Abstract
BACKGROUND AND PURPOSE:
APOE4 is the best-documented genetic risk factor for sporadic AD. Previous research showed that APOE4 is associated with increased risk of occurrence and earlier onset of AD in a gene dose–dependent manner. However, the specific role of APOE4 in processing of brain functions requires further investigation. Investigators have used fMRI to measure brain activity on the basis of the blood oxygen level–dependent contrast. This study investigates the effects of APOE4 on fMRI during n-back WM tasks in healthy middle-aged adults.
MATERIALS AND METHODS:
From 110 participants, 81 individuals without objective or subjective cognitive impairment underwent APOE genotyping. Nine APOE4 carriers and 9 age- and sex-matched non-APOE4 controls were recruited for fMRI examinations during WM tasks.
RESULTS:
Both groups displayed increased brain activation in response to increases in WM loads. During low-WM-load tasks, the APOE4 carriers recruited significantly greater additional processing resources than the non-APOE4 carriers. During moderate- and high-WM-load tasks, the APOE4 carrier group displayed fewer increases in activation than the non-APOE4 carrier group.
CONCLUSIONS:
APOE genetic polymorphisms may affect brain functioning in subjects without dementia. The patterns of brain activation during different levels of WM load suggest possible subclinical impairment of WM capacity in APOE4 carriers (ClinicalTrials.gov registration: NCT01287819).
AD is the most common cause of dementia in the world. Although the specific pathophysiology of AD remains unclear, compelling evidence has shown that genetic factors play an important role in its occurrence.1 There are 3 genes linked to familial AD, including amyloid precursor protein, presenilin-1, and presenilin-2.2 APOE4 is the best-documented genetic risk factor for sporadic AD.3 The genetic polymorphisms for the APOE gene include 3 alleles: ε2, 3, and 4. Previous research identified APOE4 as associated with increased risk of occurrence and earlier onset of AD in a gene dose–dependent manner.4,5 APOE4 carriers present with more severe memory impairment and greater reductions in regional brain volume and metabolism, in comparison with non-APOE4 carriers.6–9 These findings indicate that APOE4 is important for brain functioning, though the mechanisms by which it exerts its effects need further clarification. Genetic background can thus confer changes in brain structure, metabolism, and functioning, or even behaviors.
Investigators extensively use fMRI to evaluate brain function. Most studies use an indirect-measurement method based on the blood oxygen level–dependent contrast.10,11 Under normal physiologic conditions, the amplitude of the BOLD signal is linearly connected to neural activity and can provide task-specific information concerning neural functions and networks.12–14
WM has the ability to actively keep information for further use by prioritizing, modifying, and protecting it from interference.15 Therefore, the operations of WM consist of memory itself and retention of active information in a stable yet flexible manner.16 Most patients with AD present with progressive impairment of episodic memory, and such dysfunction constitutes the core diagnostic criterion of probable AD.17 However, several findings in the literature also unveil deficits in WM and executive function in patients with AD and mild cognitive impairment.18–20 Previous studies of fMRI documented that WM emerges from interactions among higher sensory, attentional, and mnemonic functions, with separable neural bases.21–25 WM has a fundamental cognitive function, which can be evaluated by fMRI, and thus provides a good domain for testing the differences among individuals with various genetic backgrounds. fMRI studies of APOE4 carriers have shown decreased neural activity in the medial temporal lobe and increased (probable compensatory) neural activity in prefrontal and parietal regions under tasks of encoding and retrieval of episodic memory.26,27 Neural activity alteration in WM in subjects at risk of AD is not conclusive.28 An initial study by Burggren et al29 by using a digit-span task showed no difference in activation patterns between elderly APOE4 carriers and non-APOE4 carriers. They concluded that additional cognitive effort in persons at genetic risk for AD is specific to episodic encoding. Later studies by using an n-back WM paradigm showed no difference in activation patterns between APOE4 carriers and non-APOE4 carriers in young adults but increased activation in the frontal and parietal lobe in elderly APOE4 carriers.30,31 To the best of our knowledge, there is no publication to date about neural activity alteration in WM in middle-aged subjects at risk of AD, especially with different WM loads.
