Abstract
BACKGROUND AND PURPOSE:
Waardenburg syndrome, characterized by deafness and pigmentation abnormalities, is clinically and genetically heterogeneous, consisting of 4 distinct subtypes and involving several genes. SOX10 mutations have been found both in types 2 and 4 Waardenburg syndrome and neurologic variants. The purpose of this study was to evaluate both the full spectrum and relative frequencies of inner ear malformations in these patients.
MATERIALS AND METHODS:
Fifteen patients with Waardenburg syndrome and different SOX10 mutations were studied retrospectively. Imaging was performed between February 2000 and March 2010 for cochlear implant work-up, diagnosis of hearing loss, and/or evaluation of neurologic impairment. Eleven patients had both CT and MR imaging examinations, 3 had MR imaging only, and 1 had CT only.
RESULTS:
Temporal bone abnormalities were bilateral. The most frequent pattern associated agenesis or hypoplasia of ≥1 semicircular canal, an enlarged vestibule, and a cochlea with a reduced size and occasionally an abnormal shape, but with normal partition in the 13/15 cases that could be analyzed. Three patients lacked a cochlear nerve, bilaterally in 2 patients. In addition, associated abnormalities were found when adequate MR imaging sequences were available: agenesis of the olfactory bulbs (7/8), hypoplastic or absent lacrimal glands (11/14), hypoplastic parotid glands (12/14), and white matter signal anomalies (7/13).
CONCLUSIONS:
In the appropriate clinical context, bilateral agenesis or hypoplasia of the semicircular canals or both, associated with an enlarged vestibule and a cochlear deformity, strongly suggests a diagnosis of Waardenburg syndrome linked to a SOX10 mutation.
In 1951, a Dutch ophthalmologist and geneticist described a syndrome with dystopia canthorum (lateral displacement of the inner eye corners), a high broad nasal root, confluent eyebrows, iris heterochromia, white forelock or early graying, and congenital sensorineural hearing loss, a clinical association now known as Waardenburg syndrome type 1.1 Four distinct subtypes have been described since then, all characterized by deafness and pigmentary disturbance (for review, see Pingault et al2). WS has an incidence of approximately 1/40,000 births and is responsible for 1%–3% of cases of congenital deafness. SNHL in WS is thought to be due to the absence of the melanocyte-derived intermediate cells of the stria vascularis. This absence induces endolymphatic collapse and secondary agenesis of the organ of Corti, a process that is called cochleosaccular degeneration. Results from the few histopathologic studies performed on human temporal bones are consistent with this hypothesis.3–5 Characteristic radiologic abnormalities of the temporal bone have been reported in a subset of patients,6–9 enabling imaging to contribute to the differential diagnosis of syndromic SNHL.10 Because none of these studies included molecular analyses, however, these inner ear malformations have not been linked to a particular molecular defect.
WS is clinically and genetically heterogeneous (for a review see Pingault et al2). Several genes are involved (PAX3, MITF, EDN3, EDNRB, SOX10), with SOX10 mutations causing approximately 15% of type 2 WS, which is distinguished from type 1 WS by the absence of dystopia canthorum, and >50% of type 4 WS, which is also known as WS2 + Hirschsprung disease or chronic intestinal pseudoobstruction.11,12 Patients with SOX10 mutations also frequently show severe neurologic abnormalities resulting from impaired myelination of the central and peripheral nervous systems in addition to WS. This condition is referred as PCWH.13
In 2002, agenesis of the semicircular canals was reported for the first time in 2 patients with WS and SOX10 mutations.14 Vestibular and cochlear malformations were subsequently described in other studies of patients with WS carrying SOX10 mutations,11,13,15 but only 2 detailed case reports have been published. In the first one, the authors described both the absence of all 3 SCCs and the formation of a common cavity corresponding to the saccular dilation of the vestibule.16 The second case revealed the absence of cochlear nerves, mild cochlear hypoplasia, modiolar deficiency, and the absence of olfactory bulbs.17
The purpose of this study was to report temporal bone abnormalities associated with Waardenburg syndrome due to impaired SOX10 function in 15 patients with MR imaging and/or CT scans of the inner ears. We also report associated findings seen on these imaging studies.
