Abstract
BACKGROUND AND PURPOSE:
Developmental venous anomalies are the most common intracranial vascular malformation. Increased signal-intensity on T2-FLAIR images in the areas drained by developmental venous anomalies are encountered occasionally on brain imaging studies. We evaluated diffusion and perfusion MR imaging findings of the abnormally high signal intensity associated with developmental venous anomalies to describe their pathophysiologic nature.
MATERIALS AND METHODS:
We retrospectively reviewed imaging findings of 34 subjects with signal-intensity abnormalities associated with developmental venous anomalies. All subjects underwent brain MR imaging with contrast and diffusion and perfusion MR imaging. Regions of interest were placed covering abnormally high signal intensity around developmental venous anomalies on fluid-attenuated inversion recovery imaging, and the same ROIs were drawn on the corresponding sections of the diffusion and perfusion MR imaging. We measured the apparent diffusion coefficient, relative cerebral blood volume, relative mean transit time, and time-to-peak of the signal-intensity abnormalities around developmental venous anomalies and compared them with the contralateral normal white matter. The Mann-Whitney U test was used for statistical analysis.
RESULTS:
The means of ADC, relative cerebral blood volume, relative mean transit time, and TTP of signal-intensity abnormalities around developmental venous anomalies were calculated as follows: 0.98 ± 0.13 10−3mm2/s, 195.67 ± 102.18 mL/100 g, 16.74 ± 7.38 seconds, and 11.65 ± 7.49 seconds, respectively. The values of normal WM were as follows: 0.74 ± 0.08 10−3mm2/s for ADC, 48.53 ± 22.85 mL/100 g for relative cerebral blood volume, 12.12 ± 4.27 seconds for relative mean transit time, and 8.35 ± 3.89 seconds for TTP. All values of ADC, relative cerebral blood volume, relative mean transit time, and TTP in the signal-intensity abnormalities around developmental venous anomalies were statistically higher than those of normal WM (All P < .001, respectively).
CONCLUSIONS:
The diffusion and perfusion MR imaging findings of the signal-intensity abnormalities associated with developmental venous anomaly suggest that the nature of the lesion is vasogenic edema with congestion and delayed perfusion.
Developmental venous anomalies (DVAs) are encountered frequently on brain imaging studies. DVAs are identified in up to 2% of the general population, and they are the most common intracranial vascular malformation (63% and 50% of all malformations in postmortem examinations and MR imaging series, respectively).1,2 They are composed of dilated medullary veins that drain centripetally and radially into enlarged transcortical or subependymal collector veins.3–5 DVAs serve as normal drainage routes of the brain tissue because the normal venous drainage pattern is underdeveloped in the area adjacent to the DVA. These venous channels have no malformed or neoplastic elements and are generally described as having normal intervening parenchyma.6,7 However, increased signal intensity (SI) on T2 FLAIR images in the areas drained by DVAs have been reported in 7.8%–54.1% of MR imaging investigations.8–10 Such abnormal SI related to DVAs has been explained as edema, ischemia, demyelination, gliosis, leukoaraiosis, or a combination of these conditions.11,12 Several studies have been undertaken to understand the mechanism of SI change.13–15 However, there has been no report of the diffusion and perfusion changes of abnormal SI in the area of DVAs by using diffusion- and perfusion-weighted MR imaging, to our knowledge. Therefore, the aim of this study was to characterize these SIs by using DWI and PWI. DWI would discriminate between vasogenic edema and gliosis, and PWI would demonstrate signs of outflow obstruction and venous congestion.
Materials and Methods
Patients
We searched the reports of brain MR imaging with contrast, diffusion, and perfusion studies for the terms “developmental venous anomaly” or “venous angioma” through the databank of our hospital from January 2005 to January 2013. Two hundred fifty-six consecutive patients with DVAs were found. Sixty-eight of 256 (26.6%) subjects showed SI abnormalities in the DVA drainage area. No patient had suspected or known multiple sclerosis. Within these subjects, 34 were omitted from the study group due to the following exclusion criteria: 1) area of SI abnormality <5 mm2 (n = 23), 2) the lesion not distinguishable from ischemic lesions or infarction of underlying small-vessel disease (n = 7), and 3) DVAs accompanied by cavernous malformations (n = 4). Finally, 34 subjects (13.3%, 34/256) formed the study group. Our institutional review board approved this study, and informed consent was waived in this retrospective study.
