Abstract
BACKGROUND AND PURPOSE:
Although the combined end point of partial and complete recanalization is a well-established predictor of good outcome following acute stroke intervention, few investigations have evaluated the effect of the degree of recanalization. We hypothesized that greater degrees of recanalization would be associated with a higher likelihood of favorable functional outcomes.
MATERIALS AND METHODS:
Data from MERCI and Multi MERCI—prospective single-arm trials of endovascular mechanical thrombectomy for acute stroke—were pooled. The TIMI score was used to define the degree of recanalization, and a favorable outcome was defined as an mRS score of 0–2 at 90 days.
RESULTS:
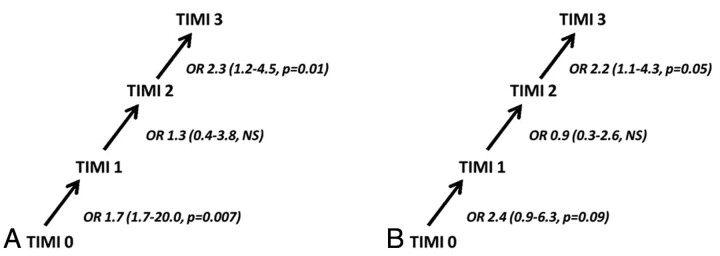
A total of 305 patients were included. Age, stroke severity, and site of arterial occlusion did not differ among groups stratified by the TIMI score. The unadjusted OR for a favorable outcome increased significantly as the TIMI score increased from 0 to 1 (OR, 5.9; 95% CI, 1.7–20.0; P = .007) and from 2 to 3 (OR. 2.3; 95% CI, 1.2–4.5; P = .01) and the likelihood of death decreased significantly as the TIMI score increased from 2 to 3 (OR, 2.2; 95% CI, 1.1–4.3; P = .05). In multivariate analysis, each increase in TIMI grade increased the odds of a good outcome 2.6-fold (95% CI, 1.9–3.4, P < .0001).
CONCLUSIONS:
Increases in the TIMI score were highly associated with improved outcomes. This finding not only provides additional evidence that restoration of blood flow improves clinical outcomes in ischemic stroke but also suggests that interventionalists should strive for complete revascularization when they provide endovascular treatment for acute ischemic stroke.
Recanalization after large-vessel stroke is the most important modifiable predictor of a favorable outcome.1–4 A recent meta-analysis of case series evaluating the effect of recanalization on outcome found a substantial decrease in mortality (OR, 0.24; 95% CI, 0.16–0.35) and an increase in the likelihood of a good functional outcome among revascularized patients (OR, 4.4; 95% CI, 3.3–5.9).5
It appears that outcomes following stroke due to large-vessel occlusion may be further improved by complete recanalization as opposed to partial recanalization. In 1 prospective trial of IV-tPA in combination with transcranial sonography, 48% of patients with complete recanalization had a favorable outcome at 90 days versus 31%–38% with partial recanalization.6 The phenomenon of better outcomes resulting from complete as opposed to partial recanalization has also been observed in retrospective case series of patients treated with IA-tPA7,8 and in a meta-analysis of studies reporting outcome after mechanical thrombectomy.9 The meta-analysis was limited, however, by the inclusion of studies with methodologic difficulties (nonconsecutive patient enrollment, absence of predefined enrollment criteria, and lack of blinded outcome assessment) and limited number of patients (n = 171).
We, therefore, sought to evaluate the effect of the degree of recanalization on outcome after large-vessel stroke among patients in the MERCI trials.10,11 We hypothesized that favorable outcomes would be more common and mortality would be reduced as the degree of recanalization increased.
Materials and Methods
All patients enrolled in the MERCI and Multi MERCI trials were included in the present pooled analysis. The interventional techniques and enrollment criteria for these trials have been described in detail previously.10,11 Essentially, patients were eligible if they developed a stroke due to large-vessel occlusion and could be treated within 8 hours of symptom onset with the Merci retriever (Concentric Medical, Mountain View, California). Key additional inclusion criteria were the following: ≥18 years of age and NIHSS score of ≥8. In the Multi MERCI trial, as opposed to MERCI, patients could be pretreated with IV-tPA before endovascular intervention. In both trials, adjuvant IA-tPA was acceptable after 6 passes with the Merci retriever alone or if inaccessible distal emboli were present.
All data used in this analysis were obtained prospectively. We included the following variables:
Presenting Features.
