mechanism of Action
HERs are cell membrane-bound glycoproteins, comprising 4 distinct receptors: EGFR, also known as HER1; HER2; HER3; and HER4.2,7,8 These receptors are divided into 3 regions: an extracellular ligand-binding region, an intracellular region with tyrosine kinase activity, and a region that spans the cell membrane and anchors the receptor to the cell. Ligand binding to the extracellular domain promotes formation of dimers, homodimers (between monomers of same receptor), or heterodimers (between the bound receptor and other members of the HER family), activating tyrosine kinase, triggering a cascade of complex cell biochemistry that regulates various cell functions, such as cell proliferation, angiogenesis, apoptosis, adhesion, and motility.7,8 Figures 1 and 2 illustrate the normal and the overexpression of the EGFR and its pathway when binding with a ligand, resulting in dimerization and initiation of an intracellular cascade.
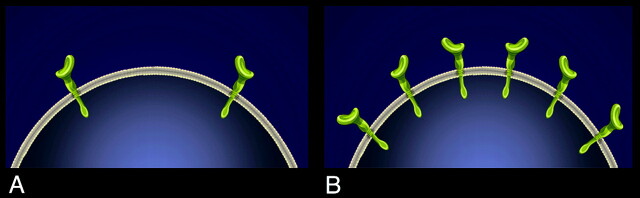
Fig 1.
Schematic representation of normal expression of EGFR (HER1) (A) and the overexpression of EGFR (B).
Fig 2.
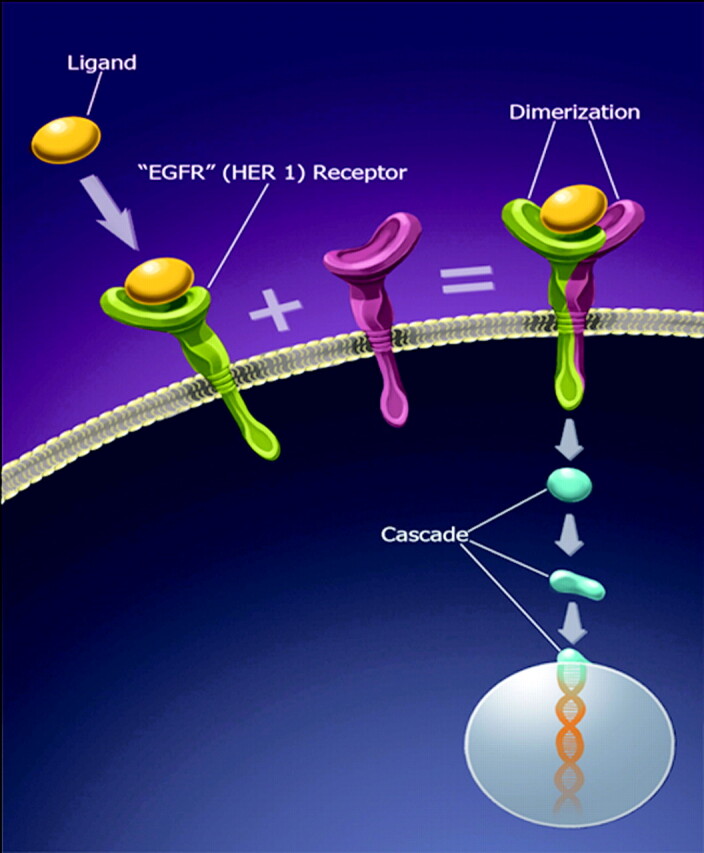
Illustration of the EGFR (HER1) pathway. Ligand binding to EGFR results in dimerization. Successful dimerization results in initiation of a cascade, which results in cell proliferation.
Cetuximab, a monoclonal antibody, binds to the extracellular domain of the EGFR, which is overexpressed in many human cancers, including head and neck and colorectal types. This process prevents the EGFR from binding with its endogenous ligand, blocking the receptor-dependent transduction pathway and providing many antitumor effects, involving cell-cycle arrest, induction of apoptosis, inhibition of angiogenesis, inhibition of metastasis, internalization and downregulation of the EGFR, and enhancement of the sensitivity to radiochemotherapy.1,4,10 Figure 3 illustrates the effect of cetuximab on the EGFR pathway.
