Abstract
BACKGROUND AND PURPOSE:
Endovascular TVE for DCCF is used for curative purposes, but serious complications can be caused with inadequate embolization. Our aim was to report clinical characteristics, angiographic findings, and results of endovascular TVE in patients presenting with DCCF.
MATERIALS AND METHODS:
We performed a retrospective analysis of 44 consecutive patients with DCCF treated by TVE. Approach routes, angiographic results, clinical outcomes, and complications were assessed.
RESULTS:
An approach via the internal jugular vein and inferior petrosal sinus was possible in 90% of patients, with complete occlusion of the fistula in 81.6% of patients. A minor residual shunt remained in 13.6% of patients, while a significant shunt remained in 4.5%. In 4 patients, add-on management with transarterial embolization was useful, and in 2 patients with residual shunt, radiosurgery was used. With long-term follow-up (6–40 months), we encountered recanalization/recurrence in 4 patients (9.1%). Complications were seen in the form of permanent morbidity in 3 patients (7%) and transient morbidity in 6 patients (14%).
CONCLUSIONS:
For endovascular treatment of DCCF, a transvenous approach was effective in most of our patients; however, some adverse effects were encountered. If AV shunts remain after transvenous treatment, additional modalities must be considered.
Intracranial dural AV fistula is a rare acquired lesion, which may present with ICH or progressive neurologic deficits. The cause is uncertain, though these lesions are often associated with intracranial venous thrombosis. The importance of this entity lies in the fact that cure is potentially achievable by using endovascular or neurosurgical procedures.1
DCCF occurs with ocular symptoms such as proptosis, chemosis, and diplopia in 80% of cases, and loss of visual acuity is also a common symptom.2 DCCF may resolve spontaneously with clinical observation alone, but this carries a risk of sudden visual deterioration in the event of acute thrombosis of the fistula.3
Manual carotid jugular compression has been advocated as a treatment option with a complete cure rate of 34%,4 but such a maneuver may precipitate a vasovagal attack, ischemic stroke, or brachial plexus injury.5 Various modalities are currently available to treat DCCF, such as endovascular procedures with transvenous, transarterial, or transarterial-transfistulous embolization,6–8 surgery to obtain access to the fistula for embolization on either the venous or arterial side or to excise the fistula,9 gamma knife surgery,10 or a combination of the 3.11 In selected cases, the lesions can also be treated conservatively.12
We retrospectively analyzed 44 consecutive cases of DCCF managed in our institution and related institutions, by using primarily the transvenous route with an emphasis on feasibility, durability, and complications encountered with the approaches used, through clinical and angiographic follow-up. The results are discussed with reference to the literature.
Materials and Methods
Subjects comprised 44 patients with angiographically confirmed DCCF, during the period between September 2003 and March 2008. Endovascular treatment was considered as first choice in this series, so all presented patients with DCCF were endovascularly treated without certain selection criteria. Retrospective analysis was performed by using information from the medical charts of the patient. Approach routes, angiographic results, complications, and clinical outcome were assessed.
Bilateral selective ICA and ECA angiography and vertebral artery angiography were performed in all patients for assessment of feeding arteries, sizes, sites, and venous drainage patterns of the fistulas. According to angiographic findings, each patient was classified into 1 of the following types of DCCF: Barrow type B, indirect fistula between the CS and dural branches of the ICA; Barrow type C, indirect fistula between the CS and dural branches of the ECA; or Barrow type D, indirect fistula between the CS and dural branches of both the ICA and ECA.
A 6F guiding catheter was navigated to the internal jugular vein, a microcatheter with a microwire was navigated coaxially to the CS via the IPS, and then contrast medium was gently injected from the microcatheter (selective venography). In cases of an occluded IPS, a 0.035-inch guidewire was used to pass through the sinus (the rolling method was manual screwlike movements of a guidewire throughout the occluded IPS to pass through it to the targeted CS). Heparin was injected after the microcatheter entered the CS. In cases of unsuccessful IPS penetration to the CS, another route was tried by using either the SOV or the facial vein.