Materials and Methods
Participants
One hundred ten subjects who underwent physical examinations at Taipei Medical University–Shuang Ho Hospital participated in this study. All participants provided written informed consent, and this study was approved by the hospital institutional review board. The participants self-completed the Alzheimer's Disease 8 (AD8) questionnaire for subjective cognitive impairment and were assessed with the MMSE for objective cognitive impairment by a neuropsychologist.32,33 Participants with AD8 >2 or MMSE <26 were excluded. Eighty-one participants without subjective or objective cognitive impairment were selected for further genetic analysis, from which 12 APOE4 carriers, all ε3/4 heterozygous, and 69 non-APOE4 carriers were found. Nine APOE4 carriers underwent fMRI analysis. Nine age-and sex-matched controls were randomly selected from the non-APOE4 carriers.
Working Memory Task
WM capacity can be tested by using a variety of tasks. A commonly used measure in fMRI is the n-back task. The participant is required to monitor a series of stimuli and to respond whenever a stimulus is presented that is the same as the one presented n trials previously. Parametric designs, comparing n = 1, n = 2, and n = 3 trials are often used. Some studies have also used a 0-back control condition. In this study, n-back WM tasks were presented by using E-Prime, Version 2.0 (http://www.pstnet.com/eprime.cfm). At the beginning, a task guide describing the rules of the study was shown. The n-back tasks comprised 4 conditions, 0-, 1-, 2-, and 3-back. The 0-back control condition had a minimal WM load, and individuals were asked to decide whether the current letter matched a single target letter that was specified before the condition began. During the 1-back condition, they were asked to decide whether the current letter matched the previous one. During the 2-back and 3-back conditions, the participants were asked to decide whether the number currently presented matched the number that had been presented 2 and 3 trials back in the sequence. Each condition was conducted in a single run, which consisted of 3 epochs. Each epoch contained 30 seconds of presentation with numbers and 30 seconds of fixation on a crosshair. Twelve numbers were presented in the 30-second period, and each consisted of a 0.5-second appearance of a black number on the screen against a white background and 2 seconds of a fixation cross. All stimuli were projected onto an overhead screen, which participants could view through a mirror located on the scanner's head coil. Participants were instructed to press a response pad with the right index finger when the currently presented number was a target or to press the middle finger when the currently presented number was not a target. The number of correct and incorrect responses was recorded.
Conditions were counterbalanced among participants: Each condition was equally often preceded and followed by each of the other conditions. Before the scans, each participant was instructed on the entire experimental procedure and practiced the tasks outside the scanner to reduce anxiety. To further reduce any group effect independent of WM processes, all participants were trained before scanning to ensure that they could perform the 1-back condition to an accuracy criterion of 70%.
Imaging Methods
Imaging was performed on a 3T MR imaging system (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin). An 8-channel head coil was used for signal reception. Care was taken to minimize the effects of movement by instructing participants to remain still and by placing foam padding around the head. Functional data were collected by using an EPI sequence (TR = 3000 ms, TE = 35 ms, flip angle = 90°, FOV = 230 mm2, matrix = 64 × 64, 40 sections, section thickness = 3 mm, and intersection gap = 1 mm) for a 3.5-minute period resulting in 70 volumes. Two dummy scans, which enabled the MR signal to achieve a steady state, were applied in each scan. A T1-weighted anatomic dataset was obtained from each subject by using brain-volume imaging (TR = 8.2 ms, TE = 3.2 ms, TI = 450 ms, flip angle = 12°, FOV = 240 mm2, matrix = 256 × 256, 160 sections, voxel size = 0.9375 × 0.9375 × 1 mm3). T2-weighted images were acquired by using fast recovery fast spin-echo (TR = 5700 ms, TE = 100 ms, FOV = 230 mm2, matrix = 416 × 416, 20 sections, section thickness = 5 mm, intersection gap = 2 mm, and NEX = 1).
Data Analysis
fMRI data were preprocessed by using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab, Version 7.9 (MathWorks, Natick, Massachusetts). To reduce motion artifacts, we realigned each participant's functional images with the first volume of the series by using the rigid-body transformation procedure and resectioned them by using fourth-degree B-spline function interpolation while adjusting for residual motion-related signal changes. All motion-corrected images were spatially normalized to the EPI template of the Montreal Neurological Institute. The normalization process involved minimizing the residual sum of squared differences among the images to be normalized. Before modeling the fMRI signal changes, normalized EPI images were spatially smoothed by convolution with a 3D Gaussian filter of 6 × 6 × 6 mm3 full width at half maximum to increase the signal-to-noise ratio, validate the statistical analysis, and reduce anatomic variations among participants.
