Abstract
BACKGROUND AND PURPOSE:
Modic type 1 degenerative signal changes can mimic/suggest infection, leading to additional costly and sometimes invasive investigations. This retrospective study analyzes the utility and accuracy of a novel, diffusion-weighted “claw sign” for distinguishing symptomatic type 1 degeneration from vertebral diskitis/osteomyelitis.
MATERIALS AND METHODS:
Seventy-three patients with imaging features resembling type 1 degeneration were classified clinically into 3 groups: true degenerative type 1 changes (n = 33), confirmed diskitis/osteomyelitis (n = 20), and radiologically suspected infection later disproved clinically (n = 20). A claw sign was defined on DWI as well-marginated, linear, regions of high signal situated within the adjacent vertebral bodies at the interface of normal with abnormal marrow. Two blinded neuroradiologists independently rated the presence of the claw sign, along with T2 disk signal and disk and endplate enhancement to determine the utility of each for identifying degeneration versus infection.
RESULTS:
When the 2 neuroradiologists identified a definite claw, 38 of 39 patients (97%) and 29 of 29 patients (100%) proved to be infection-free. When the readers identified a probable claw, 14 of 14 patients (100%) and 16 of 19 patients (84%) proved to be infection-free. Conversely, when the readers identified the absence of claw sign (diffuse DWI pattern), there was proved infection in 17 of 17 cases (100%) and 13 of 14 cases (93%).
CONCLUSIONS:
In patients with type 1 signal changes of the vertebral disk space, a claw sign is highly suggestive of degeneration and its absence strongly suggests diskitis/osteomyelitis.
Diffusion-weighted imaging is a critical tool for the evaluation of brain diseases, including ischemia, infection, and inflammation. Recently DWI has gained increasing use in diagnosing pathology in the spine, despite cited limitations,1,2 and is becoming valuable in the assessment of a variety of disease processes, including tumor and infection.3–13 Modic type 1 degenerative signal changes on conventional MR imaging sequences can mimic or suggest infection, leading to additional costly and sometimes invasive investigations.14–17 This study assesses the utility of a specific pattern of diffusion abnormality, the “claw sign,” for confirming the presence of true degenerative endplate changes and reducing concern for possible vertebral diskitis/osteomyelitis.
Materials and Methods
With prior approval by the institutional review board, the imaging studies and clinical data of patients referred for spinal MR imaging were retrospectively reviewed to select 73 patients with MR imaging features resembling Modic type 1 degeneration at a specific disk level. These patients fell into 3 groups: those with type 1 changes and 1) degeneration with no clinical or imaging suspicion of infection (n = 33); 2) clinically confirmed diskitis/osteomyelitis (n = 20); and 3) radiologically suspected infection, later disproved clinically (n = 20).
In group 2, work-up and clinical evidence of infection included bacteremia, confirmatory biopsy, and/or follow-up imaging. There were 3 biopsy-positive cases of infection with tuberculosis, oxacillin-sensitive Staphylococcus aureus, and Propionibacteria species. Positive blood cultures were used as supporting evidence in 12 patients, including 7 patients with S aureus (methicillin-resistant S aureus, n = 5; oxacillin-sensitive S aureus, n = 2), 1 with Escherichia coli, 1 with Enterococcus faecalis, 1 with Streptococcus sanguinis, and 2 with Staphylococcus epidermidis. The patient with E faecalis bacteremia was also noted to be immunocompromised. One of the patients with methicillin-resistant S aureus bacteremia had positive findings on a nuclear medicine indium-111 white blood cell scan. Progression of disease was noted on follow-up imaging of 4 patients, and 1 patient showed treatment response on subsequent imaging. Also of note, 18 of 20 patients in this group showed epidural, paraspinal, or psoas involvement, which strongly supported the diagnosis of infection.
In group 3, the cases were suspicious for infection on the basis of MR imaging signal changes beyond just type 1 changes (predominantly high T2 disk signal and sometimes endplate or disk enhancement). The interpreting radiologists either “could not exclude” or outright suspected infection. Note that in these patients, there was typically no clinical suspicion of infection, including absent symptoms, and lack of any laboratory data to support infection, including negative findings on blood cultures. In certain patients, results of additional work-up were also negative (one, nuclear imaging; another, surgical exploration yielding granulation tissue only). In several patients, while infection could not be definitively excluded on a clinical basis, there was no treatment, and follow-up imaging revealed minimal change, resolution, or evolution to Modic type 2 changes. In some cases, there was no follow-up imaging and no treatment, with presumed resolution of clinical symptoms making infection unlikely.