Materials and Methods
Patients
This retrospective multicenter study included 15 patients (14 unrelated cases and the brother of a propositus) from 8 days to 31 years of age. The patients recruited had both a SOX10 mutation that confirmed a clinical suspicion of Waardenburg syndrome and inner ear abnormalities on CT and/or MR imaging. This study was conducted in accordance with the guidelines of our institutional ethics committee for a retrospective observational review study. Imaging was performed between February 2000 and March 2010, either for SNHL (12 patients, including 10 patients in a cochlear implant work-up) or for neurologic impairment. Auditory brain stem response audiometry was performed for all the patients, and behavioral audiometry was performed if cooperation of the patient could be obtained. Six patients had an available complete vestibular evaluation.18 The age of walking was also recorded.
Molecular Analysis
Written consent for genetic testing was obtained. Genomic DNA was extracted from peripheral blood leukocytes by using standard protocols. The 3 coding exons of SOX10 were analyzed by direct sequencing as previously described,11 except for patients B and J, who were analyzed by denaturing high-performance liquid chromatography.19 Mutations were described according to the international nomenclature that is based on complementary DNA (cDNA) numbering, with +1 corresponding to the A of the ATG translation initiation codon in the cDNA reference sequence (GenBank NM_006941.3; www.ncbi.nlm.nih.gov/genbank).
Imaging
Examinations were performed at different institutions between February 2000 and March 2010. Eleven patients underwent both CT and MR imaging, 3 patients had only MR imaging (D1, F, and H) and 1 had only CT (L). For 13 patients, data were obtained by using compact disc or PACS, allowing multiplanar reconstructions for both CT and MR imaging and maximum intensity projection for MR imaging. Data from 2 patients, 1 with CT only and 1 with both CT and MR imaging, were only available on film in the axial and coronal planes.
All temporal bone CT scans were obtained on multisection CT scanners, with high-resolution helical acquisitions of ≤1-mm-thick sections. Multiplanar reconstruction in the plane of each SCC was obtained. All MR imaging scans included at least an axial 3D high-resolution T2 TSE submillimeter sequence of inner ear structures and a whole-brain study with T2-weighted sequences (FLAIR and/or T2 TSE) in ≥1 plane (coronal and/or axial).
Two radiologists (M.E.-B. and A.S., with 24 and 15 years of experience, respectively, in pediatric neuroradiology in a tertiary care university hospital) reviewed jointly the CT and MR imaging examinations. For each patient, they performed visual analysis of the following inner ear structures: cochlea (shape, number of turns, modiolus), vestibule (shape, size), SCCs (each was noted as absent or present, and when present, any anomaly in the shape and/or size was also described), and vestibular aqueduct/endolymphatic sac (size). All these data were provided by either CT or MR imaging. Measurements of inner ear structures were performed on CT. We measured the diameter of the bony canal of the cochlear nerve and the cochlear width at the level of the midturn and compared them with data from Fatterpekar et al20 and Teissier et al.21
In addition, we noted, from MR imaging, the presence or absence of the cochlear and vestibular nerves and recorded any associated abnormalities. For the 12 patients with CT, we also looked for middle ear abnormalities: hypopneumatization, ossicular deformities, round and/or oval window agenesis, and facial canal malposition.
Results
The molecular findings for the 14 independent patients included in the study are summarized in the On-line Table. They include 2 cases of WS2, 6 of WS4, and 6 of PCWH. Clinical descriptions have been previously published for patients A, F, K, and N11,14 and are provided as supplementary data (On-line Appendix) for the others, along with vestibular evaluation when available. All patients except 1 (L) had profound bilateral SNHL.
Inner ear imaging anomalies were bilateral in all cases and symmetric in 9 of the 15 cases (summarized in Table 1). The cochlea had a normal number of turns in 13/13 patients (this could not be ascertained in 2 patients, F and M, due to very hypoplastic cochleas). No incomplete partition was demonstrated, and the modiolus was present in 13/13 patients. The cochlea showed a “flattened” aspect of the midturn and apex (Figs 1 and 2) in 11/15 patients, compared with the normal mildly convex morphology of the middle and apical turns. Measurement of the CNC was possible for 10 patients, 9 of whom had CT and MR imaging. Only 1 patient (M) had bilateral CNC atresia and bilateral cochlear nerve agenesis. The mean value of the CNC diameter for our patients was 1.66 ± 0.35 mm, far below the normal value (2.13 ± 0.44 mm) found by Fatterpekar et al.20 Cochlear width was also smaller, with a mean value of 4.62 ± 0.43 mm, while the normal value reported by Teissier et al21 was 5.75 ± 0.43 mm. The vestibule was enlarged in all patients (29/30 ears), but only unilaterally in 1 case. Exact measurement was not possible due to the shape of the vestibule, which could not be isolated in many cases from the lateral SCC.