Embolization was performed with fibered and/or electrically detachable coils in all cases, 41 patients with both fibered and detachable coils and 3 patients with detachable coils only, by using real-time digital subtraction fluoroscopic mapping. We used 18F microcatheters (Excelsior 1018 and TurboTracker-18; Boston Scientific, Fremont, California) in all cases. In case of a narrow route, we used 10-sized microcatheters (Excelsior SL10, Boston Scientific) because it was not possible to insert the 18-sized microcatheters into the CS or veins. Angiography was performed immediately after completion of the procedure to check for occlusion of the fistula. Angiographic complete occlusion was defined as “complete occlusion” of the shunt; nearly complete occlusion was a small residual stagnant shunt, which was defined as a “minor residual shunt”; and incomplete occlusion was defined as the presence of “significant residual shunt.” Complete and nearly complete occlusions were considered successful angiographic results. Patients were generally followed by clinical evaluations throughout a period of 6–40 months (mean, 16.5 months). Additional imaging studies such as brain CT or MR imaging were performed during the follow-up period in complicated cases. Angiographic re-evaluation was performed if changes or deterioration of symptoms believed to be related to the DCCF was noted.
Results
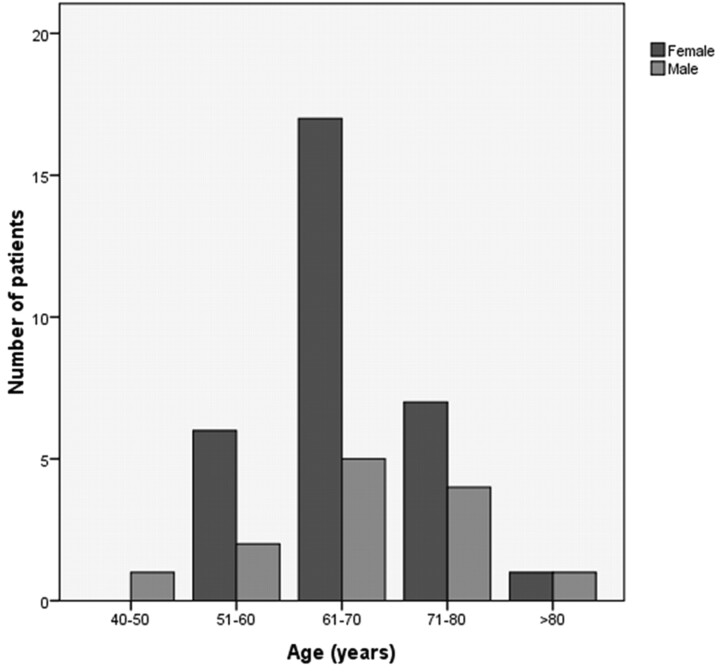
In our study, 44 patients underwent angiographic evaluation and endovascular procedures for treatment of DCCF. These included 31 women (70%) and 13 men (30%). The median age of patients was 66 years (range, 42–82 years; Fig 1).
Fig 1.
Graph showing the distribution of age and sex in the 44 patients with DCCF.
Most patients showed >1 symptom on clinical presentation, and all had chemosis. Other common findings were blurred vision, proptosis, and cranial nerve palsies. Proptosis was apparent in 6 patients (14%); chemosis, in all patients; bilateral chemosis, in 4 patients (9%); and ophthalmoparesis, in 16 patients (36%; 81% abducens nerve palsy, 19% oculomotor nerve palsy). Diminished visual acuity was found in 11% of patients. No patient in the present series presented with ICH.
The fistula was on the right side only in 41%, on the left only in 43%, and bilateral in 16% of patients. In the present series, 5 patients (11%) were type B, 4 patients (9%) were type C, and 35 patients (80%) were type D. Of the patients, 55% displayed arterial feeders from bilateral ICAs and ECAs. Venous drainage involved the SOV in all patients (bilateral in 11%), the SPS in 5%, the uncal vein in 7%, and the sylvian vein in 30% of patients. Cortical venous reflux was present in 16 patients (36%).
A transvenous approach through the IPS was successful in reaching the affected CS in 40 patients (90%). This approach failed in the remaining 4 patients (a contralateral IPS route also failed), so we tried other routes: 1 through the facial vein, 1 through the SPS, and 2 via a transarterial approach. Five patients (11%) required 2 TVE sessions to achieve good clinical outcome.
Regarding outcomes, complete occlusion was achieved in 36 patients (81.6%); significant residual shunt, in 2 patients (4.5%); and minor residual shunt, in 6 patients (13.6%). Among patients with residual shunt, add-on management with transarterial embolization proved useful in 4 and add-on management with radiosurgery was helpful in 2.
During long-term follow-up (6–40 months), recanalization/recurrence was encountered in 4 patients (9.1%). Complications were seen in 21% of patients, as either transient (14%; abducens nerve palsy, n = 4 patients, 9%; oculomotor nerve palsy, n = 2 patients, 5%) or permanent (7%; brain stem infarction, n = 2 patients, 5%; ICH/paradoxical worsening, n = 1 patient, 2%) (Table and Figs 2 and 3).
