Abstract
BACKGROUND AND PURPOSE:
Because DTI can provide good markers of white matter pathology, it could be useful in differentiating white matter changes of INPH from those of other dementias. The aim of this study was, by using DTI, to compare the characteristic white matter changes in INPH with those in AD, subcortical vascular dementia, and healthy control subjects.
MATERIALS AND METHODS:
Sixteen patients with presurgical INPH, 10 with AD, 10 with subcortical vascular dementia, and 20 healthy control subjects underwent DTI. All patients with INPH showed clinical improvement after shunt surgery, and 9 of them also underwent postshunting DTI. Regions of interest were selected at the periventricular white matter, the anterior limb of the internal capsule, the posterior limb of the internal capsule, the genu and the splenium of the corpus callosum, the superior longitudinal fasciculus, and the inferior longitudinal fasciculus. FA and MD were obtained from each region of interest and were compared among the groups.
RESULTS:
Presurgical INPH showed significantly higher FA than all the other groups in the posterior limb of the internal capsule, which was decreased after shunt surgery. Presurgical MD of the INPH group was higher than that in the AD and healthy control groups but lower than that in the subcortical vascular dementia group in the anterior periventricular white matter, the anterior limb of the internal capsule, and the superior longitudinal fasciculus. In differentiating INPH, the sensitivity and specificity of FA in the posterior limb of the internal capsule was 87.5% and 95.0%, respectively.
CONCLUSIONS:
Patients with shunt-responsive INPH showed higher FA in the posterior limb of the internal capsule compared with healthy controls and those in other groups of dementia that was reversible with shunt surgery. With this parameter, shunt-responsive INPH could be distinguished from AD, subcortical vascular dementia, and healthy conditions with high diagnostic accuracy.
Normal pressure hydrocephalus is relatively uncommon, accounting for only 6% of all cases of dementia worldwide.1,2 Accurate diagnosis of normal pressure hydrocephalus is important, however, because it is a treatable cause of dementia. Although patients with normal pressure hydrocephalus usually present with a triad of typical symptoms and signs, such as gait disturbance, progressive cognitive impairment, and urinary incontinence, the clinical decision as to whether a patient with INPH would benefit from shunt surgery is challenging. Even among patients with the classic presentation of INPH, the clinical triad combined with the radiologic signs of hydrocephalus, only 60%–70% show improvement after shunt surgery.3 Although the reason for lack of response in shunt-treated patients is often unclear, other dementias, including AD and subcortical vascular dementia, have been suggested to coexist with or be misdiagnosed as INPH.4 Patients with AD or subcortical vascular dementia may be shown to have large ventricles on CT or MR imaging as a result of cerebral atrophy; they may also have normal pressure hydrocephalus-like symptoms, such as gait disturbance or urinary incontinence, related to various degrees of white matter ischemia.
To improve the predictability of shunt-responsiveness in INPH, many invasive methods such as external lumbar drainage or long-term intracranial pressure monitoring have been applied in addition to the spinal tap test. However, these methods are associated with potentially serious complications, including central nervous system infection and hemorrhage. To develop a noninvasive and reliable diagnostic tool predicting which patients will benefit from shunt surgery, many neuroimaging studies have been conducted. Although morphologic patterns seen with CT and MR imaging have been investigated, including increased Evans index, less sulcal enlargement, white matter signal-intensity change, and aqueductal flow void, the role of structural neuroimaging in selecting surgical candidates has remained limited.5 More recently, various cerebral perfusion-imaging methods, including cerebral angiography, xenon-enhanced CT, technetium Tc99m–hexamethylpropyleneamine oxime SPECT, iodine 123 N-isopropyl-p-iodoamphetamine-SPECT, perfusion-weighted MR imaging, and H215O positron-emission tomography, have shown positive correlations between increased cerebral blood flow and clinical improvement after CSF shunt surgery.6–8 These methods, however, cannot differentiate INPH from subcortical vascular dementia, which can clinically mimic INPH despite its distinct pathologic features.
The DTI method can provide better markers of pathologic status in white matter tracts. In contrast to the large number of studies on other brain diseases by using DTI, this technique has been used in only a few investigations of white matter compression,9,10 which is a major pathologic change seen in INPH. Recent studies also suggested that DTI is a useful method in diagnosing INPH, though its diagnostic value has not been evaluated before and after shunt surgery.11,12 Thus, we investigated the characteristic white matter changes seen in INPH compared with those seen in AD, subcortical vascular dementia, and in healthy controls by using DTI, and we also compared the DTI parameters before and after shunt surgery in patients with INPH.
