Abstract
BACKGROUND AND PURPOSE:
ORN is a postradiation complication that has been well-documented in the medical literature. Most cases in the head and neck have been described in the mandible or larynx. Only a handful of cases in the hyoid bone are documented, all in the clinical literature. Our purpose is to present the clinical and imaging features of ORN involving the hyoid bone.
MATERIALS AND METHODS:
We present a case series of 13 patients with imaging findings highly suggestive of hyoid ORN after radiation therapy for head and neck cancers, in which we observed progressive features of hyoid disruption along with adjacent soft-tissue ulceration.
RESULTS:
Pretreatment imaging, when available, showed a normal hyoid. Typical postradiation imaging findings included an initial tongue base ulcerative lesion with air approaching the hyoid bone, and subsequent observation of hyoid fragmentation, often with intraosseous or peri-hyoid air and the absence of associated mass-like enhancement.
CONCLUSIONS:
Findings of hyoid fragmentation, cortical disruption, and soft tissue or intraosseous air in the postradiation therapy patient should strongly suggest the diagnosis of hyoid ORN. It is important recognize this entity because the diagnosis may preclude potentially harmful diagnostic intervention and allow more appropriate therapy.
ORN, sometimes referred to as chondroradionecrosis (in the larynx) is a well-known complication of radiation in the treatment of head and neck malignancies. Most previous reports in the literature concern ORN localized to the mandible and laryngeal cartilages.1–4 In contrast, ORN of the hyoid bone has been described in the clinical literature only, and in just a total of 7 patients.5–8 To our knowledge, no reported cases have been presented in the imaging literature. The purpose of our study was to describe the CT and clinical findings of 13 patients with hyoid ORN.
Materials and Methods
We reviewed the medical records and diagnostic images of 13 patients with head and neck malignancies treated with radiation therapy, in whom we identified characteristic imaging features of ORN, including pathologic fracture, fragmentation and disorganization of bone, and foci of air within and surrounding the hyoid. The patients ranged from 44 to 70 years of age (mean, 59.0 years) with all 13 men. All primary malignancies were biopsy-proved squamous cell carcinomas, located in the base of the tongue (n = 6); tonsil (n = 2); supraglottic larynx (n = 2); and lateral pharyngeal wall not limited to the tonsil (n = 3), including 1 patient initially radiated for neck metastasis, of unknown primary origin who subsequently developed an oropharyngeal primary and was re-irradiated (patient 3). Patient data are tabulated in the Table. Three patients received their radiation treatments at outside institutions. Three patients had initial radiation therapy courses at outside institutions and a second course at our institution (a total of 4 patients were re-irradiated). The remaining 7 patients received their radiation therapy exclusively at our institution. For the latter group, the hyoid bone was contoured, and dose-volume histograms were generated using Pinnacle software (Philips Healthcare, Milpitas, California). Mean, median, and maximal doses were evaluated. Of these patients, mean radiation doses to the hyoid bone ranged from 66.8 to 71.6 Gy. Radiation therapy doses for these patients are listed in the Table. The elapsed time between completion of radiation therapy and radiologic observation of hyoid ORN ranged between 1 and 34 months (mean, 12.7 months).
Summary of patients with hyoid ORN
| No. | Age/Sex | Dose (Gy) | Primary Adjacent to Hyoid | Post-XRT ORN DX (mo) | Preceding Soft-Tissue Ulceration | Adjacent Enhancement | Post-ORN Follow-Up (mo) |
|---|---|---|---|---|---|---|---|
| 1 | 67/M | OSH-N/A | – | 34 | No | Yes | 12 |
| 2 | 46/M | 71 | Yes | 5 | No | No | 6 |
| 3 | 66/Ma | 60, 50.2 | No | 2 | No | Yes | 23 |
| 4 | 64/M | 70.0 | Yes | 5 | Yes | No | 30 |
| 5 | 45/M | 66.8 | Yes | 5.5 | Yes | No | 1 |
| 6 | 64/M | 70.6 | Yes | 13 | No | No | 16 |
| 7 | 54/M | 70.1 | Yes | 8 | No | No | 7 |
| 8 | 49/M | 71.6 | Yes | 26 | No | No | 16 |
| 9 | 70/M | 60, 20 | – | 6 | No | No | 14 |
| 10 | 67/M | OSH-N/A | – | 1 | No | No | 21 |
| 11 | 47/M | OSH-N/A | – | 32 | Yes | Yes | 7 |
| 12 | 67/M | 41, 63 | Yes | 10 | Yes | Yes | 2 |
| 13 | 61/M | 50, 60 | – | 17 | Yes | Yes | 4 |
Patient was originally radiated for metastasis to the neck (60 Gy), primary unknown, and subsequently re-irradiated (50.2 Gy) for a pharyngeal wall squamous cell carcinoma.
