Abstract
BACKGROUND AND PURPOSE:
Patients with DMD have demonstrated functional abnormalities in the motor-related brain areas in previous PET, MRS, and TMS studies. We applied structural MR imaging and RS-fMRI in patients with DMD for the first time, and aimed to investigate the GMC and ReHo or local synchronization of spontaneous activity in the motor cortex.
MATERIALS AND METHODS:
Ten boys with DMD (6.4–14.0 years of age) and 15 healthy controls (7.9–15.1 years of age) underwent brain structural MR imaging and RS-fMRI scanning. GMC and local synchronization of spontaneous activity in the motor cortex were analyzed by using VBM and ReHo approaches, respectively.
RESULTS:
Compared with healthy controls, boys with DMD showed decreased GMC in the left PSMC and decreased ReHo in the bilateral PMSC as well as in the supplementary motor area (P < .05, corrected).
CONCLUSIONS:
The current results indicate that boys with DMD have both GMC loss and decreased local synchronization of spontaneous activity in the motor cortex, which might be due to the deficiency of dystrophin in the brain.
DMD is an X-linked recessive disease affecting 1 in 3300 males born and is the second most commonly occurring genetically inherited disease in humans. DMD is characterized by the well-known progressive skeletal muscle weakness and a different extent of cognitive impairment.1
DMD is affected by the lack of dystrophin protein, which is normally localized in skeletal muscle fiber membrane,2 cortical pyramidal neurons, and cerebellar Purkinje cells.3–5 Dystrophin protein is expressed in the postsynaptic attenuation in cerebral pyramidal neurons but is deficient in both patients with DMD and the mdx mouse (an animal model of DMD).3,5,6 In muscle, as a structural element, dystrophin protein plays an important role in stabilizing muscle fiber membrane. In the central nervous system, it may modulate synaptic terminal integrity, synaptic plasticity, and regional cellular signal-intensity integration.7 Studies on the mdx mouse found dramatic loss of corticospinal neurons8,9 due to dystrophin deficiency.
While the aforementioned studies found abnormalities at a relative microstructural level, functional neuroimaging studies have also showed alterations in patients with DMD as well as mdx mice. A 1H-MRS study found increased cerebellar choline/N-acetylaspartate ratio in patients with DMD.10 MRS studies on mdx mice showed elevated choline-containing compounds in the cerebellum and hippocampus.11 PET studies have reported an altered metabolism of glucose in the cerebellum12,13 and the right sensorimotor cortex.13 A TMS study implicated decreased excitability in the left motor cortex in patients with DMD.14
Brain MR imaging studies did not find prominent structural abnormality in DMD participants10,12,13 and mdx mice.15 The results of apparently normal structures on MR imaging in patients with DMD were obtained by visual inspection12,13 or by measuring the ventricle size.10 In fact, a modern analysis technique called VBM has been widely used in neuroimaging studies, which measures the GMC in a voxelwise way and is sensitive to subtle gray matter changes.16 Therefore, the first goal of the current study was to investigate the changes of GMC by using VBM.
Due to the radioactivity of PET, ethical constraints make it difficult to enroll healthy children.12,13 Compared with PET, BOLD fMRI has the advantages of no radioactivity and relatively high temporal resolution. However, almost all fMRI studies on neurologic disorders were conducted by using cognitive tasks to reveal specific high brain functions. It is too difficult for patients with DMD to complete these tasks well. It might be the primary reason why no such study has been carried out on patients with DMD. Recently, RS-fMRI is drawing more and more attention.17 Low-frequency (0.01–0.08 Hz) fluctuation of BOLD signal intensity has been suggested as reflecting spontaneous neuronal activity.18 The ReHo19 analytic approach for RS-fMRI data is widely used to reveal local synchronization of spontaneous activity. It has been applied in various diseases such as schizophrenia,20 early Alzheimer disease,21 children with attention deficit/hyperactivity disorder,22,23 normal aging,24 depression,25 and Parkinson disease.26 Therefore, the second goal of the current study was to reveal the functional abnormality of DMD by using a RS-fMRI and ReHo analytic approach.
Given that the motor cortex may be the most vulnerable region in the brain of patients with DMD, the current pilot study will only focus on the bilateral PSMC and SMA. We hypothesized that the patients with DMD would have both decreased GMC and decreased local synchronization of spontaneous activity. We were also interested in which of the 2 measures would be more sensitive to the abnormalities in the motor cortex of patients with DMD.