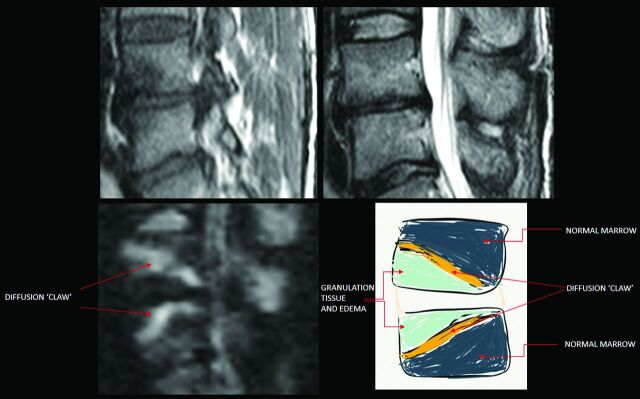
Modic et al15 described type 1 degenerative signal changes by using conventional MR imaging techniques, attributed to vascularized bone marrow and edema. The claw sign is identified on trace/combined diffusion-weighted images as well-marginated, linear, typically paired regions of high signal situated within the adjoining vertebral bodies at the boundaries between the normal bone marrow and vascularized bone marrow that lies close to the affected disk, presumed to represent a form of physiologic reactive response or induration (Figs 1–3). The claw sign was deemed absent if the diffusion findings were diffuse and not well-marginated. It seems logical that a gradual, progressive process such as degenerative disk disease would produce a well-defined border response. A destructive process such as infection might progress too quickly and diffusely infiltrate with pathogens or edema and fail to produce a defined border zone response.
Fig 1.
The claw sign is identified on trace/combined DWI as well-marginated, linear, typically paired, regions of high signal situated within adjoined vertebral bodies at the boundaries between the normal bone marrow and vascularized bone marrow.
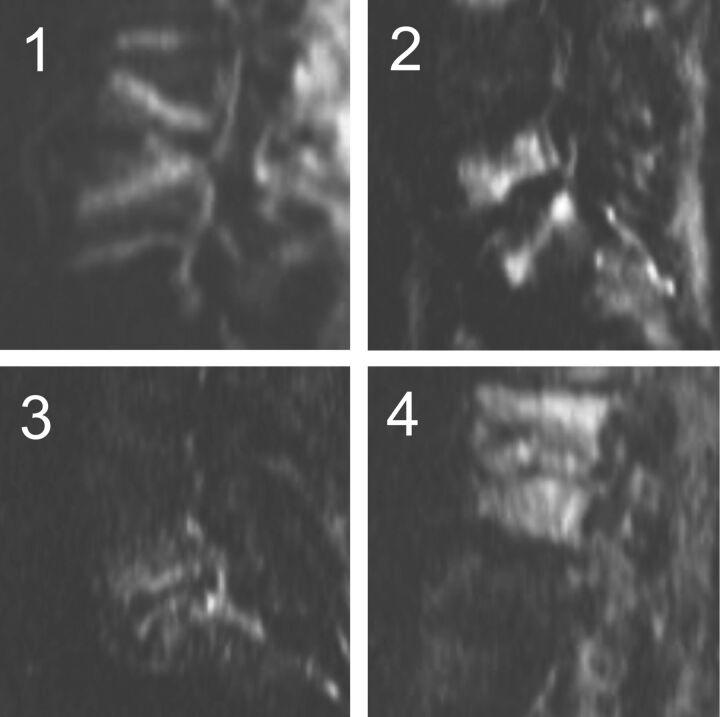
Fig 2.
Characterization of the diffusion claw sign. Exemplars of the 4 categories: 1 = definite, 2 = probable, 3 = questionable, 4 = negative (diffuse signal).