Table 1:
Inner ear imaging findings in patients included in this studya
| Patient | Age at MRI/CT | Cochlea | Cochlear Nerve | Vestibule | Posterior SCC | Superior SCC | Lateral SCC |
|---|---|---|---|---|---|---|---|
| A | ?/? | Flattened | Present | Enlarged | Agenesis | N | Large arch, thin |
| B | 32 mo/32 mo | Small | Present | Enlarged | Small arch, thick | Large arch, thin | Large arch, thin |
| C | 32 mo/32 mo | Small | Present | Enlarged | Small arch, thick | N | Small arch, thick |
| D1b | 5 wk/– | Small, flattened | Present | Enlarged | Small arch, thick | Small arch, thick | Small arch, thick |
| D2b | 2 mo/2 mo | Small, flattened | Present | Enlarged | N | R: small arch, thick L: N | Small arch, thick |
| E | 3 yr/3 yr | Flattened | Present | Enlarged | Agenesis | Agenesis | R: agenesis; L: small arch, thick |
| F | 2 mo/– | Small, flattened | Absent | Enlarged | Agenesis | Agenesis | R: agenesis; L: small, thin |
| G | 5 yr/5 yr | Flattened | Present | Enlarged | Agenesis | Agenesis | Agenesis |
| H | 18 yr/– | Small, flattened | R: present; L: present, thin | Enlarged | Agenesis | Agenesis | Small, thin |
| I | 20 mo/20 mo | Small, flattened | Present | Enlarged | Agenesis | Small arch, thick | R: small, thin, incomplete; L: small, thin |
| J | 4 yr/4 yr | Small, flattened | R: present; L: absent | Enlarged | Agenesis | Agenesis | Agenesis |
| K | 18 mo/18 mo | N | Present | Enlarged | Agenesis | Agenesis | Small, thin |
| L | –/16 yr | Small | N/A | R: enlarged; L: N | Small arch, thick | Agenesis | Small arch, thick |
| M | 8 days/ 8 days | Small, flattened | Absent | Enlarged | Agenesis | Agenesis | Agenesis |
| N | 31 yr/31 yr | Flattened | Present | Enlarged | Agenesis | Small arch, thick | Small arch, thick |
| Agenesis or absence (%) | 21 | 67 | 53 | 33 | |||
| Defect (%) | 93 | 29 | 100 | 93 | 87 | 100 |
Note:—N indicates normal; N/A, could not be analyzed; R, right; and L, left; –, not performed; ?, age unknown.
When right or left is not specified, the same findings were observed in both sides. In the case of an asymmetric defect, the percentage of absence and/or defects was calculated on the basis of the more severe side.
Brothers.
Fig 1.