Materials and Methods
Participants
Thirteen boys with DMD (age range, 4.3–14.0 years) and 15 healthy boys (age range, 7.9–15.1 years; mean age, 10.3 ± 2.4 years) participated in this study, and all the participants were right-handed. Three patients with DMD were excluded from further analysis due to excessive head motion (see “Data Analysis”); hence, there were 10 boys with DMD (6.4–14.0 years of age; mean age, 9.3 ± 2.0 years) left. The diagnosis of DMD was based on a history of progressive muscle weakness, physical symptoms, and elevated serum creatine kinase levels. The muscle biopsy showed absent dystrophin immunoreactivity in the muscle fiber membrane for each child with DMD.
We assessed the muscle strength of the upper and lower limbs in patients with DMD by using the Medical Research Council scale (0–5) as follows: grade 5, normal strength; grade 4, slight-to-moderate weakness; grade 3, muscle can move the joint against gravity but not against any added resistance; grade 2, muscle cannot move the joint against gravity but only in absence of it; grade 1, a trace of contraction; and grade 0, no visible evidence of contraction.
This study was approved by Research Ethics Committee of the Third Hospital of Hebei Medical University. Written informed consent was obtained from each participant's parent or guardian. Each child agreed to participate in this study.
MR Imaging Procedures
The imaging, data were acquired by using a 1.5T scanner (Symphony; Siemens, Erlangen, Germany) at the Third Hospital of Hebei Medical University. Participants lay supine with the head snugly fixed by a belt and foam pads to restrict head movement. T1-weighted axial images were obtained with the following parameters: 500/12 ms (TR/TE), 20 sections, 5.0/1.0 mm (thickness/gap), 220 × 220 mm FOV, 192 × 192 (resolution), and 70° flip angle. This session lasted for 76 seconds. During the resting-state scanning, participants were told not to concentrate on anything in particular, just to relax with their eyes closed. Echo-planar BOLD images were acquired axially by using the following parameters: 2000/40 ms (TR/TE), 20 sections, 5.0/1.0 mm (thickness/gap), 220 × 220 mm FOV, 64 × 64 (resolution), and 90° flip angle. The RS-fMRI scanning session lasted for 390 seconds (195 volumes). A 3D T1-weighted spoiled gradient-recalled whole brain volume was acquired sagittally by using the following parameters: 2000/2.93 ms (TR/TE), 1100 ms (TI), 112 sections, 1.3/0 mm (thickness/gap), 240 × 240 mm FOV, 512 × 512 (resolution), and 15° flip angle. This session lasted for 324 seconds.
Data Analyses
VBM Analysis.
The 3D-MR image preprocessing and VBM analyses were conducted with Statistical Parametric Mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm). Three patients with DMD were excluded due to extensive head motion by visual inspection (these 3 participants had also extensive head motion on fMRI). The individual 3D image was segmented into 3 kinds of density maps (ie, gray mater, white matter, and cerebrospinal fluid). The gray matter density (ie, the GMC map) was spatially normalized to a standard gray matter template in standard MNI space and resampled to 1 × 1 × 1 mm3. Then the GMC map was smoothed with a Gaussian kernel of 8-mm FWHM.
Preprocessing of RS-fMRI Data.
BOLD fMRI images were preprocessed with Statistical Parametric Mapping. The first 10 volumes were discarded to avoid transient signal-intensity changes before the longitudinal magnetization reached a steady state and to allow participants to get used to the fMRI scanning noise. The remaining 185 volumes were preprocessed, including section-timing, motion correction, spatial normalization to MNI space, and resampling to a spatial resolution of 3 × 3 × 3 mm3. Participants with head motion >2-mm maximum displacement in any direction of x, y, and z or 2° of any angular motion during the fMRI scan were excluded from further analysis. Therefore, 3 boys with DMD were excluded. Finally, linear detrending and band pass filtering (0.01–0.08 Hz) were performed by using an in-house software, Resting-State fMRI Data Analysis Toolkit (REST, By Song Xiaowei et al; http://www.restfmri.net).
ReHo Analysis of RS-fMRI.