Statistical Analysis
Demographic and neuropsychological test data for both groups were compared by using 2-tailed t tests or χ2 tests. For each n-back condition, the datasets were analyzed by modeling the experimental conditions by using boxcar functions convolved with a hemodynamic response function in the context of the general linear model used by SPM5. To obtain the brain-activation patterns for each group, we entered smoothed normalized scans for all participants into this general linear model and created contrast images for between 0- and 1-back (1 > 0), 1- and 2-back (2 > 1), and 2- and 3-back (3 > 2) for each group. A 1-sample t test was applied for within-group analyses. The probability threshold value was set at .01 uncorrected, with a minimum cluster extent of 3 contiguous voxels. The generated contrast images for 1- > 0-back, 2- > 1-back, and 3- > 2-back were then used for the second-level multiparticipant/between-group random effects analyses, to obtain the activation differences between APOE4 carriers and noncarriers. The 2-sample t test was applied for between-group analyses. The probability threshold value was set at .01 uncorrected, with a minimum cluster extent of 3 contiguous voxels.
Results
The Table describes the basic demographic characteristics of both groups. The groups did not differ significantly regarding sex, age, and years of education. Group accuracies for the n-back task did not differ among APOE4 carriers and non-APOE4 carriers. There was, however, a significant trend of increasing WM load associated with declines in performance accuracy in both groups (P < .001).
Demographic information, cognitive function, and accuracy rates of n-back WM in participants
| Total (n = 110) | ApoE4− (n = 9) | ApoE4+ (n = 9) |
ApoE4− versus ApoE4+ |
||
|---|---|---|---|---|---|
| t | p | ||||
| Sex (male/female) | 53:57 | 4:5 | 4:5 | – | – |
| Mean age (SD) (yr) | 57.60 | 42.78 (9.63) | 42.11 (9.13) | 0.53 | .34 |
| Mean MMSE score (SD) | 28.49 (2.65) | 29.56 (0.73) | 28.89 (1.17) | 0.42 | .38 |
| Mean AD8 score (SD) | 0.54 (1.20) | 0.44 (0.73) | 0.11 (0.33) | 0.09 | .76 |
| 0-Back accuracy rate | 90% (17) | 92% (15) | −0.19 | .85 | |
| 1-Back accuracy rate | 93% (6) | 89% (17) | 0.50 | .63 | |
| 2-Back accuracy rate | 80% (11) | 85% (9) | −0.82 | .43 | |
| 3-Back accuracy rate | 80% (9) | 85% (13) | −0.86 | .41 | |
Note:—APOE4+ indicates an APOE4 carrier; APOE4−, a non-APOE4 carrier.
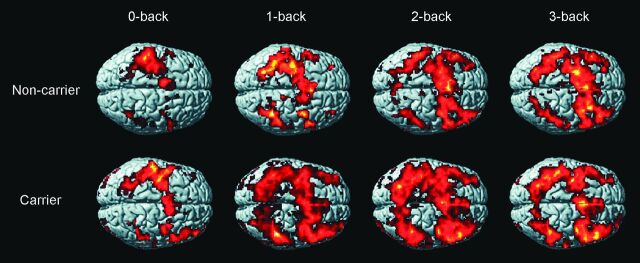
Figure 1 shows activation maps, during the n-back task, of APOE4 carriers and controls in a surface-rendered projection displayed on a standardized brain atlas (display threshold, P < .01; extent, 3 voxels). Both groups displayed increased activation in bilateral frontal and parietal regions, consistent with activation of WM circuitry.
Fig 1.
Activation maps of n-back task of APOE4 carriers and non-APOE4 carriers in a surface-rendered projection displayed on a standardized brain atlas (display threshold, P < .01; extent, 3 voxels). Increased activation in bilateral frontal and parietal regions is noted, consistent with activation of WM circuitry in both groups.