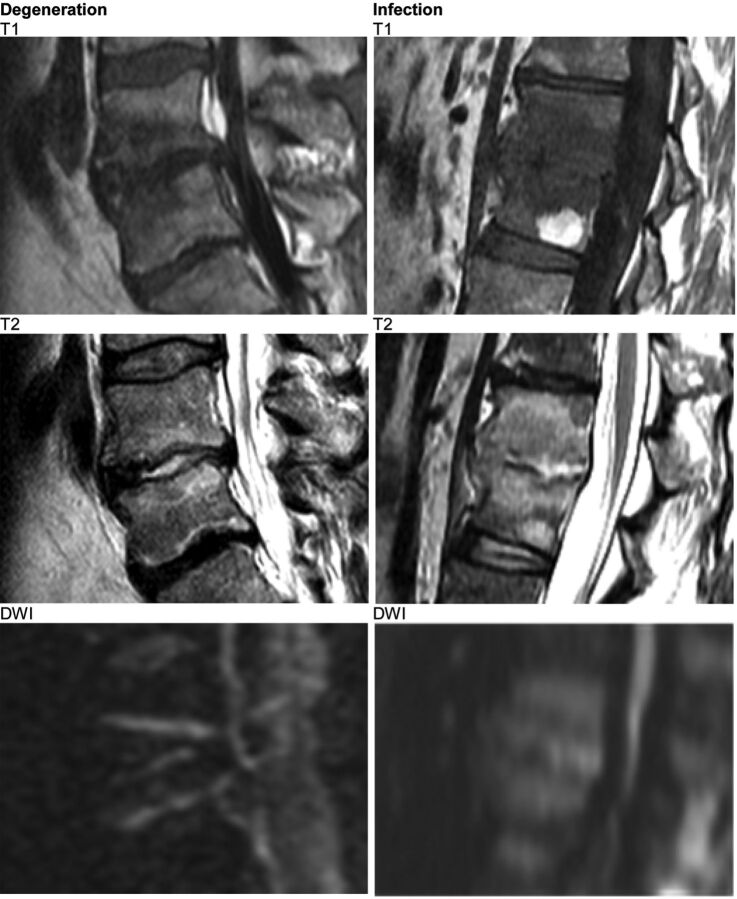
Fig 3.
Both cases show high T2 signal in the affected disk. The claw sign successfully distinguishes disk degeneration from disk infection.
The 73 patients were scanned on 3 MR imaging scanner platforms: 1.5T Avanto (Siemens, Erlangen, Germany) and 1.5T Signa HDxt, and 3T Discovery MR750 (GE Healthcare, Milwaukee, Wisconsin). Diffusion-weighted echo-planar imaging was performed in the sagittal plane, with single-shot echo-planar imaging at TR/TE, minimum; FOV, 26 cm; section thickness, 4 mm; intersection gap, 1 mm; and 2–3 excitations (averages). Conventional 6-direction, 3-direction, or tetrahedral diffusion encoding was obtained for a total sequence time of 60–90 seconds. Sagittal T2 and sagittal T1 FLAIR images were obtained in all cases. Sagittal contrast-enhanced, fat-suppressed T1WI was obtained in 49 of the 73 patients. No particular advantage of 3T over 1.5T was noted. This is largely because the diagnosis is primarily based on contrast resolution and coarse morphology of abnormality and not spatial resolution. The additional distortion-based challenges of 3T mitigate any potential SNR advantage.
At each level evaluated with type 1-like conventional signal changes, the images were scored as 1 = definite, 2 = probable, 3 = questionable, or 4 = negative or absent claw sign (Fig 2). Each neuroradiologist first assessed and scored the claw sign at a single level on each of the 73 sagittal DWIs. Then each neuroradiologist evaluated the signal intensity of the affected disk on the concurrent sagittal T2 and, when available, STIR series (n = 73) and scored the disk signal as high, normal, or low. In the 49 of 73 cases with concurrent, contrast-enhanced fat-suppressed sagittal T1 FLAIR series, the 2 neuroradiologists also noted any enhancement of the disk and any enhancement of the adjacent bone marrow. The 2 readers scored the series separately as no enhancement, mild, moderate, or marked enhancement of the disk and the endplates.
Results
The patients fell into 3 groups: 33 with degenerative Modic type 1 changes and no clinical or imaging suspicion of infection, 20 with clinically confirmed diskitis/osteomyelitis, and 20 with radiologically suspected infection later disproved clinically. For the 2 readers, the diffusion claw sign was identified as present in 26 of 33 and 19 of 33 levels with simple type 1 degenerative endplate changes; 0 of 20 and 1 of 20 patients with proved diskitis/osteomyelitis; and 12 of 20 and 10 of 20 patients with ultimately disproven radiologically suspected infection. Because the neuroradiologists had different levels of experience using the subjective claw sign in the clinical setting, scoring differed. The scoring data are thus provided for each reader separately in Tables 1–3 and Fig 4A, -B and are then summarized below.