Among the 13 cases, a total of 56 CT and 1 MR imaging examinations were reviewed. CT studies were performed on LightSpeed scanners (GE Healthcare, Milwaukee, Wisconsin) after the administration of intravenous contrast. Images were obtained in the axial plane with the following section thicknesses: 5 mm (n = 11), 2.5 mm (n = 1), 3.75 mm (n = 23), and 1.25 mm (n = 18). Bone and soft-tissue windows were evaluated in all examinations. One of the 13 patients underwent a histologic analysis of the hyoid bone as part of a total laryngectomy (patient 3). Another patient was offered biopsy but declined. The other 11 patients were not biopsied. One patient underwent a pretreatment MR imaging due to renal failure. Four patients underwent PET examinations at approximately the same time that hyoid ORN was observed.
All imaging was reviewed by a radiologist with senior American Society of Neuroradiology membership status, who is engaged exclusively in the practice of head and neck cancer imaging.
Results
Review of the clinical notes around the time of suspicion of hyoid ORN showed that the predominant symptom in 8 patients was dysphagia with or without superimposed odynophagia. Trismus was reported in 3 patients, and otalgia, in 4 patients. Nonspecific ipsilateral facial, jaw, or throat pain was identified in 6 patients.
Eight of the 13 total cases had pre-radiation therapy radiologic studies available for review. Of this group, 7 studies demonstrated malignant lesions extending to involve the mucosa at the level of the hyoid bone (Fig 1). In other cases, nodal disease was adjacent to the hyoid. The study in only 1 case showed the absence of soft-tissue malignancy in the vicinity of the hyoid bone (Table). In the patients treated elsewhere, we did not have pretreatment imaging to assess the presence of tumor, either primary or nodal, adjacent to the hyoid bone.
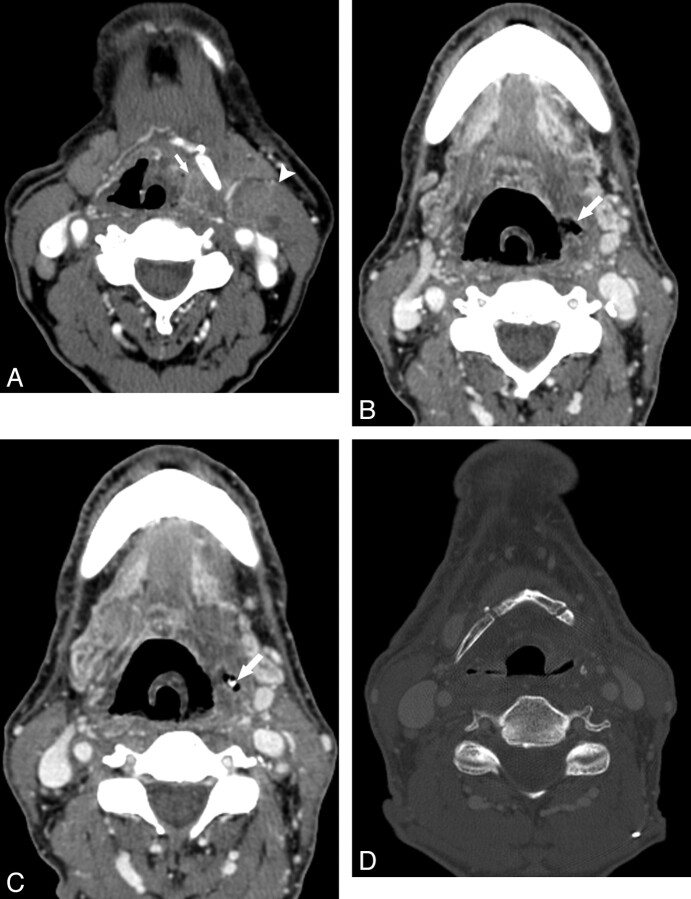
Fig 1.