Criteria of patients developing postprocedural complications
| Age (yr) | Sex | Type/ Side | Symptoms | Feeders | Cortical Refluxa | Drainage | Approach (TVE) | Outcome (AV shunt) | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 61 | M | D/Lt. | Bilat. chemosis and CN VI palsy | Lt. ICA and ECA | – | Bilat. SOV | Failure | Residual shunt (radiosurgery) | ICH/paradoxical worsening |
| 81 | M | B/Rt. | Rt. chemosis and CN VI palsy | Both ICAs | – | Rt. SOV | Success | Residual shunt (TVE) | Brain stem infarction |
| 68 | F | D/Lt. | Lt. chemosis and blurred vision | Both ICA and ECAs | – | Bilat. SOV | Success | Residual shunt (TVE) | Brain stem infarction |
| 69 | F | D/Lt. | Rt. chemosis | Both ICA and ECAs | – | Rt. SOV | Success | Success | CN III palsy |
| 42 | M | D/Rt. | Rt. chemosis | Both ICA and ECAs | + | Rt. SOV and sylvian vein | Success | Success | CN III palsy |
| 82 | F | D/Rt. | Rt. chemosis and CN VI palsy | Lt. ICA and Rt. ECA | – | Rt. SOV | Success | Success | CN VI palsy |
| 68 | F | B/Lt. | Lt. chemosis | Lt. ICA | – | Lt. SOV | Success | Success | CN VI palsy |
| 66 | F | D/Bilat. | Lt. chemosis and Lt. CN VI palsy | Both ICA and ECAs | – | Lt. SOV | Success | Success | CN VI palsy |
| 58 | F | D/Rt. | Rt. chemosis and CN VI palsy | Rt. ICA, ECA and Lt. ICA | + | Rt. SOV and SPS | Success | Success | CN VI palsy |
+ indicates with cortical reflux; −, without cortical reflux.
Fig 2.
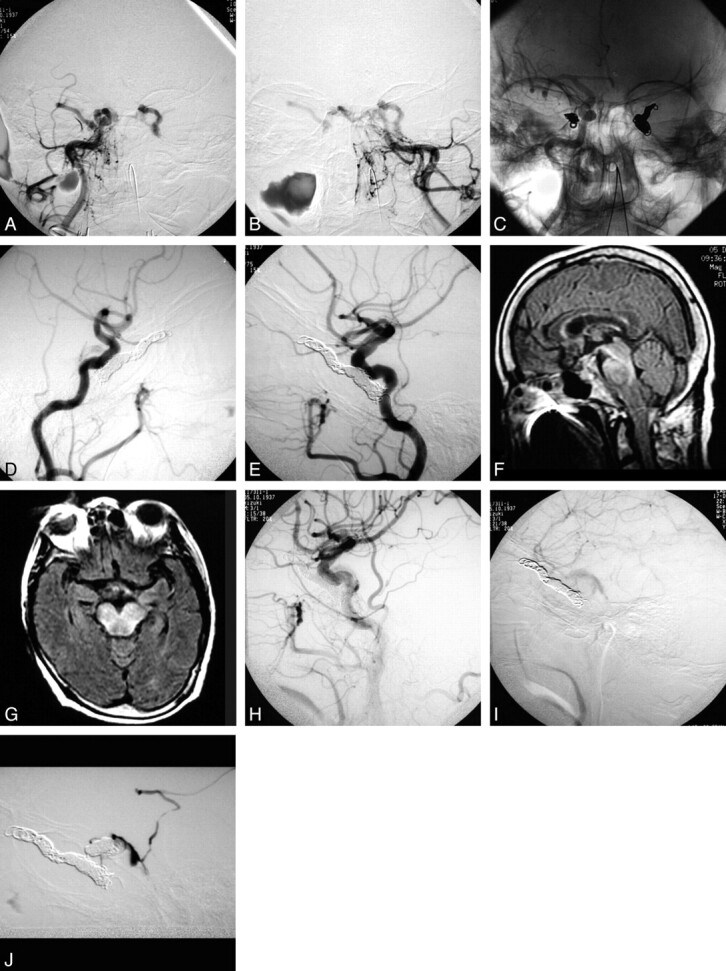
A 68-year-old woman with the complication of brain stem venous congestion with right hemiparesis, dysarthria, and left oculomotor palsy 2 days after embolization. A, Right external carotid angiogram, anteroposterior view. B, Left external carotid angiogram, anteroposterior view. C, Anteroposterior native view showing bilateral coiling. D, Lateral view of right carotid angiography. E, Lateral view of left carotid angiography. F and G, Fluid-attenuation inversion recovery MR images showing brain stem congestion 2 days later. H and I, Follow-up angiograms 2 days later. J, Selective angiogram showing a residual shunt. The patient underwent a second session of TVE and achieved gradual improvements.