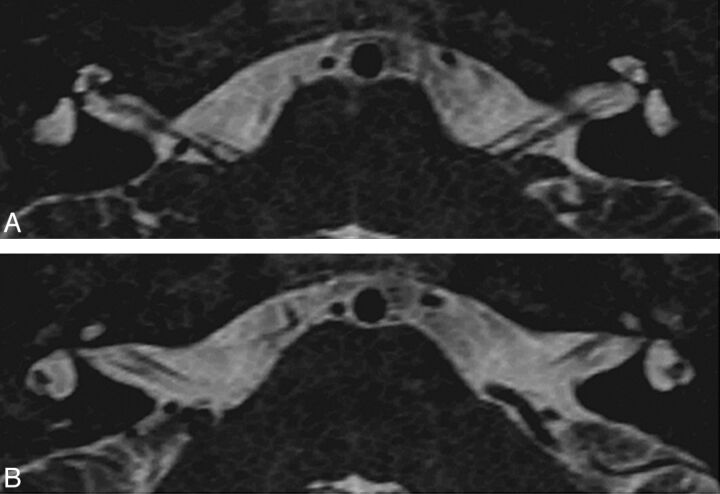
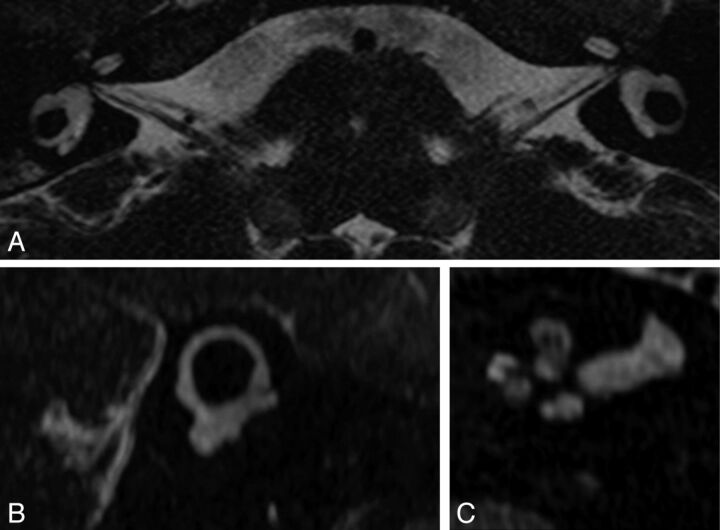
Patient H. High-resolution 3D T2 MR imaging. Axial views through the cochlea and lateral SCC show a cochlea reduced in size with a flattened aspect of the midturn and apex; an enlarged vestibule; and a lateral SCC with a small diameter, a thin arch, and a small bone island. The basal turn was normal in this patient (not shown). The left cochlea is thinner than the right; this finding was confirmed by oblique sagittal views perpendicular to the nerves (not shown).
Fig 2.
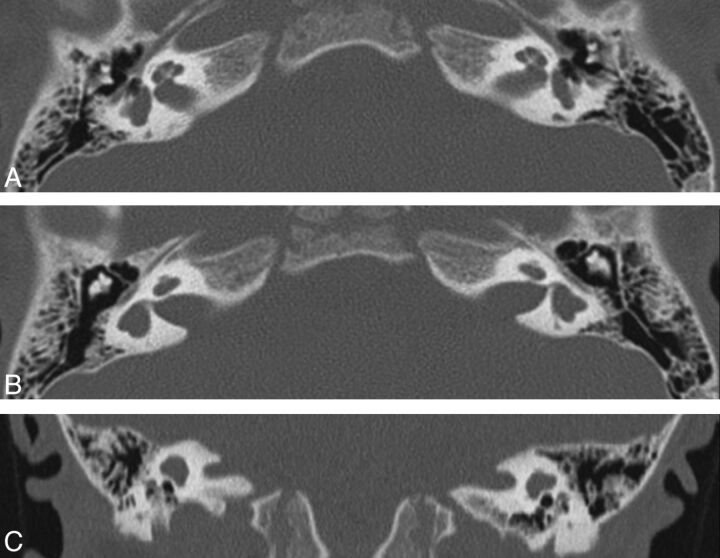
Patient J. CT. Axial views (A and B) through the cochlea and the vestibule show the flattened apex and midturn of the cochlea. The vestibular cavity is large and shows evaginations that could represent SCC anlages. The vestibular aqueduct is visible and is not dilated. Coronal view (C) favors the hypothesis of a lateral SCC anlagen. The superior SCCs are absent, but the superior margin of the vestibule is convex and a small anlagen cannot be excluded.