Materials and Methods
Subjects
A total of 56 subjects participated in the study: 16 patients with INPH, 10 with AD, 10 with subcortical vascular dementia, and 20 age-matched healthy controls. The patients with INPH were recruited prospectively from a memory disorder clinic in the department of neurology at Samsung Medical Center between October 2007 and June 2010. Initially, 43 patients who met the criteria for the probable INPH in the INPH guidelines13 and had all of the triad symptoms underwent MR imaging, including the DTI protocol, preoperatively, and 19 of them underwent ventriculoperitoneal shunt surgery. In all cases, whether to proceed to shunt surgery was determined by the presence of improvement in ≥1 triad symptom by a lumbar tap test. Among those 19 patients, we included only 16 who exhibited improvement of all the triad symptoms after shunt surgery in the INPH group, and in 9 of them, we also repeated the same DTI scan after shunt surgery. The pre- and postshunting DTIs were performed 4–15 months apart. The clinical information for the included patients with INPH is summarized in Table 1. We defined clinical improvement after shunt surgery as “fair,” “good,” or “excellent” following the scale of Black,14 which had been developed for retrospective assessment of shunt outcome. No patients with INPH showed significant white matter hyperintensities on MR imaging according to the modified criteria of Fazekas et al15 that is described below.
Table 1:
Clinical features of the 16 patients with INPH
| No. (Sex/Age, Yr) | Duration of Symptoms | White Matter Hyperintensities (Cap or Band/Deep White Matter Lesion) (mm)a | Shunt Outcomeb | Availability of Postshunt DTI | Preshunt Evans Indexc | Postshunt Evans Indexc |
|---|---|---|---|---|---|---|
| 1 (F/68) | 5 Years | 5.0/0.0 | Good | Unavailable | 0.35 | – |
| 2 (M/77) | 2.5 Years | 7.4/0.0 | Excellent | Unavailable | 0.34 | – |
| 3 (F/65) | 2 Years | 3.3/0.0 | Excellent | Available | 0.41 | 0.33 |
| 4 (M/71) | 5 Months | 2.6/0.0 | Excellent | Available | 0.33 | 0.35 |
| 5 (F/70) | 6.5 Years | 0.0/0.0 | Fair | Unavailable | 0.36 | – |
| 6 (M/70) | 3 Years | 0.0/0.0 | Good | Available | 0.33 | 0.34 |
| 7 (F/66) | 7 Years | 6.2/0.0 | Excellent | Available | 0.34 | 0.30 |
| 8 (M/65) | 6 Months | 5.5/0.0 | Excellent | Available | 0.36 | 0.29 |
| 9 (M/67) | 5 Months | 2.0/0.0 | Good | Unavailable | 0.35 | – |
| 10 (M/83) | 7 Months | 4.1/0.0 | Good | Available | 0.32 | 0.30 |
| 11 (M/71) | 6 Months | 3.5/0.0 | Good | Available | 0.30 | 0.31 |
| 12 (M/56) | 6 Months | 0.0/0.0 | Excellent | Available | 0.33 | 0.29 |
| 13 (M/71) | 1.5 Years | 0.0/0.0 | Fair | Unavailable | 0.39 | – |
| 14 (F/73) | 7 Months | 1.7/0.0 | Excellent | Available | 0.34 | 0.35 |
| 15 (F/71) | 1 Years | 3.4/0.0 | Excellent | Unavailable | 0.38 | – |
| 16 (F/74) | 2 Years | 4.1/0.0 | Good | Unavailable | 0.37 | – |
Modified criteria of Fazekas et al on MR imaging.15
From the scale of Black for assessment of shunt outcome.14
The ratio of the maximal width of the frontal horns to the maximal width of the inner skull.18
For the AD and subcortical vascular dementia groups, we selected patients whose age, sex, and MMSE scores were matched to those of the patients with INPH. The clinical diagnosis of AD followed the National Institute of Neurological and Communication Disorders and Stroke-Alzheimer Disease and Related Disorders Association criteria as described in McKhann et al.16 The patients with subcortical vascular dementia met the criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV for vascular dementia,17 and they had significant white matter hyperintensities on MR imaging in accordance with modified criteria of Fazekas et al15: a cap or band ≥10 mm and deep white matter lesion ≥25 mm. The healthy control subjects had no history of neurologic or psychiatric illnesses, and their MMSE scores were no less than 1.5 SD below the age-, sex-, and education-matched norms. All subjects provided written informed consent regarding the scientific evaluation of their data. This study was approved by the local institutional review boards at Samsung Medical Center. Demographic and clinical features of the subjects are detailed in Table 2.
Table 2:
Demographic and clinical characteristics of each groupa
| Parameters | HC (n=20) | INPH (n=16) | AD (n=10) | SVaD (n=10) | ANOVA, F or χ2 |
|---|---|---|---|---|---|
| Age (yr) | 69.8 (3.5) | 69.9 (5.9) | 70.3 (4.0) | 69.3 (5.3) | 0.08 |
| Male sex (No.) (%) | 8 (40.0) | 9 (56.3) | 4 (40.0) | 4 (40.0) | χ2=1.22 |
| MMSE | 28.5 (1.6) | 23.9 (4.3) | 21.8 (3.1) | 21.5 (2.2) | 16.56b |
| Evans indexc | 0.25 (0.03) | 0.35 (0.03) | 0.29 (0.03) | 0.28 (0.02) | 34.67b |
Variables are mean (±SD) or No. (%).
