Thromboembolic complications occur during 5–60% of all aneurysm coiling procedures and 3–6% are clinically symptomatic. These authors utilized transcranial Doppler to detect microemboli after coiling and then correlated their presence with patient demographics, aneurysm size, type of procedure and complication, medication, and clinical outcome in 123 patients. Procedures involving larger aneurysms, stent assistance, and incomplete occlusions were associated with greater risk of microemboli and heparinization played an important role in prevention.
Abstract
BACKGROUND AND PURPOSE:
Thromboembolic events after aneurysm coiling are common, but the optimal algorithm for emboli prevention remains unclear. MESs correlate with the occurrence of impending ischemic events and may be used for management guidance. This study reports the use of MES monitoring with regard to aneurysm characteristics and coiling technique after a specific anticoagulation protocol.
MATERIALS AND METHODS:
We analyzed 123 consecutive, elective endovascular procedures. Patients received intraprocedural and continuous heparin if feasible. Demographic data, aneurysm size, type of intervention/complication, medication, imaging, and clinical outcome were analyzed. MES monitoring was performed in all patients both immediately after as well as >12 hours after the procedure.
RESULTS:
Heparinization within the first 12 hours was associated with lower numbers of MESs early after coiling (3.4 versus 18.8 MESs/hr). When on heparin, larger aneurysm size, stent-assisted procedures, or incomplete occlusion did not lead to a significant increase in MESs. If the initial MES count on heparin was >10 MESs/hr, it was always safe to discontinue heparin. Inability to initiate early, continuous heparinization was associated with new neurologic deficits. Additional administration of antiplatelet agents showed lower MES counts initially, but the difference was not significant.
CONCLUSIONS:
MES monitoring is a powerful adjunct to monitor efficacy of treatment algorithms for emboli prevention after coiling. In our series, early heparinization was associated with a lower incidence of MESs. This is of particular importance in larger aneurysms, stent-assisted procedures, and incomplete occlusions, in which the thromboembolic risk is greatest early on and antiplatelet treatment alone may not suffice.
With the advent of noninvasive, high-resolution imaging techniques such as CT and MR angiography, the detection of incidental, unruptured cerebral aneurysms rose considerably within the past decade. Although the technique of treatment (coiling versus clipping) of aneurysms in the context of an acute subarachnoid hemorrhage has been the subject of many multicenter trials,1,2 the treatment of incidentally detected aneurysms in an otherwise healthy population poses a totally different challenge.3 The natural course of this disease has to be weighed carefully against the risk profile inherent to any kind of treatment intervention.
Thromboembolic events, in particular, are common after coiling and may occur in from 5% to 60% of cases.4,5 Most of these events are clinically silent and are only detected by MR imaging, but incidences of 3%–6% for permanent deficits have been quoted.6 It is still unclear whether most complications occur during the procedure itself or from early thromboembolism forming within the (incompletely) coiled aneurysm, which may be large or coiled with stent assistance. Larger aneurysms, the need for stent assistance, and incomplete occlusion of the aneurysm are generally believed to be associated with an increase in embolic risk, though it is still a matter of debate as to how one can best predict or characterize a TEE risk profile.
Different algorithms to minimize the incidence of TEE have been proposed, but consensus has only been reached regarding the application of heparin treatment during the procedure as recommended by the World Federation of Interventional and Therapeutic Neuroradiology. Heparinization may or may not be preceded or followed by antiplatelet agents. The efficacy of antiaggregational medication such as aspirin and clopidogrel is known to vary depending on the patient's drug susceptibility, as well as the dose and time of application.7 Despite various treatment and prophylaxis algorithms, the rate of thromboembolic events remains high, suggesting that not only an improvement in technique and development of new embolic materials but also an optimization of drug intervention and monitoring is mandatory.