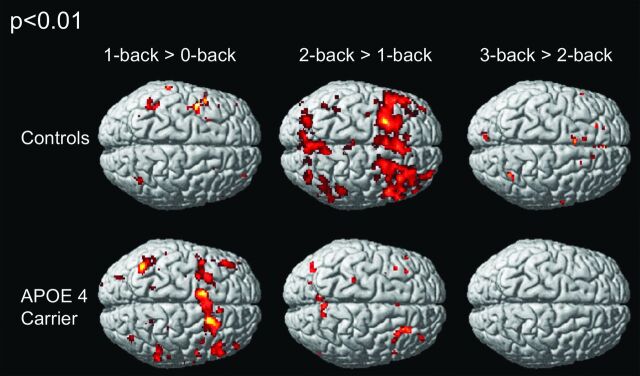
Figure 2 displays activation maps, during 1-back > 0-back, 2-back > 1-back, and 3-back > 2-back conditions, of APOE4 carriers and non-APOE4 carriers. Both groups displayed increased brain activation in response to each increase in WM load. The degree of increase in brain activation differed among the groups and was greatest in the 1-back > 0-back conditions in the APOE4 carriers and in the 2-back > 1-back conditions in the non-APOE4 carriers. Visual comparison of the between-group differences in the 1-back > 0-back conditions shows lesser activation in the non-APOE4 carrier group. In 2-back > 1-back and 3-back > 2-back conditions, the APOE4 carrier group demonstrated fewer increases in activation than the non-APOE4 carriers.
Fig 2.
Activation maps of 1-back > 0-back, 2-back > 1-back, and 3-back > 2-back conditions of APOE4 carriers and non-APOE4 carriers in a surface-rendered projection displayed on a standardized brain atlas (display threshold, P < .01; extent, 3 voxels). In both groups, an increase in brain activation occurred in response to each increase in WM load. The degree of increased brain activation was different in both groups. It was greatest in 2-back > 1-back conditions in non-APOE4 carriers, and greatest in 1-back > 0-back conditions in APOE4 carriers. A visual comparison of the between-group difference in 1-back > 0-back conditions shows less extent of activation in the noncarrier group. In 2-back 1-back and 3-back > 2-back conditions, less increase in activation is seen in the carrier group.
Discussion
APOE4 carriers displayed more rapid memory decline and mesial temporal lobe atrophy than non-APOE4 carriers.34–41 The present study recruited individuals without subjective or objective cognitive impairment, hence subjects with normal cognition, to test the effects of APOE4 on the brain functioning of WM by using BOLD fMRI. Performance of n-back WM was not different between groups (P = .41–.85), similar to the findings of a previous publication.42 fMRI analysis showed that during the low-WM-load task, functional activation significantly increased to a greater extent in the APOE4 carriers than in the non-APOE4 carriers. As the WM load increased, the non-APOE4 carriers maintained their ability to further increase activation. In contrast, the APOE4 carriers showed fewer increases in activation during the moderate- and high-WM-load conditions. One possible explanation for this phenomenon is mild impairment of WM capacity in the APOE4 carriers. In the low-WM load (1- back > 0-back conditions), the APOE4 carriers recruited additional processing resources to compensate for processing inefficiencies. In contrast, the non-APOE4 carriers were not impaired or challenged by this low-WM load and, therefore, required relatively fewer increases in processing resources to perform the task. As the WM load increased, the non-APOE4 carriers continued to show increasing activation, presumably because they still had processing reserves available to be drawn on with increasing WM demand. The APOE4 carriers, however, had already recruited most of their (assuming a finite amount) available resources, and thus little additional activation occurred.
In this study, the accuracy rate of 0-back, 2-back, and 3-back WM tasks appeared insignificantly better in the APOE4 carrier group. Among the APOE4 carriers, the accuracy rates did not dramatically decline along with the increase of memory loads when the brain activation recruitment had reached the plateau. It is speculated that there is a ceiling effect of working memory in this study. Continuing increases in memory load might enable the detection of the decline of performance in APOE4 carriers. Dickerson et al43 demonstrated increased hippocampal activation in mild cognitive impairment compared with normal aging and AD under fMRI scanning during face-name associative encoding tasks. They hypothesized that there is a phase of increased medial temporal lobe activation early in the course of prodromal Alzheimer disease. Our findings of increased functional activation under low WM load in APOE4 carriers may agree with their hypothesis and may be applied to the cognitively normal high-risk subjects. Explanations of such functional alteration include a compensatory mechanism for regional neuronal loss and disruption of the intrinsic connectivity of memory networks.44,45 Filbey et al46 also found compensatory neural activation in the medial frontal and parahippocampal gyrus in young and elderly APOE4 carriers during a 9-square-grid visual WM task, similar to our findings in low-WM load.