P < .001.
The ratio of the maximal width of the frontal horns to the maximal width of the inner skull.18
DTI Acquisition
MR imaging was performed on a 3T Intera Achieva MR imaging scanner (Philips Healthcare, Best, the Netherlands). The FOV in all MR imaging scans was 22 × 22 cm2, with a section thickness of 2 mm with no gap between sections. In-plane resolution was 1.72 × 1.72 mm. The DTI dataset was acquired by diffusion-weighted single-shot echo-planar imaging with the following parameters: TE, 60 ms; TR, 7,696 ms; flip angle, 90°; b-factor, 600 s/mm2; matrix dimensions, 128 × 128; 70 axial sections. With the baseline image without weighting, diffusion-weighted images were acquired from 45 different directions. All axial sections were acquired parallel to the anterior/posterior commissure line.
Evans Index Measurement
The Evans index was calculated on the individual baseline images without weighting. It was calculated as the ratio of the maximal width of the frontal horns to the maximal width of the inner skull.18 The pre- and/or postshunt values of each individual in the INPH group are demonstrated in Table 1. The values of the healthy control, INPH, AD, and the subcortical vascular dementia groups are demonstrated in Table 2, and these were significantly higher in the INPH group compared with the other groups.
Region of Interest–Based DTI analysis
The FA and ADC maps were used for region of interest–based analysis by using a technique modified from that used in a previous study.9 We selected regions of interest by using DTIStudio, Version 2.4.01 (Johns Hopkins University, Baltimore, Maryland)19 in 2 different ways. One method involved delineating anterior and posterior periventricular white matter tracts in the axial sections of 3 different levels, and the other method entailed selecting regions of interest at several anatomically representative tracts: the anterior limb of the internal capsule, the posterior limb of the internal capsule, the genu and splenium of the corpus callosum, the superior longitudinal fasciculus, and the inferior longitudinal fasciculus. The boundary between the anterior and the posterior periventricular white matter tracts was defined as a coronal plane passing vertically through the midpoint of the anterior/posterior commissure line. Because no significant asymmetry was expected between the left and right hemispheres, we combined the data from each hemisphere within each subject. Figure 1 demonstrates examples of region-of-interest selection in a healthy control subject (Fig 1A, -C) and an INPH subject (Fig 1B, -D). To help with region-of-interest placement, we used color-coded FA maps in which white matter structures could be identified more easily. Despite the significant displacement of the fibers by enlarged ventricles in patients with INPH, these structures could be identified. The regions of interest were drawn by a board-certified neurologist who is familiar with DTI images (M.-J.K.), and then those in the anterior periventricular white matter and the posterior limb of the internal capsule were drawn again by M.-J.K. and another rater to assess the intrarater and inter-rater reliability. Values of FA and MD in each region of interest were automatically computed by averaging the FA and MD values at each voxel location within the region of interest by using the fiber-tracking tools of the DTIStudio software.19
Fig 1.
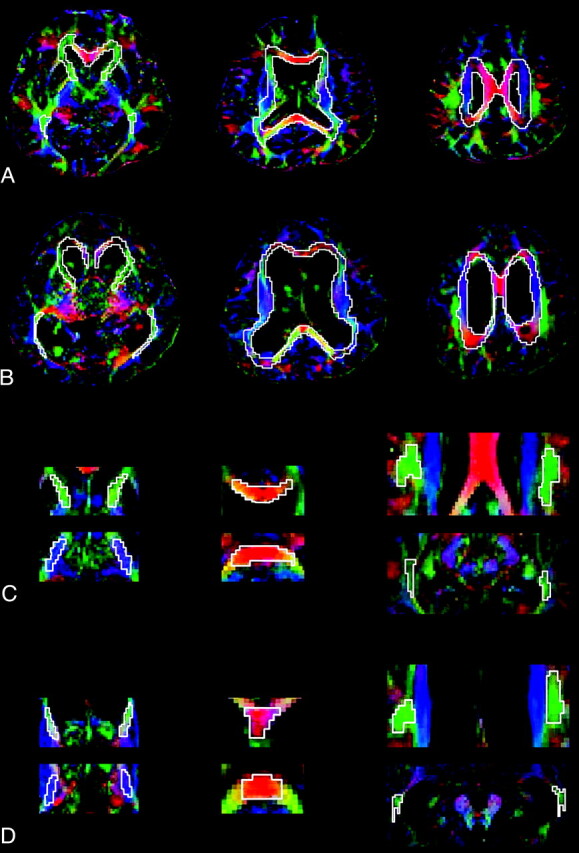
Regions of interest in a healthy control subject and a patient with INPH. A, Three representative images of color-coded FA maps in a healthy subject. Regions of interest for the periventricular white matter tracts are shown. B, The same methods are used for a patient with INPH. C, Superimposed polygons on color-coded maps in the healthy subject identify the 6 representative tracts: the anterior limb of the internal capsule and the posterior limb of the internal capsule (left), the genu and splenium of the corpus callosum (middle), and the superior longitudinal fasciculus and the inferior longitudinal fasciculus (right). D, The same methods are used for a patient with INPH. Fibers crossing from left to right are visualized in red; those crossing posteroanteriorly, in green; and those crossing inferosuperiorly, in blue.