This procedure was completed by using REST software. ReHo analysis19 was performed on each participant by calculating the Kendall coefficient concordance of the time series of a given voxel with those of its nearest neighbors (26 voxels) in a voxelwise way. The ReHo of each voxel was divided by the individual global mean ReHo within a brain mask provided in the REST software. The brain-mask was obtained by removing the tissues outside the brain by using the software MRIcro (http://www.sph.sc.edu/comd/rorden/mricro.html). This standardization procedure is the same as that used in PET studies.27 Subsequently, the ReHo maps were spatially smoothed with an 8-mm FWHM Gaussian kernel.
Group Statistical Analysis
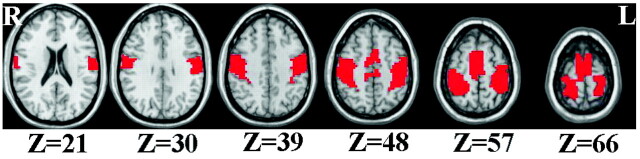
We defined bilateral PSMC and SMA as an inclusive mask based on the AAL28 template (Fig 1). Two-sample t tests were performed on the individual ReHo maps and GMC maps to reveal differences between boys with DMD and healthy controls. The resulting statistical maps were thresholded at P < .05 (t > 2.07) with a cluster size >405 mm3 by using Analysis of Functional NeuroImages29 (http://afni.nimh.nih.gov/afni). The statistical maps were transformed to TT coordinates.30
Fig 1.
The defined mask based on the AAL template includes the bilateral primary sensorimotor cortex and supplementary motor area. The numbers below the images refer to the z coordinates in the TT space. R indicates right; L, left; Z, the z coordinates.
Correlation Analysis
We analyzed the correlation between the muscular strength and the deceased ReHo values or GMCs in patients with DMD by using the Statistical Package for the Social Sciences, Version 11.5 (SPSS, Chicago, Illinois).
Results
There was no significant difference in age between the 2 groups of participants (patients with DMD, 9.3 ± 2.0 years; controls, 10.3 ± 2.4 years, P = .285). The age of patients with DMD showed significantly negative correlation with the muscle strength of the distal limbs (P = .009 in the upper limb; P = .012 in the lower limb; Table 1 and Fig 2).
Table 1:
The correlation between age and muscle strength in patients with DMD
| Muscle Strength | r | P value | |