Demographics (age, sex, race), baseline NIHSS score, site of occlusion [ICA, MCA (M1, or M2), vertebrobasilar] mRS score before stroke onset.
Procedural Details.
Time to arterial puncture, time to end of the procedure, number of passes with Merci device, use of adjuvant IA techniques, the presence of symptomatic intracerebral hemorrhage, and the presence of PH-2 hemorrhage.
Final TIMI Grade.
TIMI grade at the conclusion of intervention (final TIMI grade). TIMI score was assigned on the basis of the status of all treatable vessels according to the following definitions: TIMI 0, no flow; TIMI 1, minimal flow restoration; TIMI 2, incomplete flow restoration; TIMI 3, normal flow.
Outcome.
mRS at 90 days; death at ≤90 days.
Three primary statistical analyses were performed. First, patients were grouped according to the final TIMI grade and the presenting features, and procedural details among these groups were compared. Second, clinical outcomes were stratified by final TIMI grade, and unadjusted ORs for each increase in TIMI score (0 to 1, 1 to 2, 2 to 3) were calculated. Finally, multivariate predictors of good clinical outcome (90-day mRS score of 0–2) were identified by stepwise (forward/backward) logistic regression. Only variables present for at least 95% of patients and significant at the P < .2 level in the univariate logistic regression analysis were considered for inclusion in the multivariate analysis. The multivariate model was built by using forward/backward stepwise regression, with variables entered into the model at the P ≤ .05 level and removed at the P ≤ .10 level. All statistical analysis was performed by using SAS, Version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
A total of 305 patients were included in the analysis.
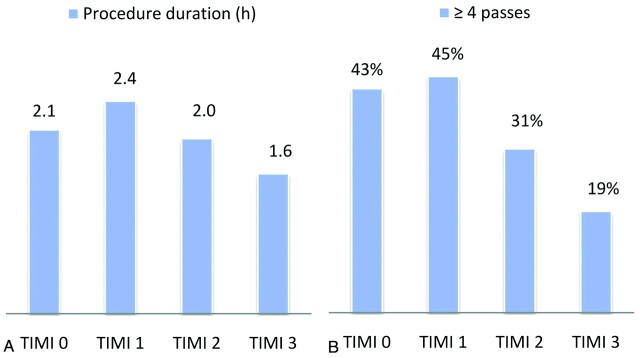
The presenting features and procedural details stratified by TIMI score are shown in Tables 1 and 2. Strokes in the MERCI trials were severe, with a median NIHSS score of 19. The median age was 72 years. The presenting features (Table 1) did not differ statistically among the groups, with no significant differences found in age, sex, stroke severity, site of occlusion, or baseline mRS score. The MCA was the most frequently involved vessel, accounting for 58% of cases; the ICA and vertebrobasilar systems were involved in 33% and 9% of cases, respectively. Patients with lower final TIMI scores (0 or 1) underwent procedures of significantly longer duration and with significantly more device passes than patients with higher TIMI scores (Table 2 and Fig 1). Nonetheless, the rates of SICH or PH-2 hemorrhage were not related to TIMI scores.
Table 1:
Presenting features
| TIMI 0 | TIMI 1 | TIMI 2 | TIMI 3 | Total | P | |
|---|---|---|---|---|---|---|
| No. of patients | 110 | 20 | 61 | 114 | 305 | |
| Age (yr) | ||||||
| Mean | 68.1 ± 15 | 64.5 ± 16 | 70.5 ± 14.9 | 66.1 ± 16.7 | 67.6 ± 15.8 | NS |
| Median (IQR) | 73 (59.0–80.0) | 66 (54.0–77.5) | 75 (63.0–81.0) | 70 (55.0–79.0) | 72 (58.0–80.0) | |
| Female | 55.5% | 55.0% | 52.5% | 48.2% | 52.1% | NS |
| Vessel involved | ||||||
| Vertebrobasilar | 6.4% | 15.0% | 6.6% | 12.3% | 9.2% | NS |
| Carotid | 32.7% | 30.0% | 27.9% | 35.1% | 32.5% | |
| MCA (M1/M2) | 60.9% | 55.0% | 65.6% | 52.6% | 58.4% | |
| NIHSS score (median) (IQR) | 20 (16.0–24.0) | 19 (14.0–22.5) | 17 (14.0–23.0) | 18 (15.0–23.0) | 19 (15.0–23.0) | NS |
| Baseline mRS (0 or 1) | 89.1% | 75.0% | 83.6% | 92.9% | 88.5% | NS |
Note:—IQR indicates interquartile range; NS, not significant.