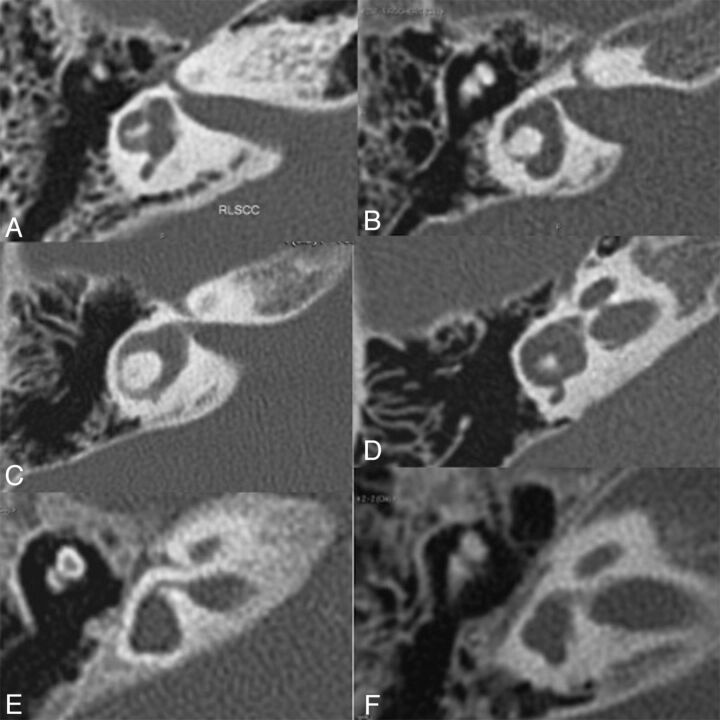
Because true agenesis of SCCs may be difficult to differentiate from a dysmorphic vestibule, an SCC was considered absent when no arch was visible, even if a small deformity of the vestibule, such as a small bud that could be an anlage, was present (Fig 3). The posterior SCCs were absent in 10 patients, had a thick arch of small diameter in 4, and were normal in 1 patient. The superior SCCs were absent in 8 patients, had a thick arch of small diameter in 4 (unilateral in 1 patient), had a thin arch of large diameter in 1, and were normal in 2. The lateral SCCs showed bilateral agenesis in 3 patients, a thick arch of small diameter in 5, a thin arch of small diameter in 3, and a thin arch of large diameter in 2. Two patients also had asymmetric findings with unilateral agenesis and a small arch on the other side. The vestibular aqueduct/endolymphatic sac was normal in all patients.
Fig 3.
Different patterns of semicircular canal abnormalities shown on high-resolution axial CT (right ear), confirmed by multiplanar reconstructions for the posterior and superior SCCs: posterior SCC with a thick arch of small diameter (A and D); posterior SCC agenesis or potential SCC anlage (B, C, E, and F); superior SCC agenesis or potential SCC anlage (E and F); lateral SCC agenesis or potential SCC anlage (E and F); lateral SCC with a thick arch of small diameter (A and D); lateral SCC with a thin arch of small diameter (B); and lateral SCC with a thin arch of large diameter (C).
The cochlear nerves were bilaterally absent in 2 of the 14 patients who underwent MR imaging and were unilaterally absent in 1 patient. Inferior and superior vestibular nerves were present in 12 patients; the inferior vestibular nerve was absent unilaterally in 1 case, and superior vestibular nerves were hardly visible in 2 neonates (F and M), who also displayed bilateral cochlear nerve agenesis.
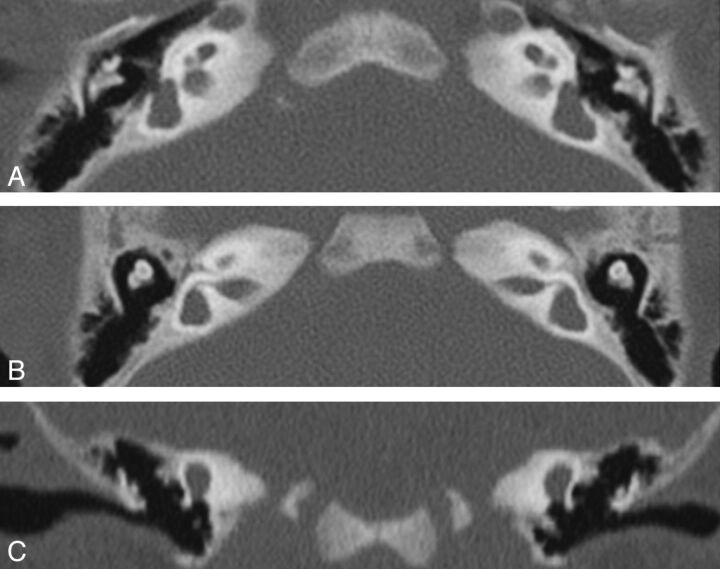
Overall, the most easily identifiable findings in patients were agenesis of or anomalies in the shape and/or size of ≥1 SCC (lateral, 100%; posterior, 93%; superior, 87%). Three patients of the 15 had agenesis of all 3 SCCs (Fig 4). None of the patients with CT had middle ear malformations.