|---|---|---|---|
| Proximal upper limb | 3.6 ± 0.8 | −0.517 | .070 |
| Distal upper limb | 4.3 ± 0.5 | −0.693 | .009 |
| Proximal lower limb | 3.3 ± 0.7 | −0.548 | .052 |
| Distal lower limb | 4.3 ± 0.4 | −0.670 | .012 |
Fig 2.
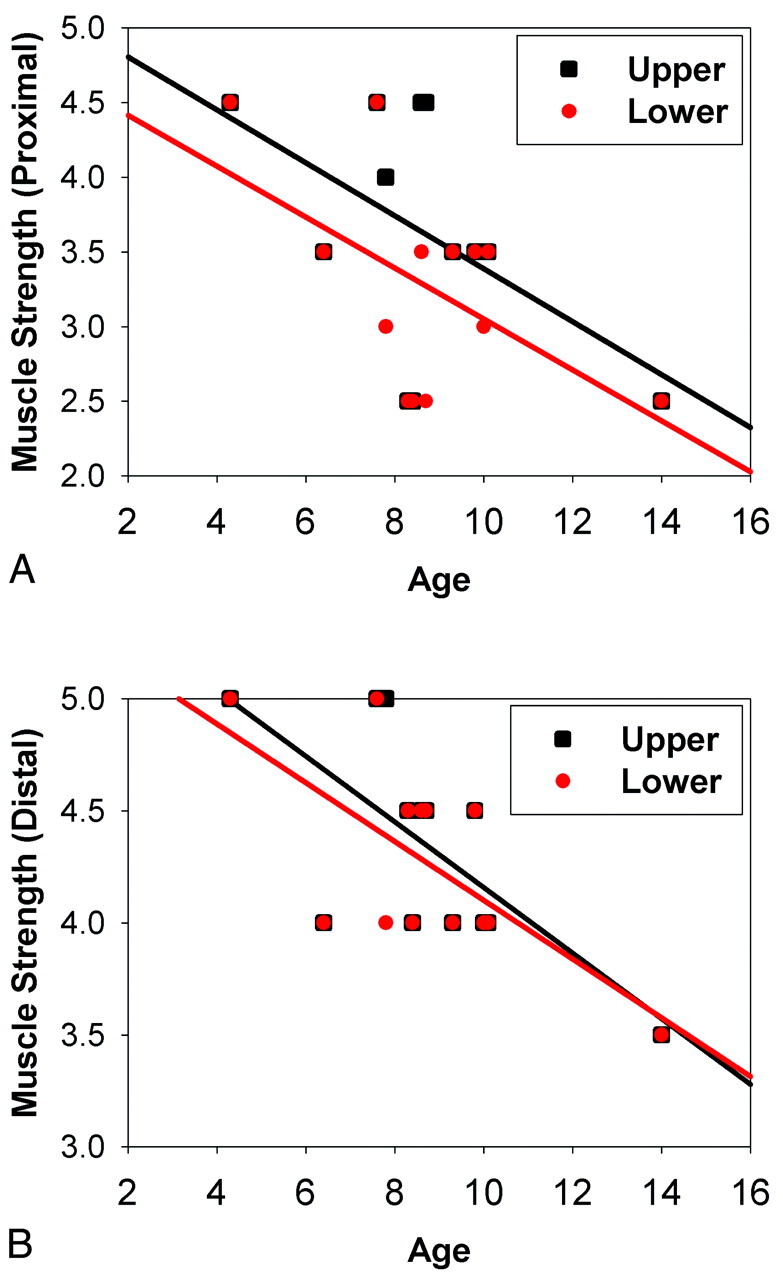
Correlation between age and muscle strength in patients with DMD. A, Proximal limb. B, Distal limb. Black squares and regression lines indicate the correlations between age and the upper limb, while red dots and regression lines indicate the correlation between age and the lower limb.
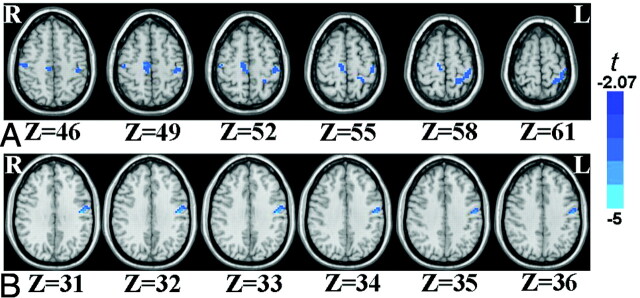
Compared with healthy controls, the boys with DMD showed decreased ReHo in the bilateral PSMCs and SMAs (Table 2 and Fig 3A). The VBM analysis showed decreased GMC in patients with DMD compared with the controls in the left PSMC (Table 2 and Fig 3B). Although t tests on ReHo have been used in many studies, there has been no normality test on ReHo, to our knowledge. We performed the Kolmogorov-Smirnov normality test on the mean ReHo value of the 4 regions shown in Table 2. All 12 tests (4 for the DMD group, 4 for the healthy group, and 4 for the total group) showed that the ReHo value is under normal distribution (P values = .052–.200). Future studies on a large group will further address this issue.
Table 2:
Brain areas of regional homogeneity and gray matter concentration difference between 2 groups in the defined motor cortex maska
| Volume (mm3) | Area | Hemisphere | BA | TT Coordinates |
Peak T Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Decreased ReHo in DMD | |||||||
| 2214 | PSMC | L | 4 | −43 | −25 | 53 | −2.73 |
| PSMC | L | 5 | −22 | −49 | 59 | −2.81 | |
| 1404 | SMA | R | 6 | 11 | −19 | 50 | −3.33 |
| 459 | PSMC | R | 4 | 47 | −19 | 47 | −2.61 |
| Decreased GMC in DMD | |||||||
| 675 | PSMC | L | 4 | −43 | −13 | 32 | −4.40 |
Note:—L indicates left; R, right; BA = Brodmann area.
P < .05.
Fig 3.
The brain areas of deceased ReHo (A) and decreased GMC (B) in boys with DMD compared with controls. The numbers below the images refer to the z coordinates in the TT space. R indicates right; L, left; Z, the z coordinates.