Statistical Analysis
The FA and MD values from the anterior and posterior periventricular white matter regions of interest at 3 different levels were averaged within each subject. Comparison of FA and MD among the groups was performed by using the 1-way ANOVA or the Kruskal-Wallis test in Sigma Plot, Version 11.0 (Systat Software, Chicago, Illinois). Pair-wise multiple comparisons were conducted by using the Holm-Sidak method when the data were normally distributed and by using the Dunn method when they were not. The ROC curve was used to determine the sensitivity and specificity of values for diagnosis of INPH. Comparisons between pre- and postshunting values were conducted by using the Wilcoxon signed rank test.
Results
The Reliability of Region-of-Interest Measurements
The ICC for intrarater reliability of FA and MD measurements was 0.86 and 0.98, respectively, in the anterior PVWN, and 0.98 and 0.99, respectively, in the posterior limb of the internal capsule. The ICC for interrater reliability of FA and MD measurements was 0.81 and 0.98, respectively, in the anterior periventricular white matter, and 0.94 and 0.96, respectively, in the posterior limb of the internal capsule. Because these ICC values were each >0.80, they constitute excellent agreement for both intra- and interrater reliability.
Comparison of DTI Parameters among Preshunting INPH and Other Groups
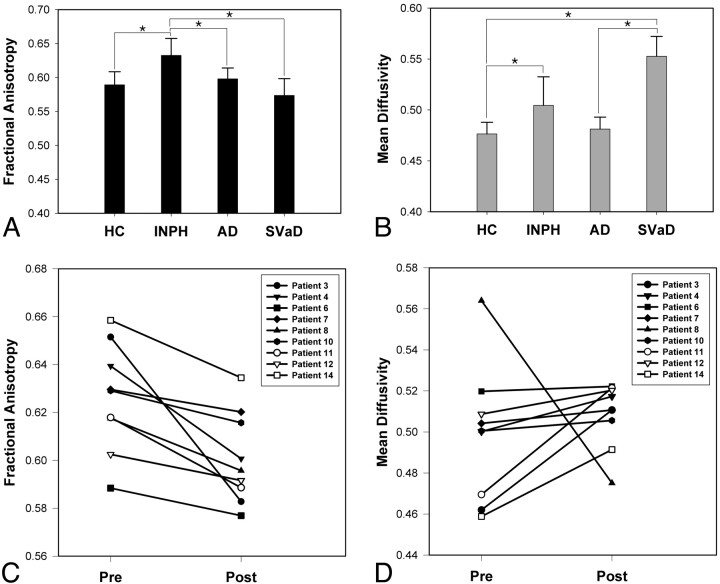
The results of ANOVA showed that both FA and MD differed significantly among the groups in all regions of interest, except in the genu of the corpus callosum (Table 3). In pair-wise comparison with the healthy control group, the INPH group showed significantly higher FA or higher MD in most of the selected tracts except in the splenium of the corpus callosum and the inferior longitudinal fasciculus. The INPH group also showed significantly higher FA than all other groups with multiple comparisons in the posterior limb of the internal capsule (Fig 2A), whereas the MD was higher than in the AD and healthy control groups but lower than that in the subcortical vascular dementia group in the anterior periventricular white matter, the anterior limb of the internal capsule, and the superior longitudinal fasciculus. The subcortical vascular dementia group showed significantly lower FA and higher MD compared with all the other groups in the anterior periventricular white matter, the anterior limb of the internal capsule, and the superior longitudinal fasciculus. Moreover, when the subcortical vascular dementia and the INPH groups were compared, FA or MD showed a significant difference in all selected tracts except in the genu of the corpus callosum. In contrast, no single value from any specific region of interest could distinguish the AD group from other groups.