Patient 4. A 64-year-old man radiated for squamous cell carcinoma of the left lateral oropharyngeal wall. A, CT examination with contrast obtained before radiation therapy shows the lower aspect of the lesion in the vallecula, lying adjacent to the hyoid bone (arrow). There is a nodal metastasis in the left neck (arrowhead), also adjacent to the hyoid. B and C, Axial contrast-enhanced CT images obtained 5 months following treatment with 70 Gy show a defect in the lateral pharyngeal wall (arrow, B) and an exposed piece of hyoid (arrow in C). At this time, the hyoid was otherwise intact. D, CT bone window on follow-up imaging shows a missing resorbed hyoid.
At a mean time of 12.7 months after completion of radiation therapy, posttreatment CT demonstrated characteristics of ORN involving the hyoid bone, including cortical disruption, pathologic fracture or fragmentation, and air within or surrounding the hyoid (Figs 1–5). In all cases in which there was pretreatment or prior posttreatment imaging, the hyoid and perihyoid findings were new. Six patients had soft-tissue ulceration resulting in exposure of the hyoid surface to air prior to the development of osseous changes (Figs 1 and 3). Eleven patients had lower oropharyngeal soft-tissue ulceration evident on images adjacent or leading to the abnormal hyoid bone (Figs 2 and 3). Only 1 of our 13 patients proceeded to biopsy (patient 3). This patient had previously been radiated for T0 squamous cell carcinoma metastatic to the left neck and was subsequently re-irradiated for a right-sided oropharyngeal primary (Fig 4). Typical exposed fragmented hyoid bone ensued, but subsequently, the patient developed progressive enhancement, which was deemed worrisome (Fig 4), as well as relentless aspiration. The decision was made to perform a total laryngectomy, whereupon histologic examination demonstrated only necrosis and inflammation.
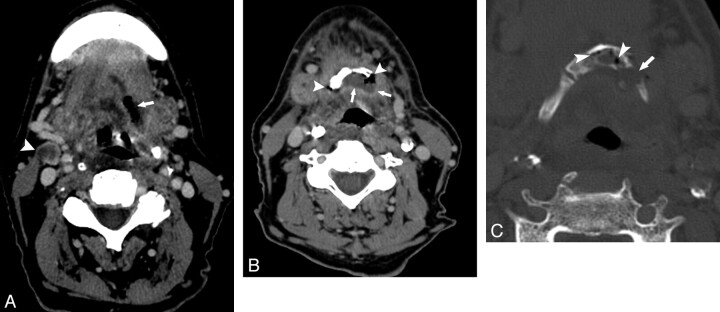
Fig 2.
Patient 10. A 67-year-old man after radiation therapy at an outside hospital for base-of-tongue cancer. A and B, Axial contrast-enhanced CT scans show an air-filled soft-tissue defect in the left posterior tongue (arrow, A). Lower down, this communicates with the hyoid (B). There is an unrelated nodal recurrence in the right neck (arrowhead, A). B, Soft-tissue CT image more inferiorly shows air (arrowheads) and fluid (arrows) surrounding the hyoid. C, CT bone window shows intraosseous air (arrowheads) and a hyoid fracture (arrow).
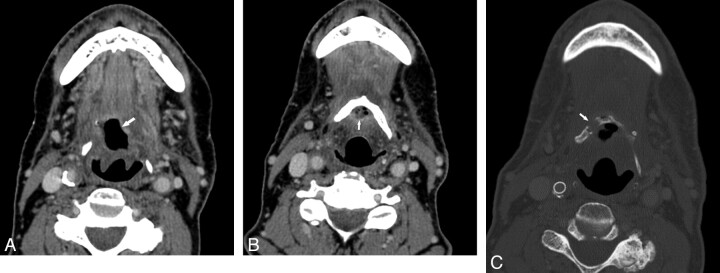
Fig 3.