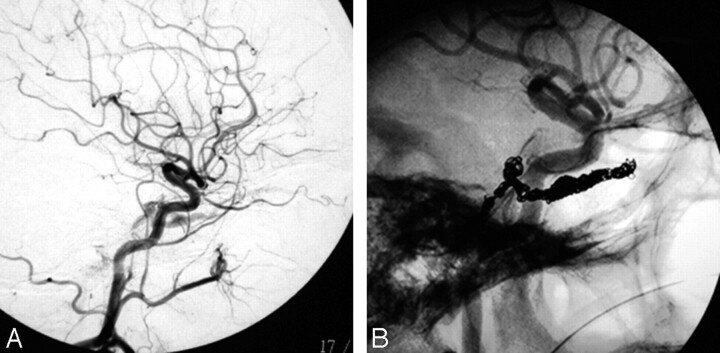
Fig 3.
An 82-year-old woman with the complication of sixth nerve palsy. A, Lateral view of right carotid angiography showing the fistula. B, Native lateral view of right carotid angiography showing coils closing the fistula.
Discussion
This study focused on the technique of endovascular management for DCCFs by using the transvenous route, stressing the feasibility of the approach and examining safety profiles in 44 consecutive patients.
Most of our patients were postmenopausal women, and most presented with orbital or neuro-ophthalmologic symptoms of moderate intensity.13,14 Clinical manifestations of patients with DCCF are related to the direction of venous drainage and blood flow through the fistula. Patients with drainage via the ophthalmic vein often display severe ophthalmic symptoms.15 In our patients, all fistulas drained via the ophthalmic vein and showed ocular symptoms, including conjunctival congestion, exophthalmos, intracranial murmur, visual disturbance, and oculomotor paralysis. Symptoms in both eyes mostly resulted from drainage of a unilateral AV fistula into the bilateral CSs, not from bilateral cavernous AV fistulas because the latter seldom occurs.16
The present results show that transvenous access of the target sinus via the IPS was successful in 90% of patients. We were forced to use other routes in the remaining 4 patients and made use of the facial vein, SOV, and transarterial routes. The major routes available for access to the CS include the following: anteriorly, the SOV and facial vein; superiorly, the superficial middle cerebral vein and sphenoparietal sinus; posteriorly, the petrosal sinuses; and inferiorly, the pterygoid plexus.9,13,17–19 Kiyosue et al20 reviewed the literature and confirmed a high success rate with TVE for DCCF, promoting a radical cure and representing a first-line curative therapy. However, Klisch et al12 reported that the approach via the internal jugular vein and IPS was possible in only 60% of patients. This approach was first described by Halbach et al,21 who used steel coils and sclerosing liquid injections into the CS via the SOV. An SPS approach has been reported as an alternative to catheterization of the IPS or SOV.17 However, the SPS must be patent, because mechanical recanalization has proved hazardous given the anatomic proximity to the vein of Labbe.22
Using a multichannel approach, we were able to achieve complete occlusion of the fistula in 81.6% of cases. These results seem compatible with other series showing rates of 71%–89%.19,21,23–25
The total incidence of complications associated with the procedure in our series was 21%, though most were transient morbidities. Approximately 7% showed a permanent deficit. This result agrees with the findings of Kim et al,23 who reported a total complication rate of 19.6%. However, Meyers et al,26 retrospectively studied a large series of 135 patients with DCCFs managed by endovascular treatment and found procedure-related permanent morbidity to be only 2.3% in followed-up patients; but as in our patients, they had no surgical mortality.
Six patients (14%) showed newly developed cranial nerve signs after transvenous coil embolizations. Cranial nerve signs after TVE may be due to progressive thrombosis of the CS, mass effect from the coils, or direct injury of the nerve by coils or the microwire/microcatheter.12,27 Overpacking of the CS may have caused transient cranial nerve symptoms in most patients. The reason for abducens nerve predilection is unclear but may be because the oculomotor, trochlear, ophthalmic, and maxillary nerves are usually located in the lateral wall of the CS, whereas the abducens nerve is located just lateral to the ICA. This anatomic position may result in increased vulnerability of the abducens nerve to stretching and mass effects of coils or thrombus within the sinus.28
We had the complication of brain stem infarction in 2 patients. In these patients, shunt surgery points were located at the posterior wall of the CS; we tried to occlude the shunts and to avoid overpacking by coils. Immediately after the procedure, we confirmed closure of the shunt, but probably a new drainage route had developed resulting in this infarction.