Table 2:
Procedural details and complications
| TIMI 0 | TIMI 1 | TIMI 2 | TIMI 3 | Total | P Value | |
|---|---|---|---|---|---|---|
| No. of patients | 110 | 20 | 61 | 114 | 305 | |
| Ictus to arterial puncture (hr) (mean) | ||||||
| Median (IQR) | 4.5 ± 2.1 | 4.5 ± 1.6 | 4.3 ± 1.6 | 4.2 ± 1.5 | 4.4 ± 1.8 | NS |
| 4.3 (3.0–5.6) | 4.1 (3.3–5.4) | 4.4 (3.2–5.7) | 4.2 (3.1–5.2) | 4.3 (3.1–5.5) | ||
| Ictus to procedure end (hr) (mean) | ||||||
| Median (IQR) | 6.6 ± 2.1 | 6.93 ± 2.0 | 6.3 ± 1.9 | 5.8 ± 1.6 | 6.3 ± 1.9 | .004 |
| 6.5 (5.5–7.8) | 7.1 (5.7–8.2) | 6.0 (5.0–7.7) | 5.8 (4.7–6.9) | 6.1 (5.0–7.5) | ||
| No. of passes | ||||||
| 1 | 9.1% | 25.0% | 31.1% | 34.2% | 23.9% | <.001 |
| 2 | 18.2% | 15.0% | 16.4% | 23.7% | 19.7% | |
| 3 | 30.0% | 15.0% | 21.3% | 22.8% | 24.6% | |
| 4 | 13.6% | 5.0% | 19.7% | 11.4% | 13.4% | |
| >4 | 29.1% | 40% | 11.5% | 7.9% | 18.4% | |
| Use of adjuvant | 40.9% | 45.0% | 32.8% | 36.0% | 37.7% | NS |
| Procedure-related clinically significant AE | 10.0% | 0.0% | 6.6% | 3.5% | 6.2% | .09 |
| Symptomatic bleeding events | 12.7% | 10.0% | 8.2% | 5.3% | 8.9% | NS |
| PH-2 events | 9.1% | 0.0% | 3.2% | 3.7% | 4.9% | NS |
Note:—IQR indicates interquartile range; NS, not significant; AE, adverse events.
Fig 1.
Procedural duration and number of passes stratified by the final TIMI score. A, Mean procedural duration (hours) stratified by final TIMI score. B, Percentage of cases in which ≥4 passes with the Merci retriever were made, stratified by final TIMI score.
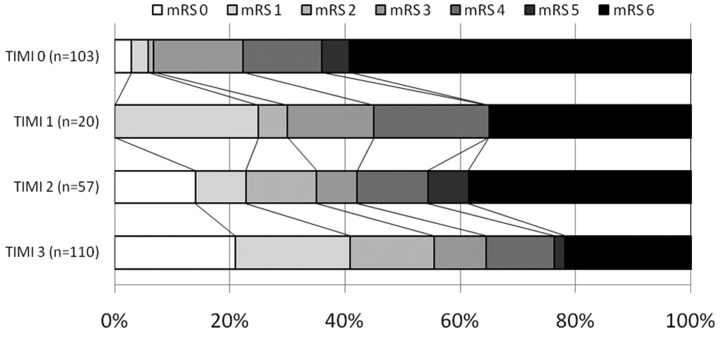
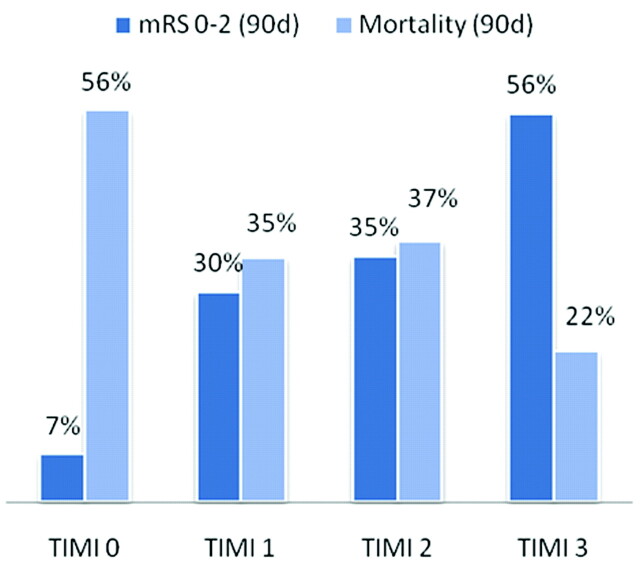
The distribution of 90-day functional outcome stratified by TIMI grade is shown in Fig 1. The likelihood of a favorable outcome or mortality at 90 days is shown graphically in Fig 2. Overall, the likelihood of a favorable outcome increased from 7% to 56% as the TIMI score increased from 0 to 3, and the likelihood of death decreased from 56% to 22% (see Fig 3). The unadjusted OR of favorable outcome increased significantly as the TIMI score increased from 0 to 1 (OR, 5.9; 95% CI, 1.7–20.0; P = .007) and from 2 to 3 (OR, 2.3; 95% CI, 1.2–4.5; P = .01) (Fig 4A). The odds of death trended toward decreasing as the TIMI score increased from 0 to 1 (OR, 2.4; 95% CI, 0.8–6.4; P = .09) and decreased significantly as the TIMI score increased from 2 to 3 (OR, 2.2; 95% CI, 1.1–4.3; P = .05) (Fig 4B).