Table 3:
Diffusion tensor values in each groupa
| Parameters | HC (n=20) | INPH (n=16) | AD (n=10) | SVaD (n=10) | ANOVA, F or H Value |
|---|---|---|---|---|---|
| Anterior PVWM | |||||
| FA | 0.53 (0.02) | 0.54 (0.03)b | 0.54 (0.02) | 0.49 (0.03) | 8.83c |
| MD | 0.50 (0.02) | 0.55 (0.03)b,d,e | 0.52 (0.02) | 0.61 (0.03) | 40.15c |
| Posterior PVWM | |||||
| FAf | 0.55 (0.54–0.55) | 0.57 (0.56–0.57)b,d | 0.56 (0.55–0.56) | 0.51 (0.50–0.53) | 24.58c |
| MD | 0.53 (0.02) | 0.55 (0.03)b | 0.53 (0.02) | 0.61 (0.03) | 25.20c |
| aIC | |||||
| FA | 0.55 (0.02) | 0.59 (0.04)b,d | 0.57 (0.03) | 0.50 (0.04) | 16.64c |
| MD | 0.49 (0.02) | 0.53 (0.04)b,d,e | 0.50 (0.03) | 0.60 (0.04) | 33.32c |
| pIC | |||||
| FA | 0.59 (0.02) | 0.63 (0.03)b,d,e | 0.60 (0.02) | 0.57 (0.02) | 18.67c |
| MDf | 0.48 (0.47–0.48) | 0.50 (0.49–0.52)d | 0.48 (0.47–0.49) | 0.56 (0.54–0.57) | 31.03c |
| Genu, CC | |||||
| FAf | 0.70 (0.64–0.71) | 0.66 (0.60–0.70) | 0.66 (0.64–0.70) | 0.64 (0.62–0.67) | 5.45 |
| MDf | 0.58 (0.55–0.61) | 0.67 (0.63–0.75)d | 0.62 (0.67–0.65) | 0.69 (0.61–0.75) | 20.67g |
| Splenium, CC | |||||
| FAf | 0.64 (0.62–0.66) | 0.64 (0.60–0.68)b | 0.66 (0.63–0.68) | 0.56 (0.52–0.63) | 11.59g |
| MD | 0.85 (0.17) | 0.82 (0.14)b | 0.88 (0.12) | 1.03 (0.16) | 4.26g |
| SLF | |||||
| FA | 0.47 (0.02) | 0.50 (0.03)b,d | 0.48 (0.02) | 0.44 (0.03) | 11.86c |
| MD | 0.48 (0.02) | 0.51 (0.02)b,d,e | 0.49 (0.02) | 0.56 (0.02) | 36.00c |
| ILF | |||||
| FA | 0.49 (0.02) | 0.47 (0.03)d | 0.49 (0.03) | 0.46 (0.02) | 4.72g |
| MDf | 0.57 (0.55–0.65) | 0.57 (0.54–0.62)b | 0.59 (0.57–0.64) | 0.64 (0.60–0.67) | 7.85g |
Variables are mean (±SD) or median (interquartile range).
P < .05, compared with the SVaD group.
P < .001.
P < .05, compared with the HC group.
P < .05, compared with the AD group.
The Dunn method was used to correct multiple comparisons. In other cases, the Holm-Sidak method was used.
P < .05.
Fig 2.
Differences among groups and postshunting changes in the posterior limb of the internal capsule. FA (A) and MD (B) are compared among the 4 groups: healthy control, INPH, AD, and subcortical vascular dementia in the posterior limb of the internal capsule. Postshunting changes of FA (C) and MD (D) in the posterior limb of the internal capsule in 9 patients with INPH (patient 3, 4, 6, 7, 8, 10, 11, 12, and 14 in Table 1) are also demonstrated. Error bars represent ±1 SD. The asterisk indicates P < .05.
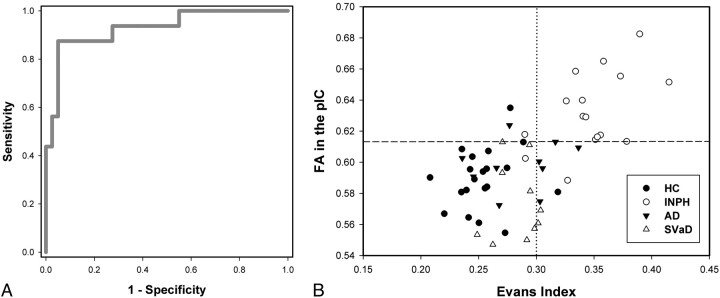
Because FA in the posterior limb of the internal capsule alone showed an extreme value in the INPH group distinct from other groups, its accuracy as a diagnostic value was assessed by using ROC (Fig 3). The area under the ROC curve was 0.93, which means that FA in the posterior limb of the internal capsule will have significant diagnostic value in differentiating patients with INPH from healthy control or other disease groups. Its sensitivity and specificity was 87.5% and 95.0%, respectively, when the cutoff value was defined as 0.613, at which the optimal values of sensitivity and specificity could be obtained on the ROC curve. On the other hand, when the guideline criterion for the Evans index (>0.30)13 was applied to our subjects, a sensitivity of 87.5% and specificity of 80.0% was obtained.
Fig 3.
Diagnostic value of FA in the posterior limb of the internal capsule. A, ROC plot of FA in the posterior limb of the internal capsule. B, Scatterplots of the Evans index and FA in the posterior limb of the internal capsule in healthy control subjects and patients with INPH, AD, and subcortical vascular dementia. The dashed line indicates the cutoff value of FA in the posterior limb of the internal capsule (0.613), and the dotted line indicates the standard cutoff value of the Evans index (0.30).