We encountered a case of venous congestion 2 days after TVE, with the patient having right hemiparesis and diplopia. MR imaging showed brain stem edema, and angiography showed venous reflux from the CS to the petrosal vein. After we performed TVE via the IPS for the petrosal vein, clinical features gradually improved. Other reports have shown rare cases of brain stem congestion in DCCF caused by shunted flow into the posterior fossa.29 Venous congestion by rerouting to the pontomesencephalic veins occurred after coil embolization in our patient, despite nearly complete occlusions of the shunts after embolization (Fig 3). Such venous rerouting is a known complication in cases of TVE in other dural sinuses.23 Avoiding simple trapping or partial embolization of the involved dural sinus is important because these can lead to diversion of shunt flow into the normal cerebral venous pathways and can ultimately result in conversion of the dural AV fistula into a more dangerous and aggressive disease.30 However, transvenous coil embolization of the complex and septate CS may sometimes be difficult and could result in unintended dangerous rerouting of the shunt.23
Conclusions
For endovascular treatment of a DCCF, a transvenous approach was effective in a most of our patients; however, some adverse effects were encountered. If AV shunts remain after transvenous treatment, additional treatment modalities must be considered.
Abbreviations
- AV
arteriovenous
- B
Barrow type B
- Bilat.
bilateral
- CN
cranial nerve
- CS
cavernous sinus
- D
Barrow type D
- DCCF
dural carotid-cavernous fistula
- ECA
external carotid artery
- ICA
internal carotid artery
- ICH
intracerebral hemorrhage
- IPS
inferior petrosal sinus
- Lt.
left
- Rt.
right
- SOV
superior ophthalmic vein
- SPS
superior petrosal sinus
- TVE
transvenous embolization
References
- 1. Wilson M, Enevoldson P, Menezes B. Intracranial dural arterio-venous fistula. Pract Neurol 2008; 8: 362–69 [DOI] [PubMed] [Google Scholar]
- 2. Cognard C, Houdart E, Casasco AE, et al. Endovascular therapy and long-term results for intracranial dural arteriovenous fistulae. In: Connors JJ, 3rd, Wojak JC. eds. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: WB Saunders; 1999: 198–214 [Google Scholar]
- 3. Sergott RC, Grossman RI, Savino PJ, et al. The syndrome of paradoxical worsening of dural-cavernous sinus arteriovenous malformations. Ophthalmology 1987; 94: 205–12 [DOI] [PubMed] [Google Scholar]
- 4. Higashida RT, Hieshima GB, Halbach VV, et al. Closure of carotid cavernous sinus fistulae by external compression of the carotid artery and jugular vein. Acta Radiol Suppl 1986; 369: 580–83 [PubMed] [Google Scholar]
- 5. Komiyama M, Nakajima H, Nishikawa M, et al. Brachial plexus and supraclavicular nerve injury caused by manual carotid compression for spontaneous carotid-cavernous sinus fistula. Surg Neurol 1999; 52: 306–09 [DOI] [PubMed] [Google Scholar]
- 6. Debrun GM. Endovascular management of carotid cavernous fistulas. In: Valavanis A. ed. Interventional Neuroradiology. Berlin, Germany: Springer-Verlag; 1993: 23–34 [Google Scholar]
- 7. Halbach VV, Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery 1997; 40: 1133–41 [DOI] [PubMed] [Google Scholar]
- 8. Ohtakara K, Murao K, Kawaguchi K, et al. Selective transvenous liquid embolization of a type 1 dural arteriovenous fistula at the junction of the transverse and sigmoid sinuses: case report. J Neurosurg 2000; 92: 1045–49 [DOI] [PubMed] [Google Scholar]
- 9. Jahan R, Gobin YP, Glenn B, et al. Transvenous embolization of a dural arteriovenous fistula of the cavernous sinus through the contralateral pterygoid plexus. Neuroradiology 1998; 40: 189–93 [DOI] [PubMed] [Google Scholar]
- 10. Guo WY, Pan DH, Wu HM, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol 1998; 19: 1081–87 [PMC free article] [PubMed] [Google Scholar]