Table 1:
Scoring of the claw sign by 2 readers
| Neuroradiologist 1 |
Neuroradiologist 2 |
||||||
|---|---|---|---|---|---|---|---|
| Claw Score | Group 1 (n = 33) | Group 2 (n = 20) | Group 3 (n = 20) | Claw Score | Group 1 (n = 33) | Group 2 (n = 20) | Group 3 (n = 20) |
| 1 | 26 | 1 | 12 | 1 | 19 | 0 | 10 |
| 2 | 7 | 0 | 7 | 2 | 10 | 3 | 6 |
| 3 | 0 | 2 | 1 | 3 | 3 | 4 | 4 |
| 4 | 0 | 17 | 0 | 4 | 1 | 13 | 0 |
Table 2:
Scoring of high T2 signal disk by 2 readers
| Neuroradiologist 1 |
Neuroradiologist 2 |
||||
|---|---|---|---|---|---|
| Claw Score | Groups 1 and 3 (n = 20) | Group 2 (n = 19) | Claw Score | Groups 1 and 3 (n = 16) | Group 2 (n = 17) |
| 1 | 15 | 1 | 1 | 10 | 0 |
| 2 | 4 | 0 | 2 | 3 | 3 |
| 3 | 1 | 2 | 3 | 3 | 4 |
| 4 | 0 | 16 | 4 | 0 | 10 |
Table 3:
Scoring of low/normal T2 signal disk by 2 readers
| Neuroradiologist 1 |
Neuroradiologist 2 |
||||
|---|---|---|---|---|---|
| Claw Score | Groups 1 and 3 (n = 33) | Group 2 (n = 1) | Claw Score | Groups 1 and 3 (n = 37) | Group 2 (n = 3) |
| 1 | 23 | 0 | 1 | 19 | 0 |
| 2 | 10 | 0 | 2 | 13 | 0 |
| 3 | 0 | 0 | 3 | 4 | 0 |
| 4 | 0 | 1 | 4 | 1 | 3 |
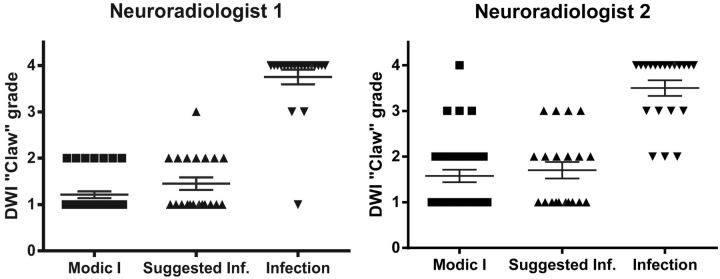
Fig 4.
A, Modic type 1 = 1.21 ± 0.07; suggested infection = 1.45 ± 0.13; proved infection = 3.75 ± 0.16. B, Modic type 1 = 1.58 ± 0.14; suggested infection = 1.70 ± 0.18; proved infection = 3.50 ± 0.17.
Diffusion Claw Sign
When a definite claw sign was identified, 38 of 39 patients (97%) and 29 of 29 patients (100%), respectively, were infection-free. When a probable claw was deemed present, 14 of 14 patients (100%) and 16 of 19 patients (84%), respectively, were infection-free. Conversely, when the 2 neuroradiologists identified diffuse signal changes within the adjoining vertebral bodies (negative or absent claw), the patient proved to have infection in 17 of 17 cases (100%) and 13 of 14 cases (93%), respectively. In the specific subgroup of 20 patients with radiologically suspected infection later disproved clinically, the diffusion claw sign was scored definite or probable in 19 of 20 (95%) and 16 of 20 (80%) of cases, respectively.
Disk Signal
High T2 signal within the disk was common in both infected and degenerated disks, reported in 39 of the 73 cases (53%) by reader 1 and in 33 of the 73 cases (45%) by reader 2 (Fig 3 and Tables 2 and 3). However, high T2 disk signal was more common at infected interspaces, 19 of 20 (95%) and 17 of 20 (85%), than in degenerative disks, 20 of 53 (38%) and 16 of 53 (30%). In the specific subgroup of 20 patients with radiologically suspected infection later disproved clinically, the T2 disk signal was scored high in 15 of 20 (75%) and 11 of 20 (55%). Low or normal signal within the disk was more common in patients without infection, 33 of 53 (62%) and 37 of 53 (70%), than in patients with infection, 1 of 20 (3%) and 3 of 20 (15%).