Patient 9. A 70-year-old man previously radiated for base-of-tongue carcinoma and subsequently re-irradiated for neck recurrence. A, Contrast-enhanced CT examination 6 months after the completion of the second course of radiation demonstrates an air-filled necrotic cavity in the tongue base without surrounding enhancement (arrow). B, CT image more inferiorly shows that the cavity contains fluid and internal air bubbles (arrow, B) and is in close contact with the hyoid. The hyoid was reported as being at risk for ORN. C, CT bone window obtained 2 months after B reveals that the hyoid is now fragmented (arrow).
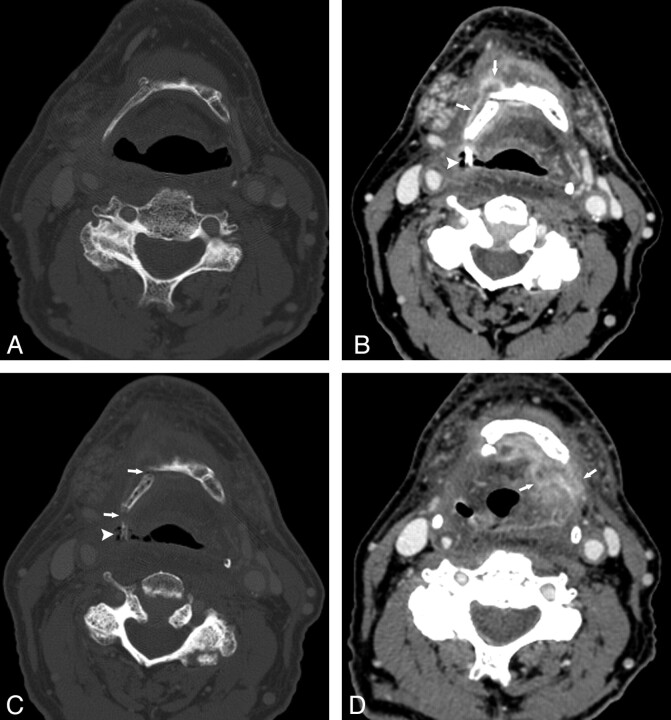
Fig 4.
Patient 3. A 66-year-old man status post 60 Gy for treatment of metastatic squamous cell carcinoma to the left neck, primary unknown, who subsequently developed a primary malignancy in the right oropharyngeal wall, for which an additional dose of 50.2 Gy was administered. A, CT bone window before XRT shows an intact hyoid. B and C, Contrast-enhanced CT, soft-tissue and bone windows, respectively, 5 months following completion of the second course of XRT. There is an enhancing process surrounding the right side of the hyoid (arrows, B) and an exposed fragment of hyoid surrounded by air (arrowhead, B and C). Hyoid fractures can be seen (arrow in C). D, Contrast-enhanced CT image 5 months after B and C shows dramatically increased enhancement extending to the left of midline (arrows). Because of these findings and relentless aspiration, a total laryngectomy was performed; at histologic examination, there was only necrosis and inflammation.
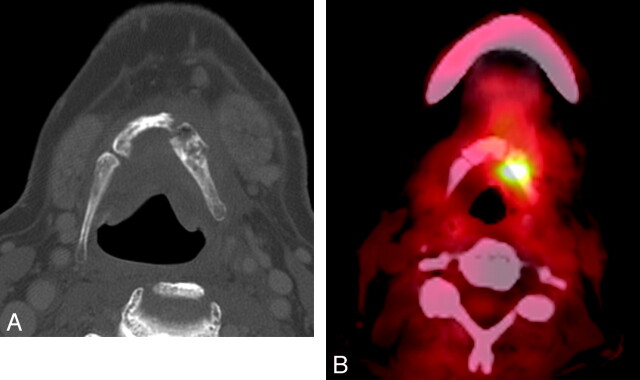
Fig 5.