The relationship between the WM fMRI findings and underlying pathology is not clear at present. It could reflect a hereditary pattern in APOE4 carriers or could be a consequence of functional decline of the subclinical brain. Using resting-state fMRI, Sheline et al47 showed that APOE4 alters the neural network in individuals with normal cognition without amyloid burden, as detected by using Pittsburgh compound B–amyloid PET. However, the long-term effects of these types of brain functional changes remain unknown.
There are a few limitations to this study. First, amyloid pathology was not completely excluded. Numerous studies have proved that amyloid burden can occur many years before objective or subjective cognitive declines, especially among APOE4 carriers.8,48,49 In the present study, amyloid pathology, which results in brain subclinical dysfunction, might potentially have contributed to the increase in BOLD. Second, the sample size of this study was small. APOE4 carrier rates are low in Asian populations relative to whites. This study recruited only 9 APOE4 carriers and 9 non-APOE4 carriers and was able to achieve statistical significance. Third, the insignificantly better performance of some n-back WM tasks in the APOE4 carrier group was unexpected and was not perfectly compatible with the fMRI presentation. Future large-scale research investigations, by using amyloid imaging and long-term follow-ups, are, therefore, warranted.
Conclusions
In summary, fMRI BOLD significantly increased in the APOE4 carriers during low-WM-load tasks but did not increase during moderate- and high-WM loads, which occurred in non-APOE4 carriers. Based on previous research and our findings, these differences could have a hereditary basis. The phenomenon could reflect an impaired WM processing resource associated with subclinical brain dysfunction. This study, therefore, also provided further evidence to confirm the effects of genetic background on brain functioning. Further studies are required to fully elucidate the underlying mechanisms.
ABBREVIATIONS:
- AD
Alzheimer disease
- APOE4
Apolipoprotein E ε4 allele
- BOLD
blood oxygen level–dependent
- MMSE
Mini-Mental State Examination
- WM
working memory
Footnotes
Disclosures: Chih-Chung Chen—RELATED: Grant: Center of Excellence for Clinical Trial and Research in Neurology and Neurosurgery.* Dean Wu—RELATED: Grant: Center of Excellence for Clinical Trial and Research in Neurology and Neurosurgery.* Po-Chih Chen—RELATED: Grant: Center of Excellence for Clinical Trial and Research in Neurology and Neurosurgery.* Hung-Wen Chiu—RELATED: Grant: Center of Excellence for Clinical Trial and Research in Neurology and Neurosurgery.* Chaur-Jong Hu—RELATED: Grant: Taiwan government,* Comments: for sponsoring this study. *Money paid to the institution.
This work was supported by the Center of Excellence for Clinical Trial and Research in Neurology and Neurosurgery.
Dr Chi-Jen Chen contribution: fMRI study design and quality control. Dr Chih-Chung Chen contribution: fMRI data analysis and manuscript preparation. Dr Dean Wu contribution: genotyping and data analysis. Dr Nai-Fang Chi contribution: neuropsychological tests. Dr Po-Chih Chen contribution: genotyping and data collection. Mr Yen-Peng Liao contribution: fMRI conduction. Dr Hung-Wen Chiu contribution: fMRI data analysis and manuscript preparation. Dr Chaur-Jong Hu contribution: study design, genotyping, data analysis, and manuscript preparation.