Comparison between Pre- and Postshunting DTI
In the 9 patients who received the repeated DTI, the presurgically high FA in the posterior limb of the internal capsule showed significant decrease after shunt surgery (Fig 2C, P =.004), while MD in the posterior limb of the internal capsule did not show significant change (Fig 2D, P =.13). Their postsurgical FA in the posterior limb of the internal capsule was still higher than that of the healthy control and the subcortical vascular dementia groups (P < .001, both), and the postsurgical MD was lower than that of the subcortical vascular dementia group (P < .001).
Discussion
In this study, we observed that FA was increased in the posterior limb of the internal capsule in patients with shunt-responsive INPH compared with healthy controls or those with other types of dementia. Furthermore, this increased FA was reversible with shunt surgery. With this parameter, the shunt-responsive INPH group could be distinguished preoperatively from the healthy control, AD, and subcortical vascular dementia groups with high sensitivity and specificity.
These findings may have clinical implications. In the early stages of INPH, ventricular compliance is particularly decreased in the frontal horns.20 Because of the compressive forces on the paracentral fibers, the patients initially experience gait disturbances or urinary incontinence.21 With time, the condition of these patients becomes less responsive to shunt surgery due to diffuse neuronal degeneration secondary to the repeated compression. Given that all patients with INPH participating in this study showed reversible gait disturbance with shunt surgery, the prominent changes in DTI parameters in the posterior limb of the internal capsule, containing the paracentral tracts running to the lower body, could be partly related to their gait symptoms. This possibility can be also supported by another recent study on INPH by using DTI, in which the changes in DTI parameters of the corticospinal tract were shown to be correlated with the severity of gait disturbance.11
The mechanisms underlying increased FA in the posterior limb of the internal capsule in INPH need to be fully clarified. As has been explained in previous studies,9,11 FA may be related to white matter compression. In 1 of the previous studies, the authors examined patients with acute hydrocephalus before and after shunt surgery and demonstrated that DTI could detect white matter changes associated with mechanical pressure by CSF.9 They hypothesized that the mechanical pressure could lead to higher fiber packing, which increases the tortuosity of the path of water molecules and could lead to both an overall reduction in measured radial diffusivity and an increase in parallel diffusivity, resulting in increased anisotropy. This mechanism may also be applied to chronic hydrocephalus as in our INPH group.
Our results, however, were different in that MD was also significantly increased in some regions of interest in the patients with INPH, whereas it remained at control levels before and after shunt surgery in the previous study on acute hydrocephalus.9 This finding could be explained by the differences in the pathologic conditions between acute and chronic hydrocephalus, as has also been suggested in another study.11 Unlike the acute condition, chronic exposure of the tissue to unnatural compression may result in tissue degeneration, including axonal loss and gliotic change, as well as higher packing of the fibers.22 These changes are expected to be most prominent in the tracts located proximal to the frontal horns, such as the anterior limb of the internal capsule, the superior longitudinal fasciculus, and the anterior periventricular white matter. We can also hypothesize that these tracts have high FA values initially due to mechanical compression, but secondary degenerative changes later decrease FA and increase MD. Because the posterior limb of the internal capsule is located relatively far from the frontal horns, it might be less affected and still have high FA and preserved MD. Our results of postsurgically decreased FA and unchanged MD in the posterior limb of the internal capsule could also support this suggestion.
In clinical settings, patients with AD or subcortical vascular dementia may have normal pressure hydrocephalus-like symptoms, and the possibility of a common mechanism for these disease categories with INPH has also been suggested.23–26 In particular, subcortical vascular dementia is considered to be more difficult to distinguish from INPH due to its characteristic cognitive impairment of a subcortical nature, higher incidence of gait and urinary symptoms, and characteristic MR imaging changes that can sometimes mimic the white matter changes seen in INPH. Histologic findings of the involved structures, however, are basically different between INPH and subcortical vascular dementia. In INPH, the most salient features are CSF diffusion into the periventricular white matter and, probably, transcapillary CSF absorption.27 The periventricular tissue is characterized by disruption of the ependyma, edema, neuronal degeneration, and gliosis, most likely related to altered extracellular fluid dynamics.28 These changes are potentially reversible in shunt-responsive INPH. On the other hand, vascular ischemic changes in subcortical vascular dementia are characterized by pronounced obliterative microangiopathy with extensive demyelination, lacunae, and widespread symmetric degeneration, which will not be reversed by shunt surgery.29,30
As a consequence, evaluating the histologic integrity of involved white matter tracts is likely critical not only in predicting shunt-responsiveness versus nonresponsiveness of INPH but also in differentiating INPH from subcortical vascular dementia. Previous DTI studies addressing ischemic white matter changes have demonstrated elevated MD and reduced FA in the anterior periventricular white matter, with MD considered to be more sensitive.31,32 A recent DTI study of subcortical vascular dementia demonstrated significant diffusion tensor changes in the anterior and posterior periventricular white matter, bilateral anterior subcortical areas, and the genu of the corpus callosum compared with control subjects,33 which is consistent with our findings in subcortical vascular dementia. In our results, the INPH group showed significantly higher FA and lower MD than the subcortical vascular dementia group in most of those areas, suggesting that INPH can be clearly distinguished from subcortical vascular dementia by using DTI parameters.
