SUMMARY:
A 7-year-old girl with a history of headaches and Gorham disease was surgically treated in infancy for Chiari I malformation. Subsequent investigation revealed that her cerebellar tonsillar ectopia was due to a long-standing spinal CSF-lymphatic fistula causing intracranial hypotension. Percutaneous fistula closure was performed several times, resulting in transient symptomatic improvement.
Gorham disease, or massive osteolysis, is a rare disorder characterized by proliferation of lymphatic channels with resulting bone destruction. Chylous pleural and pericardial effusions from extension of osseous disease into the pleural space and mediastinum and 1 case of a CSF-pleural fistula have been reported.1–4 Chiari I malformation has also been reported in 7 patients with Gorham or related diseases, though the relationship between the 2 conditions is not clear.5–8 We describe a child with Gorham disease who was initially treated for Chiari I malformation, which was, in retrospect, secondary to intracranial hypotension from a CSF fistula between a lumbar nerve root sleeve and a lymphatic malformation.
Case Report
A 1-year-old girl with no other medical history presented with crying and head holding. MR imaging demonstrated low cerebellar tonsils consistent with a Chiari I malformation, along with cervical cord syrinx. The patient underwent posterior fossa decompression and duraplasty, with improved but persistent symptoms (Fig 1 A).
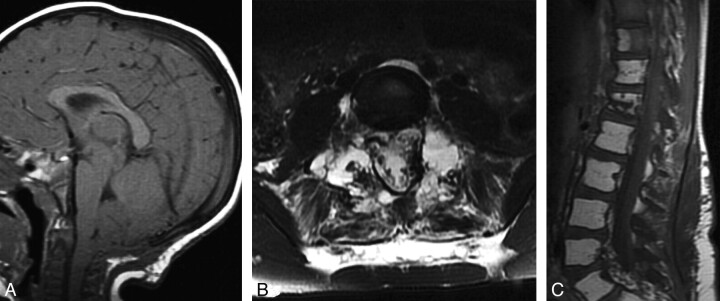
Fig 1.
A, Sagittal T1-weighted MR image obtained at 22 months of age after suboccipital decompression shows persistent cerebellar tonsillar ectopia and effacement of the prepontine cistern due to posterior fossa sagging. B and C, Axial T2-weighted (B) and sagittal T1-weighted (C) spine MR imaging at 7 years of age show extensive replacement of osseous structures by fluid-filled cystic spaces (B) and fatty marrow replacement in multiple vertebrae (C). Also, note fluid signal intensity in the paraspinous muscles and surrounding soft tissues (B).
Spine MR imaging performed to evaluate the syrinx showed a permeative osteolytic process involving the entire lumbosacral spine and pelvis, characterized by fatty marrow replacement. For several years, serial MR imaging revealed progressive replacement of bone with fluid, consistent with Gorham disease (Fig 1B, -C). The patient was treated with interferon α 2b and zoledronate (Zometa) for 1 year, with stabilization of her imaging findings. She continues to take oral vitamin D, calcium supplementation, and oral alendronate sodium (Fosamax).
Worsening postural headaches prompted a repeat MR imaging at age 7, which showed features of intracranial hypotension, including tonsillar descent, brain stem sag, draping of the floor of the third ventricle over the dorsum sellae, and diffusely enlarged dural venous sinuses. In retrospect, several of these features were also present at age 1.
A CT myelogram demonstrated rapid egress of contrast from the right L4 nerve root sleeve into the expanded L4 and L5 vertebral bodies, ultimately spreading into the paraspinous soft tissues and retroperitoneum (Fig 2 A, -B). The patient underwent 3 CT-guided transforaminal blood patches (10–20 mL) directed at the fistula, each with transient improvement of headaches. For each procedure, a 25-ga spinal needle was positioned within the thecal sac at L3, with the patient in a prone position, and rapid CT was performed during infusion of 5 mL of iohexol contrast (Omnipaque-240; GE Healthcare, Princeton, New Jersey). The needle was left in the thecal sac to monitor treatment progress.
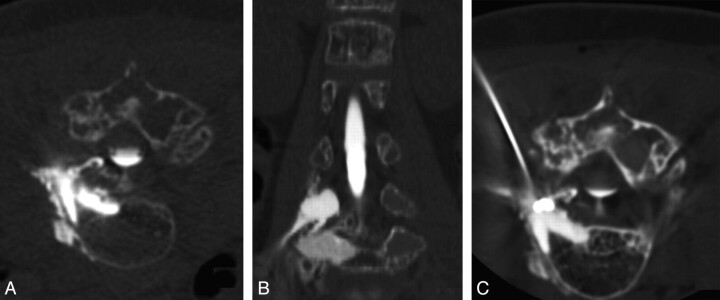
Fig 2.
CT myelogram and percutaneous fistula closure procedure. A, Axial prone image obtained during infusion of intrathecal contrast shows rapid egress of contrast material from the L4 nerve root into the expanded marrow, the adjacent vertebral body, and paraspinous malformation. B, Coronal reformatted image also shows the CSF fistula involving the posterior elements. C, Prone axial image from a CT-guided instillation of n-BCA targeting the CSF fistula at the right L4 nerve root sleeve. The focal area of highest attenuation represents n-BCA within the receiving pouch of the fistula. Hyperattenuating contrast media within the thecal sac and bone from preceding CT myelography is also seen.