Patient 1. A 67-year-old man, 34 months following an unknown dose of XRT at an outside facility for treatment of squamous cell carcinoma of the tongue base. A, Axial bone window CT scan shows typical fragmentation and intraosseous air characteristic of hyoid ORN. B, PET/CT, obtained concurrent to A, demonstrates intense focal FDG uptake (SUV=7.5), which was regarded as suspicious for tumor. The patient was offered hyoidectomy but refused, and his symptoms of dysphagia and odynophagia gradually improved with hyperbaric O2 during the follow-up period of 14 months, after which the patient was lost to follow-up.
All except 1 patient had no evidence of tumor at the time hyoid ORN was suspected, and none subsequently developed tumor in or around the hyoid. In the 1 patient who did have tumor (patient 10), it was thought that despite the presence of tumor in the tongue and neck, the findings in and around the hyoid were sufficiently characteristic of ORN for the case to be included; subsequently, this patient was lost to follow-up and presumably died of cancer.
Four patients underwent PET/CT imaging concurrent to the cross-sectional imaging that suggested hyoid ORN. Findings in 1 patient were without significant FDG activity. Three patients had focally increased FDG activity. One had an SUV = 6.8, which was attributed to a hyoid fracture erroneously regarded as traumatic in nature (it was in fact, the development of hyoid ORN) (patient 3, PET/CT obtained 3 months before Fig 4B, -C). In 2 patients, however, obvious focally increased FDG activity at the site of the observed hyoid abnormality was interpreted as suspicious for tumor recurrence (Fig 5).
Discussion
Although ORN was recognized as occurring in irradiated tissue, the earliest studies describing it implicated trauma and localized infection as the primary inciting factors.9,10 Subsequent inquiries eliminated localized infection as a major etiology by examining resected ORN bone specimens and demonstrating that micro-organisms were found only on the bone surfaces, not in the deep necrotic portions. Therefore, radiation itself is thought to be the primary inciting factor. Radiation therapy is postulated to form hypoxic and hypocellular tissue. The breakdown of collagen and cellular death overcomes the ability of the affected tissue to replicate and leads to failure of healing.11,12
Additionally, if the tumor overlies the bone, an increased rate of bone necrosis is observed.13 The prevailing thought is that the rapid tumor destruction leads to exposure of the nearby bone and increases the risk of ORN. Smoking, alcohol use, and T stage are also thought to play roles.7
Hyoid ORN is a little known entity that has only been described in a total of 7 patients whom we could identify in the clinical literature.5–8 In the reported cases, the most frequent associated symptom was odynophagia,6 which was also seen in our group. However, radiation therapy often causes mucositis in the first several months following completion. Mucositis typically produces odynophagia, so this symptom and the associated dysphagia need not by themselves suggest hyoid ORN unless they occur beyond the 2-month window. Review of the clinical notes in our patients also revealed otalgia as well as nonspecific ipsilateral pain localized to the face, jaw, or throat. Trismus was present in 3 of our patients without evidence of masticator space tumor. In the literature, it is hypothesized that trismus is the result of fibrosis of the muscles and ligaments adjacent to the temporomandibular joint and pterygoid muscles.14,15 In our patients, the symptoms of trismus and facial/jaw pain were, therefore, likely unrelated to hyoid ORN but rather were caused by the direct effect of radiation therapy on the masticator soft tissues. Therefore, the signs and symptoms associated with hyoid ORN may well occur in patients who do not have necrosis, and conversely, no specific symptom would likely suggest hyoid ORN to the clinician.
The finding of hyoid fragmentation or cortical disruption and/or air within or surrounding portions of the hyoid bone, combined with the absence of obvious enhancing soft-tissue tumor, should suggest hyoid ORN in a radiated patient. Of course, the radiologist needs to be aware of hyoid osteonecrosis so as to avoid a misdiagnosis of malignancy and to prevent unnecessary aggressive diagnostic intervention.6 There is a risk that biopsy will introduce more air and possibly micro-organisms that could lead to infection or progressive necrosis.
According to the article by Monceaux et al,7 smoking and alcohol use are thought to play roles in the development of ORN. All the patients in our case series reported significant smoking histories.