Microembolic signal intensity monitoring is a diagnostic technique that is able to detect high-intensity signals via transcranial Doppler sonography, indicating the presence of embolic material within the arterial lumen.8,9 Because of its high sensitivity and specificity10 MES monitoring has been evaluated and established as a useful adjunct during and after open carotid endarterectomy and aneurysm surgery,11 as well as after cardiac valve replacements.12 The presence and frequency of MESs have contributed to the management of stroke patients and have been established as useful predictors for the occurrence of future strokes for both symptomatic and asymptomatic carotid stenosis,13,14 but they also have been used to monitor treatment efficacy, ie, the administration of aspirin or other antiplatelet agents.11,15
Only limited data are available regarding the use of MES monitoring after coiling procedures, but such monitoring was found to be positive in up to 31% of cases in a smaller series.16 The exact role of MESs, however, and the correlation between MESs and aneurysm characteristics, coiling technique (with and without stent), result (grade of occlusion), and anticoagulation algorithms are not well defined.
This retrospective analysis aims to illustrate the use of MES monitoring after embolization of unruptured cerebral aneurysms when following a standardized anticoagulation protocol. The purpose of this analysis is to prove the validity and safety of our treatment concept as well as to illustrate the effect of aneurysm characteristics and coiling technique on emboli monitoring.
Materials and Methods
For this study, we reviewed all coiling procedures from May 2007 until June 2010 (n = 244) that were performed at our institution by J.E. To maximize the homogeneity of our study group, we excluded all patients with evidence of recent hemorrhage and previously treated patients with evidence of recanalization, pseudoaneurysm, or significant comorbidities such as fibromuscular dysplasia or profound coagulation disorders. Of the remaining 128 patients, 5 patients were excluded due to the absence of adequate cranial windows for emboli monitoring (3.9%).
Since its implementation at our institution in 2007, the electronic patient record allows complete and retrospective analysis of all pertinent patient data in an anonymous manner, including 1) patient demographics (sex, age at time of intervention); 2) aneurysm, including location, size (<10 mm, ≥10 mm), technique of embolization (coiling with and without stent), degree of occlusion achieved (complete; near complete, >90%; incomplete, <90%), and intraprocedural complications (permanent coil protrusion, rupture, distal/adjacent stenosis/occlusion/thrombosis, device-related/fracture, need for access site revision); 3) medications administered throughout hospitalization (including heparin, aspirin, and clopidogrel); and 4) diagnostic tests performed during hospitalization and on follow-up (MES count on TCD, CT postoperatively, and at time of follow-up; neurologic assessment on admission, at time of discharge, and upon follow-up).
Our periprocedural treatment protocol was observed in all patients. Previous medications, including antiplatelet agents, were continued; only warfarin (Coumadin) was stopped in time to allow for normalization of international normalized ratio. Patients may have received additional doses of clopidogrel either before or immediately after the procedure upon the surgeon's discretion (n = 95). All patients were given an initial heparin bolus of 7000–10 000 international units according to body weight upon completion of the preceding, diagnostic arteriogram. ACT was determined 5 minutes after bolus injection; additional boluses were given as needed to maintain an ACT >300 throughout the procedure. All procedures were completed under general anesthesia, after which a heparin drip was started. The drip was adapted to achieve a goal partial thromboplastin time of 60–90 seconds. A CT scan was obtained 4–6 hours after the procedure, after which the first MES monitoring (MES-1) was performed.
Emboli monitoring in the Transcranial Power M-mode transcranial Doppler system (PMD 100 or ST3; Spencer Technologies, Seattle, Washington) used a 2-MHz digital Doppler with 33 sample gates arranged in an M-mode display with Doppler signal intensity power color-coded for flow directionality. The sonography beam was aligned along the vessel axis showing blood flow from multiple depths and vessels at the same time.17 Microemboli were visible moving through the M-mode display as high intensity, slanting tracks and were frequently seen simultaneously in the spectral waveform from a single user-selected depth. All examinations were performed by registered vascular sonographers specializing in neurovascular sonography, using a head frame to stabilize the Doppler transducer (Marc 600 Headframe; Spencer Technologies). The examination was focused on the arterial segment distal to the coiled aneurysm and was performed continuously for at least 20 minutes.