Fig 3.
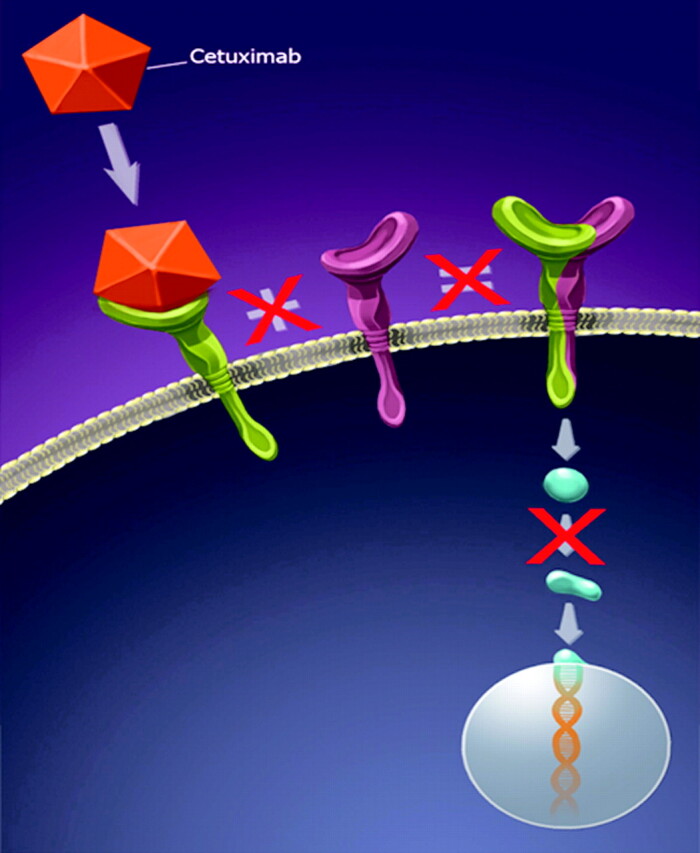
Illustration of the effect of cetuximab on the EGFR (HER1) pathway. Cetuximab binding to the EGFR prevents dimerization and the downstream cascade.
Indication and Usage
Cetuximab has been approved since 2006 for the treatment of SCCHN, in combination with radiation therapy as the initial treatment of locally or regionally advanced tumor and as a single agent for patients with platinum-resistant cancers such as recurrent or metastatic SCCHN.1,2,5,9 Figure 4 illustrates an aggressive case of SCC of the left vocal cord, treated with cetuximab, with resulting substantial reduction in the size of the lesion. Cetuximab has also been approved since 2004 for the treatment of EGFR-expressing metastatic colorectal cancer as a single agent or in combination with irinotecan for patients with chemotherapy-refractory cancers.1,2,5
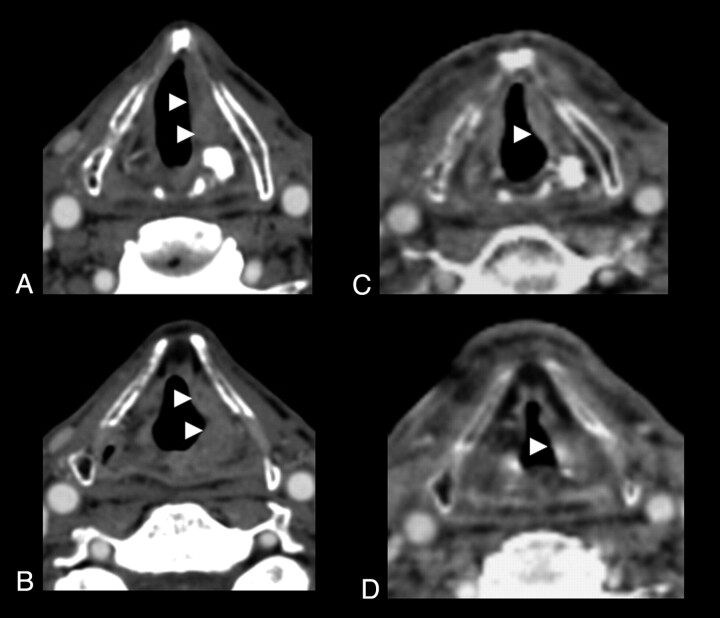
Fig 4.