Fig 2.
Distribution of functional outcome stratified by TIMI score.
Fig 3.
Unadjusted absolute likelihood of a favorable functional outcome and mortality as a function of TIMI score.
Fig 4.
The effect of each increase of TIMI score on the odds of favorable functional outcome and survival. A, Unadjusted ORs for favorable functional outcome (mRS score of 0–2) as the TIMI grade increases from 0 to 3. B, Unadjusted ORs for survival to 90 days as TIMI grade increases from 0 to 3.
The final multivariate model is shown in Table 3. In multivariate analysis, after adjusting for covariates, each increase in TIMI grade increased the odds of a good outcome 2.6-fold (95% CI, 1.9–3.4; P < .0001).
Table 3:
Final multivariate model
| OR (95% CI) | P Value | |
|---|---|---|
| Final TIMI flow (0, 1, 2, 3) | 2.6 (1.9–3.4) | <.0001 |
| NIHSS score | 0.89 (0.84–0.94) | <.0001 |
| Age (yr) | 0.97 (0.95–0.98) | .0003 |
Discussion
In a pooled analysis of data from the MERCI and Multi MERCI trials, we found that the degree of recanalization after endovascular intervention—as defined by the TIMI score—is a potent predictor of 90-day outcome. In multivariate analysis, after adjusting for covariates, each increase in TIMI grade was associated with a 2.6-fold increase in the odds of a good outcome. Furthermore, in comparison with patients with TIMI 2 flow at the conclusion of the procedure, the odds of a favorable functional outcome in patients in whom TIMI 3 flow was achieved were 2.3 times higher, and survival was increased 2.2-fold.
Intuitively, one might not expect that the increase from TIMI 2 to TIMI 3 flow would have a significant impact on cerebral ischemia. In both cases, cerebral blood flow through the vessels targeted by stroke interventions likely significantly exceeds the threshold for cerebral ischemia. Why then might improved outcomes be associated with TIMI 3 compared with TIMI 2 flow?
The answer is very likely multifactorial. Cerebral autoregulation may not be intact after restoration of blood flow to ischemic brain.12,13 Furthermore, the “no re-flow” phenomenon (absence of blood flow due to microvascular thrombi, injury to the microvasculature, and tissue edema) and the presence of distal emboli may further compromise local cerebral blood flow.14 Therefore, it is possible that prolonged tissue-level ischemia may occur even in the presence of apparently adequate blood flow through the major proximal cerebral vessels and that TIMI 3, not TIMI 2, flow is sufficient to overcome the effects of impaired cerebral autoregulation, distal embolization, and the mechanisms underlying the no re-flow phenomenon.