Out of the 13 total cases, only 1 had a biopsy performed. As mentioned above, the progressive enhancing area necessitated total laryngectomy. Pathology confirmed the diagnostic suspicion of radionecrosis. The 13 patients in our study had follow-up intervals ranging from 1 to 30 months (mean, 12.2 months) with no radiographic or clinical evidence of malignancy at the site of hyoid ORN. Even though definitive histologic diagnosis of necrosis was absent in these cases, the average year-long follow-up without evidence of malignancy at the site of ORN supports our diagnostic impression. Two of our patients with CT follow-up imaging showed stable appearance of the hyoid at intervals of 2 and 31 months. One individual had progression of osteolysis of the hyoid 2 months after initial diagnosis. Subsequent follow-up CT examinations in the same patient from 6 through 14 months showed stable findings.
Four patients demonstrated increased absorption and destruction of the hyoid with the absence of adjacent soft-tissue enhancement (Fig 1). Two patients also demonstrated progressive hyoid destruction with abnormal adjacent soft-tissue enhancement. One of these had follow-up through 11 months. Although he received hyperbaric oxygen, he reported no improvement in symptoms. The other patient in this pair was the single individual who underwent resection and biopsy. An additional 4 patients had only clinical follow-up ranging from 1 to 16 months. Two have had limited postdiagnostic visits to the clinic with no documented suspicion for local recurrence. The remaining 2 died, but without suspicion for local tumor recurrence.
Our findings suggest that tumor adjacent to the hyoid bone before radiation therapy is a factor that should be considered as putting the hyoid at risk. Eight of the cases of hyoid ORN had preradiation imaging available. Out of this subset, 7 had malignant masses that were found to abut the hyoid. In their study of mandible ORN, Beumer et al13 postulated that a primary malignancy adjacent to the bone confers a higher risk of radionecrosis. In their study, this was found especially to be the case in large adjacent masses. Presumably this is due to greater doses of radiation therapy directed to the primary site, and the consequently greater risk of mucosal breakdown and hyoid exposure. In addition, 4 of our patients were re-irradiated for recurrent tumor, and it seems plausible that with re-irradiation, an increased cumulative dose of radiation very likely puts the hyoid at higher risk for necrosis.
With respect to PET/CT imaging, it appears that a false-positive result (ie, interpreting increased FDG activity as tumor recurrence) is a potential risk if the radiologist is unfamiliar with ORN, fails to recognize the characteristic findings of hyoid ORN on the CT portion of the examination, does not have access to prior cross-sectional imaging results, or a combination of these. In a report by Liu et al,16 skull base ORN was misdiagnosed on PET/CT. The senior author has seen this with ORN of the mandible in numerous instances (L.E.G., unpublished data).
The most confounding factor in the postradiation CT studies was the presence of adjacent soft-tissue enhancement. In our group, 5 patients demonstrated adjacent soft-tissue enhancement, 1 of whom proceeded to biopsy as described above (Fig 4). A second patient among these 5 was offered biopsy, but refused, and at 1-year follow-up did not demonstrate tumor recurrence at the hyoid. The third and fourth patients were lost to follow-up. The final patient in this group has only undergone a single 1-month follow-up clinical visit, at which time throat examination did not reveal evidence of a mass. Debnam et al17 reported that in cases of soft-tissue radiation-induced ulceration and necrosis, short-term follow-up is warranted if biopsy is not performed because 4 of the 8 cases in their study with enhancement were positive for recurrent malignancy. The remaining 4 in the group either had benign biopsy results or were without tumor on follow-up. Conversely, the absence of adjacent soft-tissue enhancement favored a benign etiology, and continued CT and clinical follow-up were suggested. Chong et al2 showed that in cases of mandibular ORN, noticeable enhancement can be present in the adjacent soft tissues. However, the study noted that attention to osseous changes of ORN can steer the interpreter away from a misdiagnosis.
Conclusions
Findings of hyoid fragmentation, cortical disruption, or intraosseous air, often adjacent to a tongue-base ulceration, in the setting of previous irradiation for head and neck malignancy, should suggest to the radiologist a possible diagnosis of ORN. It is important to distinguish ORN from recurrent tumor because the diagnosis of ORN may prevent counter-productive intervention and implementation of hyperbaric O2 therapy. Such findings in the absence of adjacent soft-tissue enhancement should lead to more confidence in the diagnosis. Although adjacent soft-tissue enhancement may suggest the possibility of tumor recurrence, the characteristic osseous changes of ORN make postradiation sequelae more likely. The potential for false-positive findings in PET/CT in such cases must also be borne in mind.