- 11. Goto K, Sidipratomo P, Ogata N, et al. Combining endovascular and neurosurgical treatments of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg 1999; 90: 289–99 [DOI] [PubMed] [Google Scholar]
- 12. Klisch J, Huppertz HJ, Spetzger U, et al. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery 2003; 53: 836–56, discussion 856–57 [DOI] [PubMed] [Google Scholar]
- 13. Quinones D, Duckwiler G, Gobin PY, et al. Embolization of dural cavernous fistulas via superior ophthalmic vein approach. AJNR Am J Neuroradiol 1997; 18: 921–28 [PMC free article] [PubMed] [Google Scholar]
- 14. Annesley-Williams DJ, Goddard AJ, Brennan RP, et al. Endovascular approach to treatment of indirect carotico-cavernous fistulae. Br J Neurosurg 2001; 15: 228–33 [DOI] [PubMed] [Google Scholar]
- 15. Brown RD, Jr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg 1994; 81: 531–38 [DOI] [PubMed] [Google Scholar]
- 16. Tomsick TA. Carotid Cavernous Fistula: Transvenous Coil Embolization of CCF. Cincinnati: Digital Educational Publishing; 1997: 163–76 [Google Scholar]
- 17. Mounayer C, Piotin M, Spelle L, et al. Superior petrosal sinus catheterization for transvenous embolization of a dural carotid cavernous sinus fistula. AJNR Am J Neuroradiol 2002; 23: 1153–55 [PMC free article] [PubMed] [Google Scholar]
- 18. Kohyama S, Kaji T, Tokumaru AM, et al. Transfemoral superior ophthalmic vein approach via the facial vein for the treatment of carotid-cavernous fistulas: two case reports. Neurol Med Chir (Tokyo) 2002; 42: 18–22 [DOI] [PubMed] [Google Scholar]
- 19. Agid R, Willinsky RA, Haw C, et al. Targeted compartmental embolization of cavernous sinus dural arteriovenous fistulae using transfemoral medial and lateral facial vein approaches. Neuroradiology 2004; 46: 156–60 [DOI] [PubMed] [Google Scholar]
- 20. Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics 2004; 24: 1637–53 [DOI] [PubMed] [Google Scholar]
- 21. Halbach VV, Higashida RT, Hieshima GB, et al. Dural fistulas involving the cavernous sinus: results of treatment in 30 patients. Radiology 1987; 163: 437–42 [DOI] [PubMed] [Google Scholar]
- 22. Théaudin M, Saint-Maurice JP, Chapot R, et al. Diagnosis and treatment of dural carotid-cavernous fistulas: a consecutive series of 27 patients. J Neurol Neurosurg Psychiatry 2007; 78: 174–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DJ, Kim DI, Suh SH, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol 2006; 27: 2078–82 [PMC free article] [PubMed] [Google Scholar]
- 24. Oishi H, Arai H, Sato K, et al. Complications associated with transvenous embolisation of cavernous dural arteriovenous fistula. Acta Neurochir (Wien) 1999; 141: 1265–71 [DOI] [PubMed] [Google Scholar]
- 25. Cheng KM, Chan CM, Cheung YL. Transvenous embolisation of dural carotid-cavernous fistulas by multiple venous routes: a series of 27 cases. Acta Neurochir (Wien) 2003; 145: 17–29 [DOI] [PubMed] [Google Scholar]
- 26. Meyers PM, Halbach VV, Dowd CF, et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol 2002; 134: 85–92 [DOI] [PubMed] [Google Scholar]
- 27. Klisch J, Schipper J, Husstedt H, et al. Transsphenoidal computer-navigation assisted deflation of a balloon after endovascular occlusion of a direct carotid cavernous sinus fistula. AJNR Am J Neuroradiol 2001; 22: 537–40 [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson JE. Grant's Atlas of Anatomy. 8th ed. Baltimore: Williams & Wilkins; 1983 [Google Scholar]
- 29. Uchino A, Kato A, Kuroda Y, et al. Pontine venous congestion caused by dural carotid-cavernous fistula: report of two cases. Eur Radiol 1997; 7: 405–08 [DOI] [PubMed] [Google Scholar]
- 30. Chaloupka JC. Endovascular therapy for dural arteriovenous fistulas. In: Marks MP, Do HM. eds. Endovascular and Percutaneous Therapy of the Brain and Spine. Philadelphia: Lippincott Williams & Wilkins; 2002: 217–316 [Google Scholar]