Another possibility relates to the likelihood of reocclusion among patients with incomplete recanalization. Reocclusion after recanalization in patients treated with IV thrombolytics, IA thrombolytics, or mechanical intervention occurs in up to 34% of patients and is associated with less favorable outcomes.15–17 Reocclusion may be more likely in patients with TIMI 2 flow as opposed to TIMI 3 flow due to the persistence of thrombus. One study of patients treated with a combination of IA thrombolysis and mechanical interventions (66/91, 72%) or IA thrombolysis alone (25/91, 28%) found reocclusion in 13/41 (32%) of patients with partial recanalization compared with only 1/29 (3%) of those with complete recanalization.16
Finally, it is possible that patients with higher degrees of recanalization with mechanical thrombectomy may, in fact, differ physiologically from those with lower degrees of recanalization in ways that affect clinical outcomes. For instance, some evidence suggests that patients with relatively good collateral flow have a higher likelihood of revascularization with IV-tPA in comparison to those with poor collateral circulation.18 Furthermore, in previous analysis of the MERCI and Multi MERCI trials, increased systolic blood pressure was associated with an increased likelihood of recanalization.19 Increased blood pressure may be associated with improvements in cerebral blood flow.13 If recanalization is related to collateral circulation—or if another unrecognized factor predisposes to both recanalization and better clinical outcomes—the results of our investigation could be misleading by overestimating the effect of the TIMI grade on clinical outcome.
Regardless of the means by which outcomes are improved when TIMI 3 flow is achieved, we believe our investigation has implications for the stopping point used in endovascular interventions for acute stroke and for the evaluation of future thrombectomy systems. During stroke interventions, the degree of recanalization is typically reassessed after each attempt to re-establish flow. Because in many cases TIMI 2 flow is judged to be adequate, interventions may be stopped after TIMI 2 flow has been achieved rather than continuing in an attempt to establish TIMI 3 flow. Our data suggest that if it can be done safely, additional attempts at removal of residual thrombus are justified. Furthermore, given the tight correlation with improved outcomes, future thrombectomy trials that have recanalization as their end point should be evaluated on the basis of the success in complete (TIMI 3) recanalization, not solely on the combined end point of partial and complete (TIMI 2/3) recanalization.
Although TIMI 3 flow may be associated with superior outcomes in comparison with TIMI 2 flow, the clinical benefit may decrease with time and procedural success is likely inversely related to the number of attempts at thrombectomy. Time to treatment has been an important predictor of outcome in a number of trials of IV-tPA20 and IA- tPA,21 though it did not appear to have a significant effect on outcome in the MERCI trials.19 A separate-but-related problem is the decreased likelihood of success with each attempt at recanalization. A recent analysis from an individual center found a decreased likelihood of successful revascularization and an increase in parenchymal hematoma with ≥4 passes with the Merci retriever.22 Notably, in the present investigation, we found that procedural times tended to be longer and the number of passes greater in patients with lower TIMI scores; this finding suggests that prolonging procedures with multiple additional passes may not result in successful recanalization.
On the other hand, we also found that in 20%–30% of patients in whom partial or complete recanalization was achieved, ≥4 passes with the MERCI retriever, with or without adjuvant therapies, were required. Furthermore, the absence of a statistical relationship between TIMI flow and procedural complications or intracerebral hemorrhage in our study suggests that the strategy of additional attempts at recanalization to achieve TIMI 3 perfusion may be warranted, though the relatively small number of procedural adverse events and SICH limits the statistical power to detect an interaction between the number of device passes and adverse events. In summary, despite mixed evidence, it stands to reason that salvageable tissue declines with time and that, irrespective of stroke intervention technique, procedural risk increases and success decreases with each attempt at thrombectomy. The desirability of achieving TIMI 3 flow must, therefore, be balanced by these considerations, and the precise stopping point in any given intervention is ultimately a clinical judgment.
The present investigation has both strengths and limitations. Strengths include a relatively large number of patients and a robust data base with prospectively collected data acquired in the context of major clinical trials. Limitations relate to the definition of recanalization and the absence of evaluation of recanalization by a core laboratory. The MERCI trials used the TIMI scale as the primary means of assessing recanalization. Other definitions have been used, including the Thrombolysis in Cerebral Infarction scale, the Thrombolysis in Brain Ischemia scale, and the Arterial Occlusive Lesion scale.23 It is unclear which of these scales best predicts the extent of infarction or functional outcomes. Finally, the absence of core laboratory evaluation likely decreases the accuracy of the designation of final TIMI grade.