REFERENCES
- 1. van Duijn CM, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer's disease and related disorders: a collaborative re-analysis of case-control studies. Int J Epidemiol 1991;20(suppl 2):S13–20 [DOI] [PubMed] [Google Scholar]
- 2. Waring SC, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch Neurol 2008;65:329–34 [DOI] [PubMed] [Google Scholar]
- 3. Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 1993;90:1977–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Small GW. Neuroimaging and genetic assessment for early diagnosis of Alzheimer's disease. J Clin Psychiatry 1996;57(suppl 14):9–13 [PubMed] [Google Scholar]
- 5. Chen K, Reiman EM, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry 2007;164:916–21 [DOI] [PubMed] [Google Scholar]
- 6. Caselli RJ, Graff-Radford NR, Reiman EM, et al. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology 1999;53:201–07 [DOI] [PubMed] [Google Scholar]
- 7. Pievani M, Galluzzi S, Thompson PM, et al. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer's disease. Neuroimage 2011;55:909–19 [DOI] [PubMed] [Google Scholar]
- 8. Donix M, Burggren AC, Suthana NA, et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage 2010;53:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A 2005;102:8299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogawa S, Lee TM, Nayak AS, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990;14:68–78 [DOI] [PubMed] [Google Scholar]
- 11. Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412:150–57 [DOI] [PubMed] [Google Scholar]
- 13. Ogawa S, Lee TM, Stepnoski R, et al. An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc Natl Acad Sci U S A 2000;97:11026–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci 2000;3:716–23 [DOI] [PubMed] [Google Scholar]
- 15. Baddeley A. Working memory. Science 1992;255:556–59 [DOI] [PubMed] [Google Scholar]
- 16. Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: contributions from functional imaging studies. Behav Brain Res 2010;214:172–79 [DOI] [PubMed] [Google Scholar]
- 17. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–46 [DOI] [PubMed] [Google Scholar]
- 18. Rosen VM, Bergeson JL, Putnam K, et al. Working memory and apolipoprotein E: what's the connection? Neuropsychologia 2002;40:2226–33 [DOI] [PubMed] [Google Scholar]
- 19. Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychology 2007;21:458–69 [DOI] [PubMed] [Google Scholar]
- 20. Belleville S, Sylvain-Roy S, de Boysson C, et al. Characterizing the memory changes in persons with mild cognitive impairment. Prog Brain Res 2008;169:365–75 [DOI] [PubMed] [Google Scholar]
- 21. D'Esposito M, Postle BR, Jonides J, et al. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A 1999;96:7514–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci 2000;3:509–15 [DOI] [PubMed] [Google Scholar]
- 23. Ranganath C, Johnson MK, D'Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 2003;41:378–89 [DOI] [PubMed] [Google Scholar]
- 24. Narayanan NS, Prabhakaran V, Bunge SA, et al. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology 2005;19:223–32 [DOI] [PubMed] [Google Scholar]
- 25. Crone EA, Wendelken C, Donohue S, et al. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A 2006;103:9315–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bondi MW, Houston WS, Eyler LT, et al. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 2005;64:501–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kukolja J, Thiel CM, Eggermann T, et al. Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience 2010;168:487–97 [DOI] [PubMed] [Google Scholar]
- 28. Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-ε4 on the BOLD response. Neurobiol Aging 2012;33:323–34 [DOI] [PubMed] [Google Scholar]
- 29. Burggren AC, Small GW, Sabb FW, et al. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry 2002;10:44–51 [PubMed] [Google Scholar]
- 30. Mondadori CR, de Quervain DJ, Buchmann A, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex 2007;17:1934–47 [DOI] [PubMed] [Google Scholar]
- 31. Wishart HA, Saykin AJ, Rabin LA, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry 2006;163:1603–10 [DOI] [PubMed] [Google Scholar]
- 32. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–64 [DOI] [PubMed] [Google Scholar]
- 33. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 34. Dik MG, Jonker C, Comijs HC, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology 2001;57:2217–22 [DOI] [PubMed] [Google Scholar]
- 35. Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 2004;62:1990–95 [DOI] [PubMed] [Google Scholar]
- 36. Caselli RJ, Reiman EM, Locke DEC, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol 2007;64:1306–11 [DOI] [PubMed] [Google Scholar]
- 37. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 2009;361:255–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomann PA, Roth AS, Dos Santos V, et al. Apolipoprotein E polymorphism and brain morphology in mild cognitive impairment. Dement Geriatr Cogn Disord 2008;26:300–05 [DOI] [PubMed] [Google Scholar]
- 39. Honea RA, Vidoni E, Harsha A, et al. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J Alzheimers Dis 2009;18:553–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spampinato MV, Rumboldt Z, Hosker RJ, et al. Apolipoprotein E and gray matter volume loss in patients with mild cognitive impairment and Alzheimer disease. Radiology 2011;258:843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu PH, Thompson PM, Leow A, et al. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. J Alzheimers Dis 2011;23:433–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenwood PM, Lambert C, Sunderland T, et al. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health's BIOCARD study. Neuropsychology 2005;19:199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005;65:404–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hämäläinen A, Pihlajamäki M, Tanila H, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 2007;28:1889–903 [DOI] [PubMed] [Google Scholar]
- 45. Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med 2010;12:27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filbey FM, Chen G, Sunderland T, et al. Failing compensatory mechanisms during working memory in older apolipoprotein E-epsilon4 healthy adults. Brain Imaging Behav 2010;4:177–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 2010;30:17035–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007;130(pt 11):2837–44 [DOI] [PubMed] [Google Scholar]
- 49. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 2009;106:6820–25 [DOI] [PMC free article] [PubMed] [Google Scholar]