An ideal biomarker should detect a fundamental feature of the underlying pathophysiology of a disease and distinguish the particular condition from similar ones with a sensitivity and specificity of >80%.34 It also should be reliable, relatively noninvasive, simple to perform, and inexpensive. An Evans index of 0.30 satisfies most of these features and is highly sensitive. The recently reported specificity of the Evans index by using MR imaging, however, was as low as 74.0%.35 When the Evans index and the callosal angle measured in MR imaging (which has also been mentioned in the INPH guidelines13) were combined, the reported specificity was still 94.0%.35 From our results, however, FA in the posterior limb of the internal capsule alone could distinguish shunt-responsive INPH from other dementias with 95.0% specificity, whereas the Evans index of 0.30 was only 80.0% specific in our study. Moreover, the diagnostic specificity reached 100% when FA in the posterior limb of the internal capsule was combined with the Evans index. The sensitivity of FA in the posterior limb of the internal capsule was also as high as that of the Evans index. Another considerable advantage of DTI is its noninvasiveness and cost-effectiveness compared with other conventional procedures used to select candidates for shunt surgery.
Our study showed that the AD group did not differ from the healthy control group in most selected regions of interest. This finding could be explained by the selection of predominantly anteriorly located regions of interest, because the most common DTI changes reported in AD are reduced FA and elevated MD in posterior regions.36,37 In addition, most patients with AD in our study could be in a stage of the disease that is too early to show any changes in the selected white matter tracts.
There are several potential limitations to our study. Because we used region of interest–based analysis, the results could be operator-dependent and less reproducible than voxel-based morphometry analysis. Another limitation is that we did not compare patients with INPH who responded to shunt surgery with those who did not. From a clinical perspective, comparison within patients with INPH would be more appropriate than comparison with other forms of dementia. Likewise, because the present study was performed only with patients with INPH who underwent shunt surgery, the results could be too biased to be used with patients with general clinical conditions. Additionally, the sample size in this study was relatively small, and postshunting DTI data were partially available in the patients with INPH.
Conclusions
The clinical implication of our study is that shunt-responsive INPH could be distinguished from other conditions on the basis of higher FA in the posterior limb of the internal capsule. The diagnostic sensitivity and specificity were high enough, suggesting that DTI could be a noninvasive and valid diagnostic measure for selecting patients likely to benefit from shunt surgery. In addition, the observed reversibility of FA increase may support the currently suggested mechanism of shunt-responsiveness in INPH.
Abbreviations
- AD
Alzheimer disease
- ADC
apparent diffusion coefficient
- aIC
anterior limb of the internal capsule
- CC
corpus callosum
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- HC
healthy control
- ICC
intraclass correlation coefficient
- ILF
inferior longitudinal fasciculus
- INPH
idiopathic normal pressure hydrocephalus
- MD
mean diffusivity
- MMSE
Mini-Mental State Examination
- pIC
posterior limb of the internal capsule
- PVWM
periventricular white matter
- ROC
receiver operating characteristic analysis
- SLF
superior longitudinal fasciculus
- SPECT
single-photon emission tomography
- SVaD
subcortical vascular dementia
Footnotes
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A090629 and A050079).