Contrast Enhancement
Contrast enhancement was assessed within a subgroup of 49 of the 73 patients, 17 (35%) with infection and 32 (65%) without infection. Both readers identified at least some enhancement in the endplates in all 49 patients, infected or not. Enhancement of the disk itself was identified slightly more frequently in infected disks: 5 of 20 (25%) and 6 of 20 (30%) than in degenerative disks: 6 of 53 (11%) and 9 of 53 (17%).
Discussion
In this study, infected and degenerative disks could manifest high, normal, or low T2 signal intensity. However, high T2 signal within the affected disk strongly favored a diagnosis of infection over degeneration when Modic type 1 endplate changes were present. Specifically, high T2 disk signal was seen in 85%–95% of infected disks but in only 30%–35% of degenerative disks with type 1 endplate changes. Low-to-normal T2 signal within the disk strongly favored a diagnosis of degeneration. Specifically, low-normal T2 disk signal was seen in 62%–70% of degenerative disks but only 3%–15% of infected disks.
Contrast enhancement of the disks and endplates proved to be indeterminate findings, as reported in prior studies.14 At least some contrast enhancement of the vertebral endplates was seen in all cases but did not help to differentiate infection from degeneration. Contrast enhancement of the disk itself was seen slightly more frequently in infected disks (25%–30%) than in degenerative disks (11%–17%), but it was not a distinguishing feature. Return examinations for the sole purpose of postcontrast characterization of type 1 disk levels are advised against because of low efficacy and incremental cost. This policy does not diminish the value of contrast in delineating and characterizing epidural and paraspinal disease in cases in which these conditions are present or strongly suspected.
Of all parameters tested, the diffusion-weighted claw sign proved to be the most successful for predicting the final clinical diagnosis of degeneration versus infection. A definite claw sign accurately identified degenerative spondylosis in 97%–100% of cases. A probable claw sign identified degenerative spondylosis in 84%–100% of cases. Diffusion signal that was increased diffusely throughout the adjoining vertebral bodies (ie, a negative or absent claw sign) indicated the presence of diskitis/osteomyelitis in 93%–100% of cases with that MR imaging feature. No significant difference in diffusion abnormality between organisms was observed, including diffuse restricted diffusion in the tuberculosis, E coli, or Escherichia faecium, and S aureus.
In the specific subgroup of 20 patients with radiologically suspected infection who ultimately proved infection-free, the diffusion claw sign was scored definite or probable in 19 of 20 (95%) and 16 of 20 (80%) cases, strongly indicating degeneration. The T2 disk signal in this subgroup was scored high in 15 of 20 (75%) and 11 of 20 (55%) cases, a finding that would have favored an incorrect diagnosis of infection.
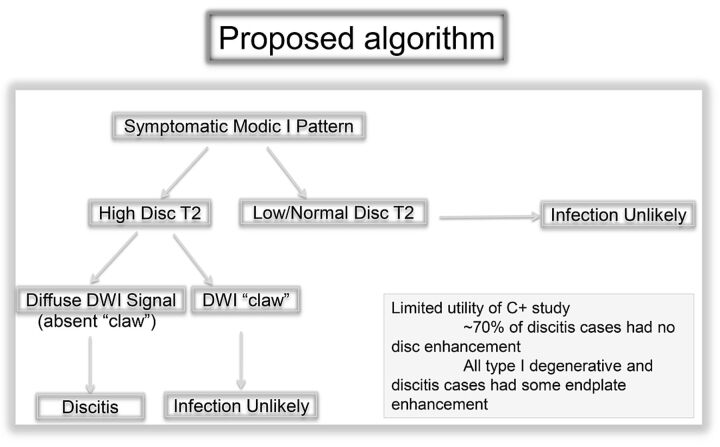
On the basis of these data, we propose an efficient algorithm for the work-up of patients with endplate changes suggestive of Modic type 1 change with degeneration versus infection (Fig 5).
Fig 5.