MR Imaging Protocol and Postprocessing
MR imaging was performed at 3T (Achieva; Philips Healthcare, Best, the Netherlands) with an 8-channel sensitivity-encoding head coil. For all patients, we obtained the following images: axial contrast-enhanced spin-echo T1-weighted images after intravenous injection of contrast material (gadoterate meglumine, Dotarem; Guerbet, Aulnay-sous-Bois, France; 0.1 mmol/kg body weight by power injector) (TR/TE = 500/10 ms, section thickness = 5 mm, acquisition matrix = 256 × 226); axial T2-weighted fluid-attenuated inversion recovery images (TR/TE = 11000/125 ms, section thickness = 5 mm, acquisition matrix = 368 × 265); spin-echo EPI DWI (TR/TE = 3000/76 ms, section thickness = 5 mm, acquisition matrix = 128 × 128, b-value = 0, 1000 s/mm2); and dynamic susceptibility contrast MR PWI (TR/TE = 1720/35 ms, flip angle = 40°, section thickness = 5 mm, acquisition matrix = 128 × 128, 50 volumes, acquisition time = 1 minute 30 seconds). All MR images were acquired with the same FOV (240 × 240 mm).
We processed the dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging by using a dedicated software package (nordicICE; NordicNeuroLab, Bergen, Norway). Perfusion maps of relative cerebral blood volume (rCBV), relative mean transit time (rMTT), and time-to-peak were made by using an acknowledged tracer kinetic model applied to first-pass data.16,17 We used γ-variate fit to diminish the recirculation effect. We performed deconvolution of the measured signal-time curves by using singular-value decomposition with the arterial input function of approximately 5 pixels retrieved from the middle cerebral artery branches. TTP was obtained by computing the arrival time of contrast material to maximum concentration.
MR Imaging Data Analysis and Statistics
Two neuroradiologists (S.T.K., H.N.J.) analyzed the MR images retrospectively. One had 22 years' experience with brain imaging, and the other had 5 years' experience with brain imaging. DVAs were identified on contrast-enhanced T1WI. We defined the drainage territory as brain parenchyma directly adjacent to the visualized medullary and collector vein of the DVA. When increased SI was seen within the drainage territory of a DVA on a T2 FLAIR image, it was defined as an SI abnormality associated with a DVA.
Regions of interest were placed covering the abnormally high SI around DVAs on T2 FLAIR imaging, not including the collecting vein because these can confound perfusion measurements. All ROIs were copied to corresponding sections of the apparent diffusion coefficient and perfusion maps. ROIs were manually drawn on the T2 FLAIR images for normal-appearing contralateral white matter, and ROIs on the ADC and perfusion maps were obtained in the same way. We measured the values of ADC, rCBV, rMTT, and TTP, respectively, and analyzed differences in values of ADC, rCBV, rMTT, and TTP between the SI abnormalities associated with the DVA and contralateral normal WM. The Mann-Whitney U test was used for statistical analysis. Null hypotheses of no difference were rejected if P values were <.05. A commercially available software program (PASW, Version 18.0; IBM, Armonk, New York) was used for all statistical analyses.
Results
The mean age of the 34 patients was 63.7 years (range, 38–81 years), and 52.9% (n = 18) were men. The mean area of the region of interest of SI abnormalities associated with DVAs was 64.5 mm2 (range, 8.8–260.2 mm2).
The values of ADC, rCBV, rMTT, and TTP of abnormal SI associated with DVAs and normal WM are summarized in the Table. The mean values of SI around DVAs were 0.98 ± 0.13 10−3mm2/s for ADC, 195.67 ± 102.18 mL/100 g for rCBV, 16.74 ± 7.38 seconds for rMTT, and 11.65 ± 7.49 seconds for TTP, respectively. The mean ADC, rCBV, rMTT, and TTP values of normal WM were as follows: 0.74 ± 0.08 10−3mm2/s, 48.53 ± 22.85 mL/100 g, 12.12 ± 4.27 seconds, and 8.35 ± 3.89 seconds. There was a significant difference between the SI abnormalities associated with DVAs and normal WM in all terms of ADC, rCBV, rMTT, and TTP (all P < .001, respectively) (Fig 1).