If the MES-1 showed <10 MESs/hr, heparin was discontinued at 6 am the following morning, and the MES-2 monitoring was performed no sooner than 4 hours after discontinuation of the drip to allow for adequate wear-off according to the half-life of heparin. If the first scan showed >9 MESs/hr, heparin was continued until the next monitoring, and the decision for discontinuation was based upon the MES count of the second scan on heparin. If the MES count had decreased, heparinization was stopped and the MESs repeated to verify absence of persistent emboli. All patients were discharged home on either 1 or 2 antiplatelet agents (aspirin, clopidogrel, or both) that either had been continued from preoperatively or had been started the day of the procedure.
Statistics
Values are presented as means ± SDs. Student t test for quantitative and Fisher exact test for qualitative, dichotomized parameters were used as indicated, with the P value for significance being set at <.05 (significant), <.01 (highly significant), and <.001 (extremely significant), respectively.
Results
In total, 123 consecutive cases were included for analysis (72% women, 28% men; mean age, 57.7 ± 11.9 years). Table 1 illustrates location, size, and percentage of stent placement for all aneurysms treated. All patients were adequately heparinized during the procedure.
Table 1:
More than 50% of aneurysms were originating from the ICA, followed by ACA, MCA, and BA/VA
| Aneurysm Location | n (%) | Aneurysm Diameter ± SD (mm) | Stent (%) |
|---|---|---|---|
| All | 123 (100) | 6.58 ± 3.7 | 26.8 |
| ICA | 62 (50.4) | 6.91 ± 4.35 | 41.9 |
| ACA | 22 (17.9) | 4.93 ± 1.70 | 0 |
| MCA | 14 (11.4) | 5.21 ± 1.27 | 0 |
| PcomA | 11 (8.9) | 8.59 ± 4.36 | 18.2 |
| BA/VA | 14 (11.4) | 7.46 ± 3.02 | 35.7 |
Note:—Less than 10% of aneurysms originated from PcomA. Average diameter was 6.58 mm, and 26.8% of aneurysms required concomitant stent placement.
Intraprocedural complications were noted in 11 patients (8.9%). We observed a trend toward a higher overall complication rate for stent-assisted procedures (n = 6; 18.2%), but the increase did not reach statistical significance (P = .07).
All patients underwent MES monitoring both within 12 and 24 hours after the intervention. A summary of MES data stratified by type of intervention, medication, and grade of occlusion is given in Table 2.
Table 2:
Incidence of MES on MES-1 and MES-2 scans is listed according to the following variables: modality of treatment, heparin, clopidogrel, heparin ± clopidogrel, extent of occlusion ± heparin, and size of aneurysm ± heparin
| Variable | MES-1 Scan |
MES-2 Scan |
New Infarct n(% of Group) | New Symptom at Discharge n(% of Group) | ||||
|---|---|---|---|---|---|---|---|---|
| n(% of Total) | MES Mean (SD) | t Test | n (% of Total) | MES Mean (SD) | t Test | |||
| Total | 123 (100) | 3.9 (12.4) | 123 (100) | 0.8 (2.8) | 2 (1.6) | 1 (0.8) | ||
| With stent | 33 (26.8) | 6.8 (18.2) | 33 (26.8) | 1.6 (4.4) | 2 (6.1) | 1 (3.0) | ||
| Heparin | ||||||||
| On heparin | 119 (96.7) | 3.4 (10.9) | P <. 05 | 10 (8.1) | 6 (7.2) | P < .001 | 1 (0.9) | 0 (0) |
| Off heparin | 4 (3.3) | 18.8 (35.5) | 113 (91.9) | 0.4 (1.3) | 1 (25) | 1 (25) | ||