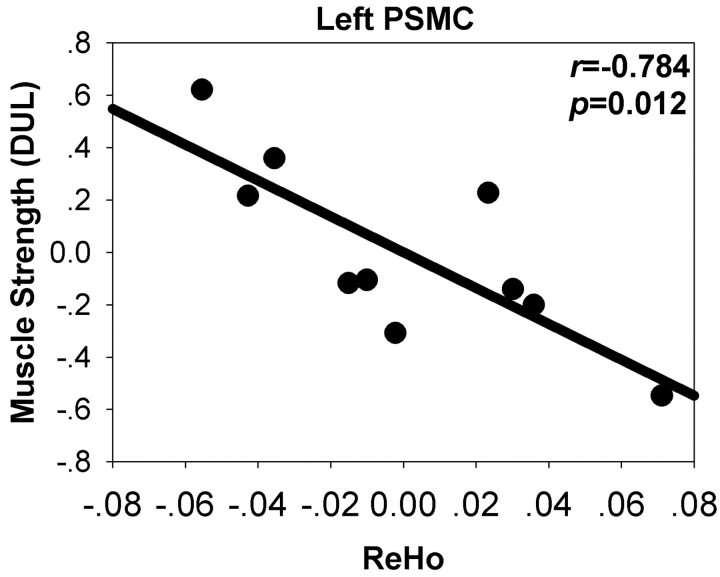
There was a significant negative correlation between the ReHo value in the left PSMC and the muscle strength of the distal upper limb when age was taken as a covariate (r = −0.784, P = .012; Table 3 and Fig 4). No other significant correlations were found between the muscle strength and ReHo value or GMC.
Table 3:
The correlation between MR imaging measures and the muscle strength in patients with DMD
| Area | ReHo Value |
GMC Value | ||||
|---|---|---|---|---|---|---|
| L-PSMC (BA 4) | L-PSMC (BA 5) | R-SMA (BA 6) | R-PSMC (BA 4) | L-PSMC (BA 4) | ||
| Proximal upper limb | r | −0.347 | −0.388 | 0.353 | 0.260 | −0.550 |
| P | 0.360 | 0.302 | 0.352 | 0.500 | 0.125 | |
| Distal upper limb | r | −0.784 | −0.235 | 0.482 | −0.407 | −0.626 |
| P | 0.012 | 0.543 | 0.189 | 0.277 | 0.071 | |
| Proximal lower limb | r | −0.105 | 0.208 | −0.530 | 0.012 | 0.248 |
| P | 0.789 | 0.592 | 0.142 | 0.976 | 0.520 | |
| Distal lower limb | r | −0.428 | −0.435 | 0.094 | −0.271 | −0.520 |
| P | 0.250 | 0.242 | 0.810 | 0.481 | 0.151 | |
Note:—L indicates left; R, right; BA, Brodmann area.
Fig 4.
Correlation between ReHo at the left PSMC (BA 4) and muscle strength of the distal upper limb (DUL) with age as covariance. The x- and y-axes are the residuals of the original ReHo and muscle strength of the DUL, respectively, with age regressed out.
Discussion
By using both structural and functional MR imaging, the current study found decreased GMC and decreased local synchronization of spontaneous activity in the motor cortex of patients with DMD compared with healthy controls.
In this study, we found deceased GMC in the left PSMC in patients with DMD compared with age-matched controls by using VBM. A few previous MR imaging studies could not find structural abnormalities in the brains of patients with DMD by visual inspection,12,13 or by measuring the ventricle size.10 VBM is a modern analysis technique for measuring the GMC in a voxelwise way and is sensitive to subtle gray matter changes.16,31 The current study provided direct evidence of gray matter loss in the motor cortex of patients with DMD.
We also found deceased ReHo (ie, decreased intraregional or local synchronization) in the PSMC and SMA in patients with DMD compared with age-matched controls. To the best of our knowledge, this is the first fMRI study of patients with DMD. The current results are partly consistent with previous results of studies of functional abnormalities in the motor-related brain areas of patients with DMD. A PET study13 compared 10 boys with DMD (5.2–16.2 years of age, with a mean age of 11.8 years) with 17 healthy adults (21.1–38.2 years of age, with a mean age of 27.6 years) and found decreased glucose metabolism in patients with DMD in the right sensorimotor cortex. A TMS study implicated decreased excitability in the left motor cortex in patients with DMD (n = 4).14 Taken together, all the above functional techniques including PET, TMS, and RS-fMRI suggested prominent functional abnormality in the motor cortex of patients with DMD. In addition, the current results indicate that the functional abnormality as revealed by ReHo is more extensive than the structural abnormality obtained by VBM (Fig 3A versus Fig 3B).