Abbreviations
- DX
diagnosis
- FDG
fluorodeoxyglucose
- N/A
not applicable
- ORN
osteoradionecrosis
- OSH
outside hospital, records unavailable
- PET
positron-emission tomography
- SUV
standard uptake value
- XRT
radiotherapy
References
- 1. Bedwinick JM, Shukovsky LJ, Fletcher GH, et al. Osteonecrosis in patients treated with definitive radiotherapy for squamous cell carcinomas of the oral cavity and naso- and oro-pharynx. Radiology 1976; 119: 665–67 [DOI] [PubMed] [Google Scholar]
- 2. Chong J, Hinckley LK, Ginsberg LE. Masticator space abnormalities associated with mandibular osteoradionecrosis: MR and CT findings in five patients. AJNR Am J Neuroradiol 2000; 21: 175–78 [PMC free article] [PubMed] [Google Scholar]
- 3. MacDougall JA, Evans AM, Lindsay RK. Osteoradionecrosis of the mandible and its treatment. Am J Surg 1963; 106: 816–18 [DOI] [PubMed] [Google Scholar]
- 4. Hermans R, Pameijer FA, Mancuso AA, et al. CT findings in chondroradionecrosis of the larynx. AJNR Am J Neuroradiol 1998; 19: 711–18 [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatia PL, Dutta NK, Sanasam JC. Osteonecrosis of hyoid bone and thyroid cartilage. Arch Otolaryngol 1979; 105: 553–54 [DOI] [PubMed] [Google Scholar]
- 6. Smith WK, Pfleiderer AG, Billet B. Osteoradionecrosis of the hyoid presenting as a cause of intractable neck pain following radiotherapy and the role of magnetic resonance image scanning to aid diagnosis. J Laryngol Otol 2003; 117: 1003–05 [DOI] [PubMed] [Google Scholar]
- 7. Monceaux G, Perie S, Montravers F, et al. Osteoradionecrosis of the hyoid bone: a report of 3 cases. Am J Otolaryngol 1999; 20: 400–04 [DOI] [PubMed] [Google Scholar]
- 8. Robertson JS, Frauenhoffer EE, Stryker J, et al. Osteoradionecrosis of the hyoid induced by combined modality therapy for laryngeal carcinoma. Ear Nose Throat J 1995; 74: 578–81 [PubMed] [Google Scholar]
- 9. Meyer I. Infectious diseases of the jaws. J Oral Surg 1970; 28: 17–26 [PubMed] [Google Scholar]
- 10. Bump RL, Young JM, David JC, et al. Hyperbaric oxygen: treatment of osteoradionecrosis and osteomyelitis. Med Serv Digest 1976; 26: 17 [Google Scholar]
- 11. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41: 283–88 [DOI] [PubMed] [Google Scholar]
- 12. Morrish RB, Chan E, Silverman S, et al. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer 1981; 47: 1980–83 [DOI] [PubMed] [Google Scholar]
- 13. Beumer J, Harrison R, Sanders B, et al. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg 1984; 6: 819–27 [DOI] [PubMed] [Google Scholar]
- 14. Steelman R, Sokol J. Quantification of trismus following irradiation of the temporomandibular joint. MO Dent J 1986; 66: 21–23 [PubMed] [Google Scholar]
- 15. Goldstein M, Maxymiw WG, Cummings BJ, et al. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 365–73 [DOI] [PubMed] [Google Scholar]
- 16. Liu SH, Chang JT, Ng SH, et al. False positive fluorine-18 fluorodeoxy-D-glucose positron emission tomography finding caused by osteoradionecrosis in a nasopharyngeal carcinoma patient. Br J Radiol 2004; 77: 257–60 [DOI] [PubMed] [Google Scholar]
- 17. Debnam JM, Garden AS, Ginsberg LE. Benign ulceration as a manifestation of soft tissue radiation necrosis: imaging findings. AJNR Am J Neuroradiol 2008; 29: 558–62 [DOI] [PMC free article] [PubMed] [Google Scholar]