| Clopidogrel | ||||||||
| On clopidogrel | 94 (76.4) | 3.5 (11.8) | NS | 105 (85.4) | 0.9 (3.0) | NS | 2 (2.1) | 1 (1.1) |
| Off clopidogrel | 28 (22.7) | 5.3 (14.4) | 18 (14.6) | 0.3 (1.4) | 0 (0) | 0 (0) | ||
| Heparin ± clopidogrel | ||||||||
| On heparin, on clopidogrel | 93 (75.6) | 2.8 (9.5) | NS | 10 (8.1) | 6 (7.2) | 1 (1.1) | 0 (0) | |
| On heparin, off clopidogrel | 26 (21.1) | 5.6 (14.9) | 0 (0) | 0 (0) | 0 (0) | |||
| Extent of occlusion | On heparin | Off heparin | All | |||||
| Complete (100%) | 91 (74.0) | 3.4 (11.0) | NS | 85 (69.1) | 0.4 (1.4) | NS | 1 (0.8) | 0 (0) |
| Near complete (>90%) | 24 (19.5) | 25 (20.3) | ||||||
| Incomplete (<90%) | 4 (3.3) | 1.5 (3) | 3 (2.4) | 0 (0) | 1 (20) | 1 (20) | ||
| Size of aneurysm | On heparin | Off heparin | All | |||||
| <10 mm | 97 (78.9) | 3.0 (9.4) | NS | 91 (74.0) | 0.3 (1.0) | NS | 0 (0) | 0 (0) |
| ≥10 mm | 22 (17.9) | 5.1 (16.0) | 22 (17.9) | 0.7 (2.3) | 2 (8.3) | 1 (4.2) | ||
| Stent-assisted | On heparin | Off heparin | All | |||||
| With stent | 32 (26.0) | 4.8 (14.2) | NS | 29 (23.6) | 0.3 (1.2) | NS | 2 (6.1) | 1 (3.0) |
| Without stent | 87 (70.7) | 2.9 (9.4) | 84 (68.3) | 0.4 (1.4) | 0 (0) | 0 (0) | ||
Note:—Total number of patients and relative percentage of total are given. Significance of differences for the absolute number of occurrences is calculated by a Student t test. Total number of new infarcts or new symptoms at the time of discharge is shown, with relative percentage of respective group.
In total, 119 patients (96.7%) were on a heparin drip during the MES-1 scan. Among those patients, the total number of MESs observed was lower than in cases where heparin was not continued (3.4 versus 18.8 MESs/hr). Of those 4 cases, where a heparin drip was not initiated after the procedure, 2 patients had evidence of contrast extravasation on the postprocedural CT scan, 1 patient had evidence of a hemodynamically relevant retroperitoneal hematoma, and 1 patient had refused treatment with heparin (Fig 1, case illustration).
Fig 1.
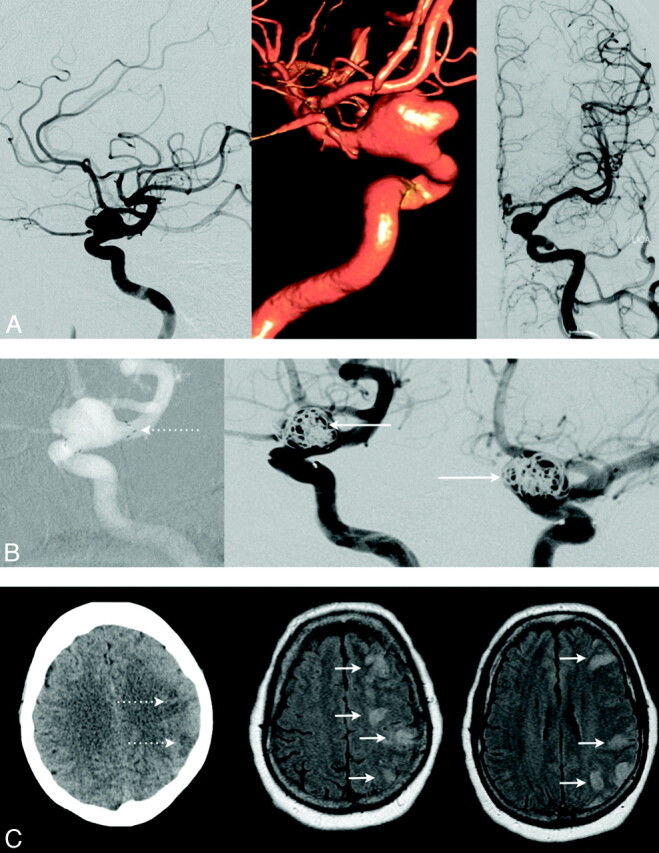
The case of a patient on clopidogrel with a large, broad-based, left-sided ophthalmic aneurysm is presented (A). A stent was placed across the neck of the aneurysm (B; left, dotted arrow); however, only incomplete embolization could be achieved (B; middle and right, full arrows). The procedure was uneventful; no focal neurologic deficits were noted after procedure. However, the patient then developed severe agitation and refused heparinization. The next morning, the patient presented with new receptive aphasia and a profound weakness of her right upper and lower extremity. Follow-up MES monitoring performed at this time (12 hours after the procedure) showed 72 MESs/hr, indicating that antiplatelet agents alone may not suffice early after this particular intervention. When we were able to initiate the heparin drip later on, repeat MES monitoring 6 hours later showed a reduction to 12 MESs/hr, but unfortunately, CT already confirmed multiple areas of embolic infarction (C; left, dotted arrows), which also were demonstrated by MR imaging later that day (C; middle and right, full arrows).