Axial contrast-enhanced CT scans demonstrate an aggressive SCC involving the left true vocal cord (A), which extends superiorly to involve the left aryepiglottic fold (B) (arrowheads). The patient was treated with cetuximab and radiation therapy. C and D, Following treatment, there is a substantial reduction in the size of the lesion (arrowhead) compared with the pretreatment study.
Administration and Side Effects
Cetuximab is a prescription-only drug administered intravenously, usually with an H1 antagonist, in a dose of 400 mg/m2, with a subsequent weekly dose of 250 mg/m2. Intravenous administration of cetuximab has several side effects: The most serious are infusion reactions, cardiopulmonary arrest, dermatologic toxicity and radiation dermatitis, sepsis, renal failure, interstitial lung disease, and pulmonary embolus.1,2,4,10,11 Across all studies, cetuximab was interrupted in 3%–10% of patients because of adverse reactions.5
Economic and Clinical Issues
Cetuximab is expensive with the cost approximating $30,000 per 8 weeks of treatment per patient.14 This definitively raises many questions by health professionals and economists about the cost-effectiveness of this drug. An economic analysis in 5 European countries in 2008 suggested a good value for the money of cetuximab when combined with radiation therapy for locally advanced head and neck cancer.1,9,12 Its cost-effectiveness appears to be more debated when used for colorectal cancer, with the incremental cost-effectiveness ratio being approximately Can$199,742 (US $188,708) per life-year gained, as recently evaluated by the National Cancer Institute of Canada.13
Abbreviations
- EGFR
epidermal growth factor receptor
- FDA
US Food and Drug Administration
- HER
human epidermal growth factor receptor
- SCC
squamous cell carcinoma
- SCCHN
SCC of the head and neck
References
- 1. Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs 2007; 67: 2585–607 [DOI] [PubMed] [Google Scholar]
- 2. Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin Ther 2005; 27: 684–94 [DOI] [PubMed] [Google Scholar]
- 3. Steiner P, Joynes C, Bassi R, et al. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res 2007; 13: 1540–51 [DOI] [PubMed] [Google Scholar]
- 4. Martinelli E, De Palma R, Orditura M, et al. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol 2009; 158: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erbitux (Cetuximab); [package insert]. New York and Princeton, New Jersey: ImClone Systems and Bristol-Myers Squibb; 2009 [Google Scholar]
- 6. Ettinger DS. Emerging profile of cetuximab in non-small cell lung cancer. Lung Cancer 2009. September 25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004; 59 (2 suppl): 21–26 [DOI] [PubMed] [Google Scholar]
- 8. Pérez-Soler R. HER1/EGFR targeting: refining the strategy. Oncologist 2004; 9: 58–67 [DOI] [PubMed] [Google Scholar]
- 9. Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 2009; 101: 1044–48. Epub 2009 Jun 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koukourakis MI, Tsoutsou PG, Karpouzis A, et al. Radiochemotherapy with cetuximab, cisplatin, and amifostine for locally advanced head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys 2009. September 8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11. Birnbaum A, Dipetrillo T, Rathore R, et al. Cetuximab, paclitaxel, carboplatin, and radiation for head and neck cancer: a toxicity analysis. Am J Clin Oncol 2009. September 25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12. Brown B, Diamantopoulos A, Bernier J, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008; 11: 791–99. Epub 2008 Jan 11 [DOI] [PubMed] [Google Scholar]
- 13. Mittmann N, Au HJ, Tu D, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst 2009; 101: 1182–92. Epub 2009 Aug 7 [DOI] [PubMed] [Google Scholar]
- 14. Holmer AF. Cetuximab in colon cancer. N Engl J Med 2004; 351: 1575–76 [DOI] [PubMed] [Google Scholar]