The neural mechanisms underlying the gray matter loss and decreased local synchronization of spontaneous activity in the motor cortex of patients with DMD remain unclear. Dystrophin has been found to be absent in the postsynaptic attenuation of patients with DMD or the mdx model's brain.3,5,6 To what extent the absence of dystrophin has contributed to the decreased ReHo and decreased GMC in patients with DMD needs to be elucidated in the future studies.
The current study found that the patients' ages had significant negative correlations with the muscle strength of distal limbs (Table 1). This result was very consistent with that in the literature,32 indicating that the deceased muscle strength is closely related to the progressive deterioration of the disease. We performed correlation analysis between muscle strength and neuroimaging indices (ReHo and GMC value). There were a total of 20 pairs of correlation analysis (Table 3), but only 1 pair (ie, the ReHo value in the left PSMC) showed significant negative correlation (r = −0.784, P = .012) with the muscle strength of the distal upper limb. This negative correlation seems to be counterintuitive because both the ReHo value and muscle strength are significantly decreased in patients with DMD. However, an mdx model study found that the number of parvalbumin-positive and calbindin-positive neurons increased more selectively in the sensorimotor cortex in mdx mice than in wild-type ones, implying a functional compensation process.33 Two TMS studies34,35 found that patients with other types of muscular dystrophy showed reduced inhibition in the primary motor cortex, indicating a compensatory mechanism. Thus, given the combined results of the negative correlation between ReHo and muscle strength, the current study might suggest that though the regional functional synchronization in motor cortex decreased significantly in patients with DMD, local functional compensation may also exist due to muscle-strength impairment. However, such a P value (.012) would no longer be significant when taking multiple comparison correction into account by using the Bonferroni method (ie, .05/20 = .0025). Future studies with a larger sample size will further investigate the correlations between brain imaging measures and the severity of symptoms, including cognitive measurements.
In this study, we were only interested in the motor cortex, including the PSMC and SMA; therefore, we used a less strict threshold (P < .05). Due to the relatively small sample size, the statistical power will be a concern if the entire brain (ie, a huge number of voxels) is analyzed. The results of the current pilot study need to be validated in future studies.
Conclusions
Boys with DMD showed decreased local synchronization of spontaneous activity and decreased GMC in the motor cortex, in addition to skeletal muscle dysfunctions. Furthermore, we found that the functional abnormality is more extensive than structural abnormality, indicating that the functional index might be more sensitive. Future MR imaging studies on a larger sample will be helpful in exploring the brain mechanisms of DMD.
ABBREVIATIONS
- AAL
automated anatomical labeling
- BOLD
blood oxygen level–dependent
- DMD
Duchenne muscular dystrophy
- FWHM
full width at half maximum
- GMC
gray matter concentration
- MNI
Montreal Neurological Institute
- PSMC
primary sensorimotor cortex
- ReHo
regional homogeneity
- RS
resting-state
- SMA
supplementary motor area
- TMS
transcranial magnetic stimulation
- TT
Talairach and Tournoux
- VBM
voxel-based morphometry
Footnotes
This work was supported by the Scientific Research Foundation of Health Bureau of Hebei Province (No. 08133) and the Natural Science Foundation of China (81020108022).