One hundred patients (81.3%) had received antiplatelet agents (aspirin, clopidogrel, or both) before the MES-1 scan. If patients had received either aspirin, clopidogrel, or both in addition to heparin before the first monitoring session, MES-1 was less likely to be positive, and the total number of MESs observed was lower, but the difference was not significant.
In 113 of the patients on heparin (91.9%), we were able to stop the heparin drip the next morning before the MES-2 scan. In this group, a highly significant decrease in mean MESs from 1.4 ± 3.1 to 0.4 ± 1.3 MESs/hr was observed (P < .001). MES-2 was negative in 103 cases (91.2%), with the 10 remaining patients ranging from 2 to 9 MESs/hr (mean, 4.2 ± 2.1 MESs/hr).
As was to be expected, because the result of the MES-1 monitoring determined whether to continue or discontinue the heparin drip, MESs in those patients who had to be continued on heparin up to the next scan (n = 10) were found to be higher (31.8 versus 1.4 MESs/hr). All of these patients were on heparin and on anticoagulants at the time of the MES-2 scan, and mean MESs had significantly decreased to 6 MESs/hr (P < .05), so the heparin drip could be stopped with the MES-3 scan being negative in all cases.
Grade of Obliteration
When on heparin for MES-1 (n = 119), no significant increase in MESs was observed if the aneurysm was only incompletely obliterated (MES-1, 1.5 versus 3.4; P = .72). Of the 4 patients without heparin drip, 1 patient with an incompletely coiled aneurysm was found to have 72 MESs/hr, and the mean MES-1 for the remaining patients with complete or near complete occlusion (n = 3) was 1 ± 1.7 MESs/hr.
Size of Aneurysm
On heparin, MES count was higher in patients with larger aneurysms (MES-1, 5.1 ± 16.0 versus 3.0 ± 9.4), but the difference did not reach significance (P = .43). In the remaining 4 patients off heparin, MES count was higher in larger aneurysms (MES-1, 36 ± 50.9 versus 1.5 ± 2.1 MESs/hr), though probably due to the very small sample size, and the difference was not significant.
Stent-Assisted Procedures
In patients on heparin early after the procedure, more emboli were observed after stent-assisted interventions (MES-1, 4.8 ± 14.2 versus 2.9 ± 9.4), but the difference was not significant. Heparin could be stopped in most cases, and no difference in MESs was observed the next day off heparin (MES-2, 0.4 ± 1.4 versus 0.3 ± 1.2). One patient underwent a stent-assisted procedure, could not get started on a heparin drip, and was found to have 72 MESs/hr early after the treatment (Fig 1, case illustration).
CT scans were performed within our timeframe of 4–6 hours after the procedure in 117 of 123 patients, and newly infarcted areas were identified in 2 cases (1.7%). Three patients developed a new neurologic deficit after the procedure (2.4%), one of which was found to be permanent on follow-up (0.8%).
When dichotomized for heparin (yes/no), clopidogrel (yes/no), heparin + clopidogrel (yes/no), heparin + any anticoagulant (yes/no), stent (yes/no), size (<10 mm, ≥10 mm), or grade of occlusion (complete and nearly complete/incomplete), no significant influence could be detected on whether emboli monitoring was positive (≥1 MESs/hr) or negative.