The ADC, rCBV, rMTT, and TTP values of signal-intensity abnormalities associated with developmental venous anomalies and contralateral normal white matter
| DVAs | Normal WM | P Valuesa | |
|---|---|---|---|
| ADCb | 0.98 ± 0.13 | 0.74 ± 0.08 | <.001 |
| rCBVc | 195.67 ± 102.18 | 48.53 ± 22.85 | <.001 |
| rMTTd | 16.74 ± 7.38 | 12.12 ± 4.27 | <.001 |
| TTPe | 11.65 ± 7.49 | 8.35 ± 3.89 | <.001 |
Mann-Whitney U test.
Apparent diffusion coefficient is expressed as 10−3mm2/s.
Relative cerebral blood volume is expressed as mL/100 g.
Relative mean transit time is expressed as seconds.
Time-to-peak is expressed as seconds.
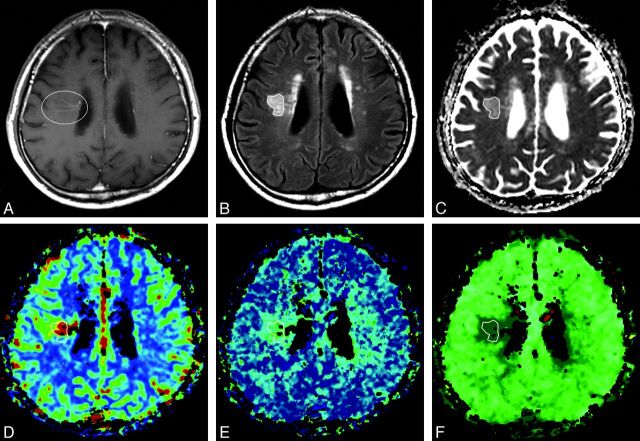
Fig 1.
A 60-year-old man with blurred vision and headache. A, Postcontrast T1-weighted axial image shows a dilated medullary vein draining into the subependymal collecting vein in the right corona radiata (circle), representing a developmental venous anomaly. B, Abnormal signal intensity is seen in the area of the DVA (polygon) on the axial T2 fluid-attenuated inversion recovery imaging. C, Apparent diffusion coefficient. D, Relative cerebral blood volume. E, Relative mean transit time. F, Time-to-peak map demonstrates increased values of the corresponding area compared with contralateral normal white matter. These findings suggest that the nature of the SI abnormalities around DVAs is vasogenic edema with congestion and delayed perfusion.
Discussion
In the present study, SI abnormalities associated with DVAs were seen in 13.3% of DVAs (34/256), and this value is in the range of prevalence of 7.8%–54.1% that is reported in previous studies.8–10 Abnormal SI associated with DVAs is not a rare finding in an imaging study, and some possibilities have been proposed as the etiology. Among them, considerable studies have supported venous congestion as the cause of SI change around a DVA.3,8,10,18–25 Some studies have shown that DVAs may have thickened and hyalinized vessel walls without a smooth-muscle layer or elastic lamina.11,18–20 Dillon21 substantiated increased venous pressure within a DVA by measuring a 15-mm Hg pressure gradient across the stenotic collecting vein of a DVA in 1 patient. Truwit3 reported that a focal stenosis of the draining vein may be seen at the point where it penetrates the dura to enter the dural sinus, resulting in delayed filling and emptying of the DVA. Narrowing of the veins can cause lessened compliance, increase resistance to flow, and reduce the capacity of the vessel to adjust to pressure changes.10 Besides, a single collecting vessel in a DVA drains an abnormally large parenchymal territory, resulting in relative volume overload and chronic cerebral edema or ischemia.10,22 Impaired cerebral blood flow attributed to venous congestion has been reported in the drainage vicinity of the DVA.23–25 In recent CT and MR perfusion studies, increased CBV, CBF, and MTT can be observed in the region of the DVA.26,27
Abnormal SI lesions are understood as vascular-induced leukoaraiosis associated with chronic cerebral ischemia, resulting from venous congestion.10 In addition, it seems reasonable that the underlying venous hypertension may be a causative factor in hemorrhage, one of the rare complications of DVAs, as well as ischemia. A recent study revealed a significant relationship between abnormal SI in the drainage territory of DVAs and hypointense foci on phase-sensitive MR imaging, indicating microhemorrhage or cavernous malformation.8 Venous hypertension would overload the vessel wall and cause injury, resulting in microhemorrhage from the weakest point and reactive angiogenesis.28,29 For the angiogenesis, lack of vasoregulatory capacity of the neovasculature leads to repeated hemorrhage and formation of cavernous malformations, eventually.30,31
The results of the present study correspond well with the aforementioned studies. SI abnormalities in the draining area of DVAs showed higher ADC value, increased rCBV, and perfusion delay, compared with the normal white matter, representing venous congestion. The ADC value would increase in reactive gliosis—disruption of cell membranes, loss of myelin, or any process that may alter the integrity of axons that would reduce the restriction of water motion.32 However, gliosis alone did not cause increased rCBV and perfusion delay. A previous study showed that areas of gliosis demonstrated significantly higher ADC values (1.76 ± 0.09 × 10−3 mm2/s) than areas of vasogenic edema (1.35 ± 0.06 × 10−3 mm2/s) without overlap.32 The ADC value for SI abnormalities in the draining area of DVAs was 0.98 ± 0.13 × 10−3 mm2/s, which is lower than that of gliosis. It is difficult to simply compare the ADC value in our study with that in the references because there is an absence of comparable gliosis evaluation and the area range of 34 cases in our study is quite broad. However, the alteration of perfusion parameters in SI abnormalities around DVAs compared with normal white matter could suggest that it has at least a component of vasogenic edema rather than gliosis alone.