References
- 1. Clarfield AM. The reversible dementias: do they reverse?. Ann Intern Med 1988;109:476–86 [DOI] [PubMed] [Google Scholar]
- 2. Larson EB, Reifler BV, Featherstone HJ, et al. Dementia in elderly outpatients: a prospective study. Ann Intern Med 1984;100:417–23 [DOI] [PubMed] [Google Scholar]
- 3. Chen YF, Wang YH, Hsiao JK, et al. Normal pressure hydrocephalus: cerebral hemodynamic, metabolism measurement, discharge score, and long-term outcome. Surg Neurol 2008;70:S69–77 [DOI] [PubMed] [Google Scholar]
- 4. Savolainen S, Hurskainen H, Paljärvi L, et al. Five-year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir 2002;144:515–23 [DOI] [PubMed] [Google Scholar]
- 5. Tarnaris A, Kitchen ND, Watkins LD. Noninvasive biomarkers in normal pressure hydrocephalus: evidence for the role of neuroimaging. J Neurosurg 2009;110:837–51 [DOI] [PubMed] [Google Scholar]
- 6. Hertel F, Walter C, Schmitt M, et al. Is a combination of Tc-SPECT or perfusion weighted magnetic resonance imaging with spinal tap test helpful in the diagnosis of normal pressure hydrocephalus? J Neurol Neurosurg Psychiatry 2003;74:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klinge PM, Brooks DJ, Samii A, et al. Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting: a retrospective analysis of [(15)O]H(2)O PET-CBF studies in 65 patients. Clin Neurol Neurosurg 2008;110:369–75. Epub 2008 Feb 11 [DOI] [PubMed] [Google Scholar]
- 8. Momjian S, Owler BK, Czosnyka Z, et al. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004;127:965–72 [DOI] [PubMed] [Google Scholar]
- 9. Assaf Y, Ben-Sira L, Constantini S, et al. Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol 2006;27:1717–24 [PMC free article] [PubMed] [Google Scholar]
- 10. Wieshmann UC, Symms MR, Parker GJ, et al. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry 2000;68:501–03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hattingen E, Jurcoane A, Melber J, et al. Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery 2010;66:917–24 [DOI] [PubMed] [Google Scholar]
- 12. Hong YJ, Yoon B, Shim YS, et al. Differences in microstructural alterations of the hippocampus in Alzheimer disease and idiopathic normal pressure hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol 2010;31:1867–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:S2-4–S2-16 [DOI] [PubMed] [Google Scholar]
- 14. Black PM. Idiopathic normal-pressure hydrocephalus: results of shunting in 62 patients. J Neurosurg 1980;52:371–77 [DOI] [PubMed] [Google Scholar]
- 15. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–56 [DOI] [PubMed] [Google Scholar]
- 16. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 17. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 18. Evans WA. An encephalographic ratio for estimating ventricular and cerebral atrophy. Arch Neurol Psychiatry 1942;47:931–37 [Google Scholar]
- 19. Jiang H, van Zijl PC, Kim J, et al. DTIStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006;81:106–16 [DOI] [PubMed] [Google Scholar]
- 20. Mendez MF, Cummings JL. Miscellaneous dementia syndromes. In: Mendez MF, Cummings JL, eds. Dementia: A Clinical Approach. Philadelphia: Butterworth Heinemann; 2003:510 [Google Scholar]
- 21. Bradley WJ. MR prediction of shunt response in NPH: CSF morphology versus physiology. AJNR Am J Neuroradiol 1998;19:1285–86 [PMC free article] [PubMed] [Google Scholar]
- 22. Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 2003;53:337–46 [DOI] [PubMed] [Google Scholar]
- 23. Bech RA, Juhler M, Waldemar G, et al. Frontal brain and leptomeningeal biopsy specimens correlated with cerebrospinal fluid outflow resistance and B-wave activity in patients suspected of normal-pressure hydrocephalus. Neurosurgery 1997;40:497–502 [DOI] [PubMed] [Google Scholar]
- 24. Golomb J, Wisoff J, Miller DC, et al. Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry 2000;68:778–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silverberg GD. Normal pressure hydrocephalus (NPH): ischaemia, CSF stagnation or both. Brain 2004;127 (pt 5)947–48 [DOI] [PubMed] [Google Scholar]
- 26. Bradley WG, Whittemore AR, Watanabe AS, et al. Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible origin of normal-pressure hydrocephalus. AJNR Am J Neuroradiol 1991;12:31–39 [PMC free article] [PubMed] [Google Scholar]
- 27. Deo-Narine V, Gomez DG, Vullo T, et al. Direct in vivo observation of transventricular absorption in the hydrocephalic dog using magnetic resonance imaging. Invest Radiol 1994;29:287–93 [DOI] [PubMed] [Google Scholar]
- 28. Akai K, Uchigasaki S, Tanaka U, et al. Normal pressure hydrocephalus: neuropathological study. Acta Pathol Jpn 1987;37:97–110 [PubMed] [Google Scholar]
- 29. Tullberg M, Hultin L, Ekholm S, et al. White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand 2002;105:417–26 [DOI] [PubMed] [Google Scholar]
- 30. Kalaria RN, Erkinjuntti T. Small vessel disease and subcortical vascular dementia. J Clin Neurol 2006;2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Sullivan M, Summers PE, Jones DK, et al. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 2001;57:2307–10 [DOI] [PubMed] [Google Scholar]
- 32. Holtmannspötter M, Peters N, Opherk C, et al. Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study. Stroke 2005;36:2559–65. Epub 2005 Nov 3 [DOI] [PubMed] [Google Scholar]
- 33. Chen TF, Lin CC, Chen YF, et al. Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res 2009;173:15–21 [DOI] [PubMed] [Google Scholar]
- 34. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer's Disease”: The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging 1998;19:109–16 [PubMed] [Google Scholar]
- 35. Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008;18:2678–83 [DOI] [PubMed] [Google Scholar]
- 36. Chua TC, Wen W, Slavin MJ, et al. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol 2008;21:83–92 [DOI] [PubMed] [Google Scholar]
- 37. Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. J Magn Reson Imaging 2008;27:20–26 [DOI] [PubMed] [Google Scholar]