Follow-up examinations were available in 79 cases (64%; mean, 5.5 months since procedure). One patient had persistent postembolization symptoms (1.3%), which had been present since the time of discharge. Twenty-nine patients were available for follow-up emboli monitoring, all of which were negative.
Discussion
TEE, although fortunately seldom clinically apparent, are commonly found on MR imaging scans after endovascular procedures and are thought to develop more readily if the aneurysm is large, incompletely coiled due to a greater volume of turbulent flow and possible clot formation, or both. Stent-assisted coiling, in contrast, is thought to contribute significantly to the increase in embolic risk because of the increase in thrombogenic surface (similar to that of a protruding coil segment) as well as vessel wall manipulation. No standardized risk stratification has been identified, and no clear consensus has been reached as to what may represent the “ideal” prophylactic treatment. Heparin is probably the most commonly used intraprocedural medication, thought to minimize the risk of clot formation during the intervention, though its treatment effect has not yet been quantified. The duration of administration and the addition of further antiaggregational therapy also remain a matter of debate.
Detection of microemboli via TCD has recently emerged as a powerful, noninvasive monitoring tool that can be applied in a variety of cerebrovascular diseases. A decrease in MES as a result of antithrombotic treatment has been related to a reduced risk of overall recurrent ischemic attacks in cerebral steno-occlusive disease.18 Furthermore, MESs very recently has been found to facilitate risk stratification for patients with asymptomatic carotid stenosis, allowing for more individualized treatment recommendations.14 In a smaller series looking at patients undergoing coiling procedures (n = 35), MESs were found to be higher in clinically symptomatic patients.16 Another study also found TEE to be common after coiling (MR imaging positive in 57%) but fortunately largely silent (National Institutes of Health Stroke Scale score unchanged in 93%).19 In our series of 123 procedures, we found an overall intraprocedural complication and occlusion rate comparable with that of other studies.20,21 In another investigation, heparin was stopped immediately after the procedure, and the incidence of long-term neurologic deficit was only 0.27%.22 The study did, however, only include smaller aneurysms (≤7 mm in diameter), and the risk profile for early discontinuation of heparin may indeed be distinctly different in larger aneurysms in which clot fragments can form more easily, particularly if occlusion is incomplete.
In our observation, early and continuous administration of heparin was associated with a low occurrence of MESs, which correlates with the incidence of ischemic sequelae. Although the comparative group where heparin could not be given was very small, MESs were found to be higher without heparin. If patients were on heparin, additional antiaggregational treatment did lower the incidence of MES, but the difference was not significant. Under the current regimen, use of stents did not increase MESs significantly, which is of importance because stent placement is known to significantly increase the rate of not only intraprocedural complications but also of delayed ischemia.23 Size and configuration of aneurysms also are associated with an increase in thromboembolic events detected by diffusion-weighted imaging.4 Our analysis indicates that concomitant treatment with heparin may be able to ameliorate the inherent, immediate risk for thromboembolic events in larger and/or incompletely occluded aneurysms due to manipulation and clot dislocation, because no significant change in MESs was observed for either size or grade of occlusion. Clot formation in or adjacent to the aneurysm or to the stent seems to occur mostly within the first 6–12 hours after the coiling, which is indirectly demonstrated by higher numbers of MESs. During early heparinization, fewer occurrences of emboli were noted. Consolidation of the thrombus should be confirmed by a decrease or absence of MESs before prophylaxis with antiplatelet agents alone is deemed sufficient. The risk persists later after the treatment (>12 hours) but probably does not reach clinical relevance, because we demonstrate that the total amount of MESs is then already very low, and new adverse events were not observed at that time.
We routinely stopped the heparin drip in patients where MES-1 showed <10 MESs/hr. Despite discontinuation of heparin, MESs were highly significantly decreased on follow-up examination the next morning. If MES-1 count was elevated, continuation of the heparin drip in addition to anticoagulants led to a significant decrease of embolic signals on MES-2. Once a patient qualified for discontinuation of the drip, the next monitoring scan was negative in most cases and never exceeded our critical threshold of 9 MESs/hr. A secondary increase was not observed.