References
- 1. Anderson JL, Head SI, Rae C, et al. Brain function in Duchenne muscular dystrophy. Brain 2002;125:4–13 [DOI] [PubMed] [Google Scholar]
- 2. Zubrzycka-Gaarn EE, Bulman DE, Karpati G, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 1988;333:466–69 [DOI] [PubMed] [Google Scholar]
- 3. Kim TW, Wu K, Xu JL, et al. Detection of dystrophin in the postsynaptic density of rat brain and deficiency in a mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 1992;89:11642–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lidov HG, Byers TJ, Kunkel LM. The distribution of dystrophin in the murine central nervous system: an immunocytochemical study. Neuroscience 1993;54:167–87 [DOI] [PubMed] [Google Scholar]
- 5. Lidov HG, Byers TJ, Watkins SC, et al. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature 1990;348:725–28 [DOI] [PubMed] [Google Scholar]
- 6. Kim TW, Wu K, Black IB. Deficiency of brain synaptic dystrophin in human Duchenne muscular dystrophy. Ann Neurol 1995;38:446–49 [DOI] [PubMed] [Google Scholar]
- 7. Mehler MF. Brain dystrophin, neurogenetics and mental retardation. Brain Res Brain Res Rev 2000;32:277–307 [DOI] [PubMed] [Google Scholar]
- 8. Carretta D, Santarelli M, Vanni D, et al. The organisation of spinal projecting brainstem neurons in an animal model of muscular dystrophy: a retrograde tracing study on mdx mutant mice. Brain Res 2001;895:213–22 [DOI] [PubMed] [Google Scholar]
- 9. Sbriccoli A, Santarelli M, Carretta D, et al. Architectural changes of the cortico-spinal system in the dystrophin defective mdx mouse. Neurosci Lett 1995;200:53–56 [DOI] [PubMed] [Google Scholar]
- 10. Rae C, Scott RB, Thompson CH, et al. Brain biochemistry in Duchenne muscular dystrophy: a 1H magnetic resonance and neuropsychological study. J Neurol Sci 1998;160:148–57 [DOI] [PubMed] [Google Scholar]
- 11. Rae C, Griffin JL, Blair DH, et al. Abnormalities in brain biochemistry associated with lack of dystrophin: studies of the mdx mouse. Neuromuscul Disord 2002;12:121–29 [DOI] [PubMed] [Google Scholar]
- 12. Bresolin N, Castelli E, Comi GP, et al. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul Disord 1994;4:359–69 [DOI] [PubMed] [Google Scholar]
- 13. Lee JS, Pfund Z, Juhasz C, et al. Altered regional brain glucose metabolism in Duchenne muscular dystrophy: a PET study. Muscle Nerve 2002;26:506–12 [DOI] [PubMed] [Google Scholar]
- 14. Di Lazzaro V, Restuccia D, Servidei S, et al. Functional involvement of cerebral cortex in Duchenne muscular dystrophy. Muscle Nerve 1998;21:662–64 [DOI] [PubMed] [Google Scholar]
- 15. Dunn JF, Zaim-Wadghiri Y. Quantitative magnetic resonance imaging of the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve 1999;22:1367–71 [DOI] [PubMed] [Google Scholar]
- 16. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 17. Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol 2010;6:15–28 [DOI] [PubMed] [Google Scholar]
- 18. Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412:150–57 [DOI] [PubMed] [Google Scholar]
- 19. Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22:394–400 [DOI] [PubMed] [Google Scholar]
- 20. Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport 2006;17:19–22 [DOI] [PubMed] [Google Scholar]
- 21. He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage 2007;35:488–500 [DOI] [PubMed] [Google Scholar]
- 22. Cao Q, Zang Y, Sun L, et al. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport 2006;17:1033–36 [DOI] [PubMed] [Google Scholar]
- 23. Zhu CZ, Zang YF, Cao QJ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage 2008;40:110–20 [DOI] [PubMed] [Google Scholar]
- 24. Wu T, Zang Y, Wang L, et al. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett 2007;423:189–93 [DOI] [PubMed] [Google Scholar]
- 25. Yuan Y, Zhang Z, Bai F, et al. Abnormal neural activity in the patients with remitted geriatric depression: a resting-state functional magnetic resonance imaging study. J Affect Disord 2008;111:145–52 [DOI] [PubMed] [Google Scholar]
- 26. Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 2009;30:1502–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89 [DOI] [PubMed] [Google Scholar]
- 29. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73 [DOI] [PubMed] [Google Scholar]
- 30. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988 [Google Scholar]
- 31. Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 1995;2:244–52 [DOI] [PubMed] [Google Scholar]
- 32. Carretta D, Santarelli M, Vanni D, et al. Cortical and brainstem neurons containing calcium-binding proteins in a murine model of Duchenne's muscular dystrophy: selective changes in the sensorimotor cortex. J Comp Neurol 2003;456:48–59 [DOI] [PubMed] [Google Scholar]
- 33. Uchikawa K, Liu M, Hanayama K, et al. Functional status and muscle strength in people with Duchenne muscular dystrophy living in the community. J Rehabil Med 2004;36:124–29 [DOI] [PubMed] [Google Scholar]
- 34. Liepert J, Schoser BG, Weiller C. Motor excitability in myopathy. Clin Neurophysiol 2004;115:85–89 [DOI] [PubMed] [Google Scholar]
- 35. Schwenkreis P, Voigt M, Hasenbring M, et al. Central mechanisms during fatiguing muscle exercise in muscular dystrophy and fibromyalgia syndrome: a study with transcranial magnetic stimulation. Muscle Nerve 2011;43:479–84 [DOI] [PubMed] [Google Scholar]