Proposed algorithm for the approach to symptomatic Modic type 1 pattern on spine MR imaging.
Earlier Work
Eguchi et al18 evaluated diffusion-weighted imaging in 15 healthy volunteers and 16 patients with vertebral abnormalities. In 11 patients with 20 levels of disk degeneration (7 Modic 1, 7 Modic 2, and 6 Modic 3), the authors reported no high-diffusion signal “at the site of endplate abnormalities in any patients with degenerative changes.” In 5 other patients with 9 levels of spinal infection, high diffusion signal was seen at all infected levels.
The apparent discordance of their results versus ours most likely stems from the following factors:
1) We studied only Modic type 1 endplate degeneration, whereas most of the endplate changes included in study of Eguchi et al were types 2 and 3: The degenerative disk illustrated in that study (their Fig 2) was specifically stated to be Modic type 3.
2) We characterized a specific form of diffusion abnormality (the diffusion claw sign), whereas Eguchi et al appear to have taken any form of increased diffusion as positive.
3) We focused on the changes at the interface between the normal marrow and vascularized bone marrow close to the affected disk, whereas Eguchi et al appear to have focused on the disks and the endplates themselves.
Limitations
The present study has a number of limitations. First, the sample size was small. Second, most patients were categorized by their final clinical diagnoses, not tissue diagnosis. Because of the difficulty of ruling out infection, this necessarily raises concern for the group classified as radiologically suspected infection later disproved clinically. Misclassification of such patients could influence the perceived accuracy of the signs described. Nonetheless, the results reported indicate a real utility of applying the diffusion claw sign in assessing patients with possible infection versus degenerative disk disease with Modic type 1 endplate changes.
ADC values have been cited as useful in quantitative assessment of bone marrow lesions.19 ADC values were not included in our evaluation because the main focus was evaluating the morphology of the detected diffusion trace signal. ADC values ranged from slightly low to high, but image quality and SNR on these studies were poor. With the legacy diffusion techniques used in this study, noise and distortion challenges rendered ADC images unreliable. As multishot and restricted FOV EPI techniques gain widespread use and availability, resolution, SNR, and overall quality will improve, making ADC assessment more practical in day-to-day practice.
The claw pattern on diffusion is a qualitative and morphologic finding. Experience with teaching this subjective sign to clinical readers shows that there is a learning curve. Readers with greater experience apply the sign with greater ease and accuracy. In most cases, it is clear-cut and easy to recognize, even for the recently instructed, and perhaps it is no greater challenge than confirming or excluding the diagnosis of infection with traditional MR imaging indicators. With any subjective signal-based changes based on a physiologic abnormality, equivocal cases can occur. The reader is then left using the preponderance of conventional MR imaging evidence to make the definitive diagnosis. In this trial, a recently initiated reader performed nearly as well as the more experienced reader who had been considering the sign in practice for some time.
This study has defined and validated the diffusion claw sign in symptomatic patients with type 1 endplate changes and degenerative disk disease or infection. The claw sign has not been evaluated at disk levels that manifest type 2 or type 3 endplate changes, but experience suggests it will have limited utility.
Conclusions
The present study introduces and illustrates a distinct pattern of diffusion abnormality, the claw sign, that is useful and accurate for distinguishing degenerative spondylosis from diskitis/osteomyelitis in patients with Modic type 1 endplate changes. At any suspicious level, a definite diffusion claw sign signifies very high likelihood of degenerative disease (97%–100%) rather than infection. A probable claw sign is highly suggestive of degenerative disk disease (85%–100%) rather than infection. Conversely, a pattern of diffusely increased diffusion signal (negative claw sign) signifies infection in 93%–100% of patients, rather than disk degeneration with Modic type 1 endplate changes. The data additionally suggest that routine use of contrast may not be cost-effective in assisting in the primary diagnosis of infection of the spine.
The diffusion claw sign supplements classic imaging features such as disk signal and can increase accuracy and confidence in the differential diagnosis of degenerative spondylosis versus diskitis/osteomyelitis. The use of the claw sign in day-to-day practice may reduce cost by eliminating or reducing concern for infection in symptomatic patients manifesting type 1 changes, which might otherwise provoke invasive testing and contrast-enhanced and follow-up examinations. With variable experience, there will be variable certainty in the confidence of the presence of the “sign” to mitigate infection. Absent a confident determination, typical decision factors and investigations will apply.