Within our treatment algorithm, no patient required heparin for >24 hours, and emboli monitoring was negative in all patients who were available for follow-up (n = 29). This helps to illustrate the need for continuous heparinization early after the intervention, when formation is an immediate, though probably transient risk.
We found that the total number of MESs per hour was more sensitive to detect treatment effects, because differences were less apparent when dichotomizing for presence or absence of MES only. We use an empirical limit of 10 MESs/hr in our algorithm, and discontinuation of heparin was safe in all cases in which this critical threshold was not exceeded. Incidentally, this threshold corroborates findings observed for ipsilateral stroke/transient ischemic attack after carotid endarterectomy.24
Study Limitations
Although this is the first comprehensive investigation analyzing the characteristics of MESs after aneurysm coiling, our analysis is retrospective and represents a single-institution, single-surgeon observation. All patients were treated according to a standardized protocol, and objective criteria were collected for analysis, but prospective recruitment of a well-matched group of patients after different coagulation algorithms is warranted to fully characterize the relationship of MES counts and heparinization. The number of patients where a heparin drip could not be started after the intervention is too low (n = 4) to allow for a meaningful statistical comparison of groups with or without heparin. This observation alone cannot determine the efficacy of heparin in lowering the MESs, because groups to be compared are not randomized or evenly matched. However, we observed a decrease of MESs beneath a threshold of 9 MESs/hr when our algorithm was observed, which might indicate a relative protective effect of heparinization in a phase where the risk for thromboembolic complications is highest.
We are able to correlate our treatment algorithm with symptomatic infarction, which is most relevant to the patient, but reliable correlation with morphologic, clinically silent occurrence of infarction could not be achieved, because MR imaging was not performed routinely. CT, however, allowed for detection of serious complications such as subarachnoid hemorrhage, indicating an intraprocedural vessel perforation that in turn may alter the postoperative treatment course.
To our knowledge, this is the first observational analysis of the use of MES monitoring after coiling of elective aneurysms. In our experience, MES monitoring is a useful, noninvasive monitoring technique that is able to safely guide a treatment algorithm after endovascular procedures. Configuration of the aneurysm and the technique and grade of occlusion are known to determine the specific need for anticoagulation because they correlate with occurrence of ischemic complications. Through quantification of microemboli, we are now able to indirectly assess the risk for thromboembolic complications that in turn allows for a more individualized, safer approach to each patient.
Conclusions
MES monitoring is a powerful adjunct to monitor efficacy of treatment algorithms aimed at the prevention of embolic complications after coiling. In our observational analysis of 123 consecutive endovascular procedures, early heparinization was associated with a low incidence of MESs. This is of particular importance in larger aneurysms, stent-assisted procedures, and incomplete occlusions in which the thromboembolic risk is greatest early on and antiplatelet treatment alone may not suffice. In our experience, it was always safe to discontinue heparinization if TCD showed <10 MESs/hr.
Abbreviations
- ACA
anterior cerebral artery
- ACT
activated clotting time
- BA/VA
basilar/vertebral artery
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MES
microembolic signal
- PcomA
posterior communicating artery
- TCD
transcranial Doppler
- TEE
thromboembolic event
Footnotes
Disclosures: Colleen Douville, Speaker Bureau: IAME, Details: Speaks 1 or 2 times per year at the Institute of Advanced Medical Education Symposia on Transcranial Doppler for which I receive an honorarium of US$1000 and travel expenses. Joe Eskridge: Ownership Interest: Pulsar Vascular Inc. Details: I own 10% of the company as of October 2010. Other Financial Relationships: Boston Scientific, Details: I own 10,000 shares of stock in Boston Scientific Corp.