Our study has several limitations. First, this was a retrospective study. Second, it had a small sample size. Third, the prevalence of SI abnormalities in the territory of DVAs in our study may not be entirely accurate and may potentially have selection bias because cases with DVAs were detected from reports of brain MR imaging with contrast and diffusion and perfusion studies. Fourth, we did not include the collecting vein in ROIs to avoid confusion of values of the perfusion measurements. However, the tributary veins were included in the ROIs, and this inclusion may have affected the results. Fifth, there was a lack of information about the values of DWI and PWI in the vicinity of the DVA without SI change. Further research is needed from 2 perspectives: Radiologically, diffusion tensor imaging could determine the pathophysiology of abnormally high signal intensity of white matter around DVAs in more detail. The clinical correlations, detailed follow-up, and management in clinical practice would be outside the scope of this imaging-based study.
Conclusions
The prevalence of abnormal SI associated with DVAs is not low, and vasogenic edema and venous congestion are the primary pathophysiologic characteristics of the SI abnormalities in the draining territory of DVAs.
ABBREVIATIONS:
- DVA
developmental venous anomaly
- rCBV
relative cerebral blood volume
- rMTT
relative mean transit time
- SI
signal intensity
REFERENCES
- 1. Garner TB, Del Curling O, Jr, Kelly DL, Jr, et al. The natural history of intracranial venous angiomas. J Neurosurg 1991;75:715–22 [DOI] [PubMed] [Google Scholar]
- 2. Sarwar M, McCormick WF. Intracerebral venous angioma: case report and review. Arch Neurol 1978;35:323–25 [DOI] [PubMed] [Google Scholar]
- 3. Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol 1992;159:1299–307 [DOI] [PubMed] [Google Scholar]
- 4. Mateos JH, Dorfsman J, Lombardorivera L. Vascular malformations of the spinal cord [in Spanish]. Rev Med Hosp Gen (Mex) 1963;26:851–60 [PubMed] [Google Scholar]
- 5. Calem WS, Jimenez FA. Vascular malformations of the intestine: their role as a source of hemorrhage. Arch Surg 1963;86:571–79 [DOI] [PubMed] [Google Scholar]
- 6. Aimes A. Vascular malformations and lesions in pathology [in French]. Angeiologie 1963;15:33–39 [PubMed] [Google Scholar]
- 7. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev 1986;9:233–42 [DOI] [PubMed] [Google Scholar]
- 8. Takasugi M, Fujii S, Shinohara Y, et al. Parenchymal hypointense foci associated with developmental venous anomalies: evaluation by phase-sensitive MR imaging at 3T. AJNR Am J Neuroradiol 2013;34:1940–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santucci GM, Leach JL, Ying J, et al. Brain parenchymal signal abnormalities associated with developmental venous anomalies: detailed MR imaging assessment. AJNR Am J Neuroradiol 2008;29:1317–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. San Millán Ruíz D, Delavelle J, Yilmaz H, et al. Parenchymal abnormalities associated with developmental venous anomalies. Neuroradiology 2007;49:987–95 [DOI] [PubMed] [Google Scholar]
- 11. Courville CB. Morphology of small vascular malformations of the brain: with particular reference to the mechanism of their drainage. J Neuropathol Exp Neurol 1963;22:274–84 [DOI] [PubMed] [Google Scholar]
- 12. Djindjian R, Faure C. Neuro-radiological investigations (arteriography and phlebography) in vascular malformations of the spinal cord [in French]. Rontgeneur Radiodiagn Clin Eur 1963;158:171–95 [PubMed] [Google Scholar]