Conclusions
We evaluated the effect of the degree of recanalization on outcome after endovascular mechanical thrombectomy for the treatment of acute ischemic stroke. After adjusting for covariates, we found that each increase in TIMI grade was associated with a 2.6-fold increase in the odds of a favorable outcome at 90 days. These results provide support for the hypothesis that patient outcomes in the context of stroke interventions may be improved by additional attempts to increase the TIMI grade during stroke interventions. Because patients with different TIMI grades may differ from each other at baseline, this hypothesis would require validation in a randomized trial. An old surgical adage holds that “better is the enemy of good”; applied angiographically, the supposition is that the pursuit of a perfect angiographic result may not be necessary clinically and may result in clinical harm from additional complications. In the case of stroke intervention, our data suggest that better may be better than good.
ABBREVIATIONS
- CI
confidence interval
- IA
intra-arterial
- MERCI
Mechanical Embolus Removal in Cerebral Ischemia
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OR
odds ratio
- PH-2
parenchymal hematoma type 2
- SICH
symptomatic intracranial hemorrhage
- TIMI
Thrombolysis in Myocardial Infarction
Footnotes
Disclosures: Helmi Lutsep, Consultant: Concentric Medical Inc, Co-Axia; Details: Concentric Medical Inc: executive committee (coprincipal investigator) for the Thrombectomy Revascularization of Large Vessel Occlusions (TREVO 2) trial, modest; Co-Axia: DSMB chair for the Safety and Efficacy of Neuroflo for Treatmentof Ischemic Stroke (SENTIS) trial completed in the summer of 2010, significant (>$10 000). Wade Smith, Consultant: Concentric Medical Inc, Ornim Inc. Details: Medical Advisor; Ownership Interest: Concentric Medical Inc, Ornim Inc. Details: stock ownership
References
- 1. Zangerle A, Kiechl S, Spiegel M, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology 2007;68:39–44 [DOI] [PubMed] [Google Scholar]
- 2. Wunderlich MT, Goertler M, Postert T, et al. Recanalization after intravenous thrombolysis: does a recanalization time window exist? Neurology 2007;68:1364–68 [DOI] [PubMed] [Google Scholar]
- 3. Brekenfeld C, Remonda L, Nedeltchev K, et al. Endovascular neuroradiological treatment of acute ischemic stroke: techniques and results in 350 patients. Neurol Res 2005;27(suppl 1):S29–35 [DOI] [PubMed] [Google Scholar]
- 4. Fields JD, Lindsay K, Liu KC, et al. Mechanical thrombectomy for the treatment of acute ischemic stroke. Expert Rev Cardiovasc Ther 2010;8:581–92 [DOI] [PubMed] [Google Scholar]
- 5. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 6. Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–54 [DOI] [PubMed] [Google Scholar]
- 7. Arnold M, Nedeltchev K, Remonda L, et al. Recanalisation of middle cerebral artery occlusion after intra-arterial thrombolysis: different recanalisation grading systems and clinical functional outcome. J Neurol Neurosurg Psychiatry 2005;76:1373–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaidat OO, Suarez JI, Sunshine JL, et al. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol 2005;26:880–84 [PMC free article] [PubMed] [Google Scholar]
- 9. Stead LG, Gilmore RM, Bellolio MF, et al. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol 2008;65:1024–30 [DOI] [PubMed] [Google Scholar]
- 10. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 11. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 12. Dohmen C, Bosche B, Graf R, et al. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke 2007;38:56–61 [DOI] [PubMed] [Google Scholar]
- 13. Shin HK, Nishimura M, Jones PB, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke 2008;39:1548–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke 2009;40:S24–27 [DOI] [PubMed] [Google Scholar]
- 15. Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–67 [DOI] [PubMed] [Google Scholar]
- 16. Janjua N, Alkawi A, Suri MF, et al. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol 2008;29:253–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saqqur M, Molina CA, Salam A, et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke 2007;38:69–74 [DOI] [PubMed] [Google Scholar]
- 18. Jovin TG, Gupta R, Horowitz MB, et al. Pretreatment ipsilateral regional cortical blood flow influences vessel recanalization in intra-arterial thrombolysis for MCA occlusion. AJNR Am J Neuroradiol 2007;28:164–67 [PMC free article] [PubMed] [Google Scholar]
- 19. Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 2009;40:3777–83 [DOI] [PubMed] [Google Scholar]
- 20. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703 [DOI] [PubMed] [Google Scholar]
- 21. Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73:1066–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loh Y, Jahan R, McArthur DL, et al. Recanalization rates decrease with increasing thrombectomy attempts. AJNR Am J Neuroradiol 2010;31:935–39. Epub 2010 Jan 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol 2007;28:382–84 [PMC free article] [PubMed] [Google Scholar]