References
- 1. Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74. [DOI] [PubMed] [Google Scholar]
- 2. Bakker NA, Metzemaekers JD, Groen RJ, et al. International subarachnoid aneurysm trial 2009: endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery 2010;66:961–62 [DOI] [PubMed] [Google Scholar]
- 3. Gerlach R, Beck J, Setzer M, et al. Treatment related morbidity of unruptured intracranial aneurysms: results of a prospective single centre series with an interdisciplinary approach over a 6 year period (1999–2005). J Neurol Neurosurg Psychiatry 2007;78:864–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003;24:127–32 [PMC free article] [PubMed] [Google Scholar]
- 5. Koebbe CJ, Horowitz MB, Levy EI, et al. Intraarterial thrombolysis for thromboemboli associated with endovascular aneurysm coiling. Report of 5 cases. Interv Neuroradiol 2002;8:151–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spelle L, Pierot L. Endovascular treatment of non-ruptured intracranial aneurysms: critical analysis of the literature. J Neuroradiol 2008;35:116–20. [DOI] [PubMed] [Google Scholar]
- 7. Pandya DJ, Fitzsimmons BF, Wolfe TJ, et al. Measurement of antiplatelet inhibition during neurointerventional procedures: the effect of antithrombotic duration and loading dose. J Neuroimaging 2010;20:64–69 [DOI] [PubMed] [Google Scholar]
- 8. Spencer MP, Campbell SD, Sealey JL, et al. Experiments on decompression bubbles in the circulation using ultrasonic and electromagnetic flowmeters. J Occup Med 1969;11:238–44 [PubMed] [Google Scholar]
- 9. Newell DW. Transcranial Doppler ultrasonography. Neurosurg Clin N Am 1994;5:619–31 [PubMed] [Google Scholar]
- 10. Russell D, Madden KP, Clark WM, et al. Detection of arterial emboli using Doppler ultrasound in rabbits. Stroke 1991;22:253–58 [DOI] [PubMed] [Google Scholar]
- 11. Goertler M, Blaser T, Krueger S, et al. Acetylsalicylic acid and microembolic events detected by transcranial Doppler in symptomatic arterial stenoses. Cerebrovasc Dis 2001;11:324–29 [DOI] [PubMed] [Google Scholar]
- 12. Georgiadis D, Grosset DG, Kelman A, et al. Prevalence and characteristics of intracranial microemboli signals in patients with different types of prosthetic cardiac valves. Stroke 1994;25:587–92 [DOI] [PubMed] [Google Scholar]
- 13. King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and meta-analysis. Stroke 2009;40:3711–17 [DOI] [PubMed] [Google Scholar]
- 14. Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010;9:663–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong KS. Is the measurement of cerebral microembolic signals a good surrogate marker for evaluating the efficacy of antiplatelet agents in the prevention of stroke? Eur Neurol 2005;53:132–39 [DOI] [PubMed] [Google Scholar]
- 16. Klötzsch C, Nahser HC, Henkes H, et al. Detection of microemboli distal to cerebral aneurysms before and after therapeutic embolization. AJNR Am J Neuroradiol 1998;19:1315–18 [PMC free article] [PubMed] [Google Scholar]
- 17. Moehring MA, Spencer MP. Power M-mode Doppler (PMD) for observing cerebral blood flow and tracking emboli. Ultrasound Med Biol 2002;28:49–57 [DOI] [PubMed] [Google Scholar]
- 18. Goertler M, Blaser T, Krueger S, et al. Cessation of embolic signals after antithrombotic prevention is related to reduced risk of recurrent arterioembolic transient ischaemic attack and stroke. J Neurol Neurosurg Psychiatry 2002;72:338–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rordorf G, Bellon RJ, Budzik RE, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:5–10 [PMC free article] [PubMed] [Google Scholar]
- 20. Benes V, Mitchell P, Molyneux AJ, et al. Endovascular coiling in 131 patients with low complication rate justifies treating most unruptured intracranial aneurysms. Cen Eur Neurosurg 2010;71:1–7 [DOI] [PubMed] [Google Scholar]
- 21. Pierot L, Spelle L, Vitry F, et al. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39:2497–504 [DOI] [PubMed] [Google Scholar]
- 22. Im SH, Han MH, Kwon OK, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol 2009;30:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010;41:110–15 [DOI] [PubMed] [Google Scholar]
- 24. Abbott AL, Levi CR, Stork JL, et al. Timing of clinically significant microembolism after carotid endarterectomy. Cerebrovasc Dis 2007;23:362–67 [DOI] [PubMed] [Google Scholar]