- 13. Munoz DG, Hastak SM, Harper B, et al. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch Neurol 1993;50:492–97 [DOI] [PubMed] [Google Scholar]
- 14. Noran HH. Intracranial vascular tumors and malformations. Arch Pathol 1945;39:393–416 [Google Scholar]
- 15. Moody DM, Brown WR, Challa VR, et al. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995;194:469–76 [DOI] [PubMed] [Google Scholar]
- 16. Galambos C, Nodit L. Identification of lymphatic endothelium in pediatric vascular tumors and malformations. Pediatr Dev Pathol 2005;8:181–89 [DOI] [PubMed] [Google Scholar]
- 17. Koch G. Hereditary of vascular tumors and malformations of the brain [in undetermined language]. J Med (Oporto) 1953;21:657–70 [PubMed] [Google Scholar]
- 18. McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg 1966;24:807–16 [DOI] [PubMed] [Google Scholar]
- 19. Koussa A, Chiras J, Poirier B, et al. X-ray computed tomographic and angiographic aspects of venous angiomas of the brain: apropos of 15 cases [in French]. Neurochirurgie 1985;31:161–68 [PubMed] [Google Scholar]
- 20. Abe M, Hagihara N, Tabuchi K, et al. Histologically classified venous angiomas of the brain: a controversy. Neurol Med Chir (Tokyo) 2003;43:1–10, discussion 11 [DOI] [PubMed] [Google Scholar]
- 21. Dillon WP. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol 1997;18:1839–46 [PMC free article] [PubMed] [Google Scholar]
- 22. Pereira VM, Geibprasert S, Krings T, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke 2008;39:3201–15 [DOI] [PubMed] [Google Scholar]
- 23. Matsuda H, Terada T, Katoh M, et al. Brain perfusion SPECT in a patient with a subtle venous angioma. Clin Nucl Med 1994;19:785–88 [DOI] [PubMed] [Google Scholar]
- 24. Uchida K, Tamura K, Takayama H, et al. Xenon-enhanced CT CBF measurements in intracranial vascular malformations [in Japanese]. No Shinkei Geka 1989;17:239–46 [PubMed] [Google Scholar]
- 25. Tomura N, Inugami A, Uemura K, et al. Multiple medullary venous malformations decreasing cerebral blood flow: case report. Surg Neurol 1991;35:131–35 [DOI] [PubMed] [Google Scholar]
- 26. Kroll H, Soares BP, Saloner D, et al. Perfusion-CT of developmental venous anomalies: typical and atypical hemodynamic patterns. J Neuroradiol 2010;37:239–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma A, Zipfel GJ, Hildebolt C, et al. Hemodynamic effects of developmental venous anomalies with and without cavernous malformations. AJNR Am J Neuroradiol 2013;34:1746–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perrini P, Lanzino G. The association of venous developmental anomalies and cavernous malformations: pathophysiological, diagnostic, and surgical considerations. Neurosurg Focus 2006;21:e5. [DOI] [PubMed] [Google Scholar]
- 29. Awad IA, Robinson JR, Jr, Mohanty S, et al. Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery 1993;33:179–88, discussion 88 [DOI] [PubMed] [Google Scholar]
- 30. Rammos SK, Maina R, Lanzino G. Developmental venous anomalies: current concepts and implications for management. Neurosurgery 2009;65:20–29, discussion 29–30 [DOI] [PubMed] [Google Scholar]
- 31. Hong YJ, Chung TS, Suh SH, et al. The angioarchitectural factors of the cerebral developmental venous anomaly; can they be the causes of concurrent sporadic cavernous malformation? Neuroradiology 2010;52:883–91 [DOI] [PubMed] [Google Scholar]
- 32. Hagen T, Ahlhelm F, Reiche W. Apparent diffusion coefficient in vasogenic edema and reactive astrogliosis. Neuroradiology 2007;49:921–26 [DOI] [PubMed] [Google Scholar]