Footnotes
Disclosures: Lawrence Tanenbaum—speaker for GE and Siemens, neither of which were involved in the project beyond the use of their scanners.
Paper previously presented at: Annual Meeting of the European Congress of Radiology, March 1–5, 2012, Vienna, Austria; Annual Meeting of the American Society of Neuroradiology Meeting and the Foundation of the ASNR Symposium, April 21–26, 2012, New York, New York; Annual Symposium of the American Society of Spine Radiology, February 21–24, 2013, Scottsdale, Arizona (1st place Mentor Award); and Annual Meeting of the American Society of Neuroradiology, May 18–23, 2013, San Diego, California.
REFERENCES
- 1. Castillo M, Arbelaez A, Smith JK, et al. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol 2000;21:948–53 [PMC free article] [PubMed] [Google Scholar]
- 2. Castillo M. Diffusion-weighted imaging of the spine: is it reliable? AJNR Am J Neuroradiol 2003;24:1251–53 [PMC free article] [PubMed] [Google Scholar]
- 3. Dietrich O, Biffar A, Reiser MF, et al. Diffusion-weighted imaging of bone marrow. Semin Musculoskeletal Radiol 2009;13:134–44 [DOI] [PubMed] [Google Scholar]
- 4. Baur A, Dietrich O, Reiser M. Diffusion-weighted imaging of the spinal column. Neuroimaging Clin N Am 2002;12:147–60 [DOI] [PubMed] [Google Scholar]
- 5. Byun WM, Shin SO, Chang Y, et al. Diffusion-weighted MR imaging of metastatic disease to the spine: assessment of response to therapy. AJNR Am J Neuroradiol 2002;23:906–12 [PMC free article] [PubMed] [Google Scholar]
- 6. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:162–63 [DOI] [PubMed] [Google Scholar]
- 7. Park MJ, Cha ES, Kang BJ, et al. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol 2007;8:390–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimofusa R, Fujimoto H, Akamata H, et al. Diffusion-weighted imaging of prostate cancer. J Comput Assist Tomogr 2005;29:149–53 [DOI] [PubMed] [Google Scholar]
- 9. Park SW, Lee JH, Ehara S, et al. Single shot fast spin echo diffusion-weighted MR imaging of the spine: is it useful in differentiating malignant metastatic tumor infiltration from benign fracture edema? Clin Imaging 2004;28:102–08 [DOI] [PubMed] [Google Scholar]
- 10. Baur A, Stabler A, Brunning R, et al. Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology 1998;207:349–56 [DOI] [PubMed] [Google Scholar]
- 11. Finelli DA. Diffusion-weighted imaging of acute vertebral compressions: specific diagnosis of benign versus malignant pathologic fractures. AJNR Am J Neuroradiol 2001;22:241–42 [PMC free article] [PubMed] [Google Scholar]
- 12. Karchevsky M, Babb JS, Schweitzer ME. Can diffusion-weighted imaging be used to differentiate benign from pathologic fractures? A meta-analysis. Skeletal Radiol 2008;37:791–95 [DOI] [PubMed] [Google Scholar]
- 13. Eastwood JD, Vollmer RT, Provenzale JM. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol 2002;23:496–98 [PMC free article] [PubMed] [Google Scholar]
- 14. Oztekin O, Calli C, Kitis O, et al. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol 2010;44:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193–99 [DOI] [PubMed] [Google Scholar]
- 16. Dunbar JA, Sandoe JA, Rao AS, et al. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol 2010;65:974–81 [DOI] [PubMed] [Google Scholar]
- 17. Dihlmann W. Hemispherical spondylosclerosis: a polyetiologic syndrome. Skeletal Radiol 1981;7:99–106 [DOI] [PubMed] [Google Scholar]
- 18. Eguchi Y, Ohtori S, Yamashita M, et al. Diffusion magnetic resonance imaging to differentiate degenerative from infectious endplate abnormalities in the lumbar spine. Spine (Phila Pa 1976) 2011; 36:E198–202 [DOI] [PubMed] [Google Scholar]
- 19. Balliu E, Vilanova JC, Pelaez I, et al. Diagnostic value of apparent diffusion coefficients to differentiate benign from malignant vertebral bone marrow lesions. Eur J Radiol 2009;69:560–66 [DOI] [PubMed] [Google Scholar]