Follow-up MR imaging showed improvement in findings of CSF hypotension. Because of recrudescent headaches and persistence of the fistula, CT-guided placement of 0.8 mL of n-BCA liquid embolic into the receiving cavity of the fistula was performed, with documentation of reduction of flow through the fistula (Fig 2C). Several days following treatment, the patient's headaches significantly improved. This improvement lasted 6 months, but she continues to have mild positional headaches and evidence of intracranial hypotension on MR imaging.
Discussion
The pathophysiology of Gorham disease, also known as “vanishing bone disease” or “massive osteolysis,” remains poorly understood. In the early stage, resorbed bone is replaced by non-neoplastic hypervascular angiomatous and fibrous connective tissue, with proliferative vessels showing either capillary, sinusoidal, or cavernous histology. In the later stages, progressive dissolution of bone occurs, with replacement by fibrous tissue.1,9 Although the exact nature of the disease remains unknown, the effects of hyperemia, changes in local pH-stimulating hydrolytic enzyme activity, and increased osteoclast activity have all been proposed as contributing factors.10
Gorham disease occurs in men or women of any age group but is usually discovered before 40 years of age. It may involve any bone within the axial or appendicular skeleton, though spine and visceral involvement have higher morbidity and mortality.1,3,4 Chylothorax from accumulation of lymph or thoracic duct disruption may also be associated with significant morbidity.1,4,11 Bone destruction tends to be regional, unimpeded by joint or disk spaces, and progressive. Medical treatment options include antiosteoclastic agents (bisphosphonates), radiation therapy, and interferon α 2b.10,11 Although resection has been attempted in localized disease, no single therapy is effective. MR imaging typically shows replacement of normal bone marrow with high T1 signal intensity from fat. In our case, very high signal intensity within lumbar vertebral bodies was also visible on T2-weighted images due to CSF accumulation from the fistula.
Cerebellar tonsillar ectopia in our patient was a manifestation of intracranial hypotension caused by a high-volume lumbar CSF fistula, with syrinx formation secondary to foramen magnum obstruction. Chiari I malformation has been described in 5 other patients with Gorham disease and in 2 with similar conditions.5–8 Agrawal et al4 reported a duropleural fistula in a patient with Gorham disease with a Chiari I malformation, but a potential relationship was not discussed. The likely etiology of the CSF fistula in our patient is similar to that proposed in previously reported cases: dural erosion by the angioproliferative process in the adjacent bone.11
In any patient with suspected Chiari I malformation, MR images should be examined before decompression for signs of intracranial hypotension, such as sagging of posterior fossa structures, inferior displacement of the third ventricle, dural venous sinus enlargement, and diffuse dural enhancement. These findings should prompt an investigation to identify the source of the CSF leak. Direct treatment of the leak is the appropriate step, rather than suboccipital decompression surgery. The cumulative treatment effects of repeat blood patching and embolization in this case were sufficient to improve imaging findings of CSF hypotension and symptoms. Percutaneous injection of autologous blood or liquid embolics such as n-BCA can be performed safely under low-radiation-dose CT guidance. Laminectomy or fusion was not attempted due to fear of spinous instability or vertebral collapse.
ABBREVIATIONS
- n-BCA
n-butyl cyanoacrylate
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 16–21, 2009; Vancouver, British Columbia, Canada.
References
- 1. Patel DV. Gorham's disease or massive osteolysis. Clin Med Res 2005;3:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swelstad MR, Frumiento C, Garry-McCoy A, et al. Chylotamponade: an unusual presentation of Gorham's syndrome. Ann Thorac Surg 2003;75:1650–52 [DOI] [PubMed] [Google Scholar]
- 3. Fujiu K, Kanno R, Suzuki H, et al. Chylothorax associated with massive osteolysis (Gorham's syndrome). Ann Thorac Surg 2002;73:1956–57 [DOI] [PubMed] [Google Scholar]
- 4. Agrawal R, Mohammed I, Reilly PG. Duropleural fistula as a consequence of Gorham-Stout syndrome: a combination of 2 rare conditions. J Thorac Cardiovasc Surg 2006;131:1205–06 [DOI] [PubMed] [Google Scholar]
- 5. Hughes BD, Grant GA, Cummings TJ, et al. Disappearing bone disease and Chiari I malformation. Pediatr Neurosurg 2010;46:58–61 [DOI] [PubMed] [Google Scholar]
- 6. Jea A, McNeil A, Bhatia S, et al. A rare case of lymphangiomatosis of the craniocervical spine in conjunction with a Chiari I malformation. Pediatr Neurosurg 2003;39:212–15 [DOI] [PubMed] [Google Scholar]
- 7. Pavanello M, Piatelli G, Ravegnani M, et al. Cystic angiomatosis of the craniocervical junction associated with Chiari I malformation: case report and review of the literature. Childs Nerv Syst 2007;23:697–700 [DOI] [PubMed] [Google Scholar]
- 8. Sferopoulos NK, Anagnostopoulos D, Webb JK. Cystic angiomatosis of bone with massive osteolysis of the cervical spine. Eur Spine J 1998;7:257–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radhakrishnan K, Rockson SG. Gorham's disease: an osseous disease of lymphangiogenesis? Ann N Y Acad Sci 2008;1131:203–05 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg 2005;40:E47–50 [DOI] [PubMed] [Google Scholar]
- 11. Kose M, Pekcan S, Dogru D, et al. Gorham-Stout syndrome with chylothorax: successful remission by interferon alpha-2b. Pediatr Pulmonol 2009;44:613–15 [DOI] [PubMed] [Google Scholar]