Fig 4.
Patient M. CT. Axial views (A and B) through the cochlea and the vestibule show a small cochlea, flattened with a partition hardly visible and atresia of cochlear nerve canals, an enlarged vestibular cavity, and agenesis of all of the semicircular canals. Coronal view (C) confirms the absence of the superior and lateral SCC. Posterior deformity of the vestibule cannot exclude an anlage.
Additional imaging findings are summarized in Table 2. The facial nerve was unilaterally absent in 1 of the patients with bilateral agenesis of the cochlear nerve. White matter signal anomalies were found in 7 patients (5 with PCWH, 1 with WS4, and 1 with WS2), involving the supratentorial and/or infratentorial white matter (Fig 5). Agenesis of the olfactory bulbs was found in 7 of the 8 patients whose olfactory apparatus could be analyzed on the supplied MR images. Incidentally, there was also parotid (12/14) and lacrimal (11/14) gland absence or hypoplasia.
Table 2:
Summary of other imaging findings in patients included in this study
| Patient | Age at MRI/CT | Facial Nerve | Posterior Fossa | White Matter | Olfactory Bulbs | Lacrimal Glands | Parotid Glands |
|---|---|---|---|---|---|---|---|
| A | ?/? | Present | N | N | N/A | Hypoplastic | N/A |
| B | 32 mo/32 mo | Present | ICP | Abnormal | Agenesis ×2 | Hypoplastic | Hypoplastic |
| C | 32 mo/32 mo | Present | N | N | Agenesis ×2 | N | N |
| D1a | 5 wk/– | Present | N | Abnormal | N/A | Hypoplastic | Hypoplastic |
| D2a | 2 mo/2 mo | Present | N | N | N/A | Hypoplastic | Hypoplastic |
| E | 3 yr/3 yr | Present | N | N | Agenesis ×2 | Hypoplastic | Hypoplastic |
| F | 2 mo/– | Right agenesis | Hypoplastic brain stem | Abnormal | Agenesis ×2 | Absent | Hypoplastic |
| G | 5 yr/5 yr | Present | N | N | N/A | N | Hypoplastic |
| H | 18 yr/– | Present | N | Large VRS | Agenesis ×2 | Absent | Hypoplastic |
| I | 20 mo/20 mo | Present | N | Abnormal | Agenesis ×2 | N | N |
| J | 4 yr/4 yr | Present | N | N | N/A | Hypoplastic | Hypoplastic |
| K | 18 mo/18 mo | Present | N | Abnormal | N/A | Hypoplastic | Hypoplastic |
| L | –/16 yr | N/A | N/A | N/A | N/A | N/A | Hypoplastic |
| M | 8 days/ 8 days | Present | N | Abnormal | Presents | Hypoplastic | Hypoplastic |
| N | 31 yr/31 yr | Present | N | N/A | Agenesis ×2 | Hypoplastic | Hypoplastic |
| Agenesis or absence (%) | 7 | 88 | 14 | ||||
| Defect (%) | 14 | 50 | 79 | 85 |
Note:—N indicates normal; N/A, could not be analyzed; R, right; and L, left; –, not performed; ?, age unknown; ICP, inferior cerebellar peduncles; VRS, Virchow Robin spaces; ×2, similar findings on both sides.
Brothers.
Fig 5.
Patient B. 3D T2 TSE MR image driven equilibrium (Philips). Reformatted axial view in the lateral SCC plane (A) and maximum intensity projection of the superior and posterior SCC (B and C). Same patient as in Fig 2C. Axial view shows a short linear structure along the posterior aspect of the lateral SCC, but multiplanar reformations and maximum intensity projection demonstrate a deformity of the posterior aspect of the vestibule and no posterior SCC. The superior SCC has a thin arch with a large diameter. Hypersignal of the inferior cerebellar peduncles is noticeable due to hypomyelination.
Discussion
Abnormalities of the labyrinth have previously been reported in WS. In a review of the literature combined with their own data, Oysu et al9 found abnormalities in 6 of 36 cases in radiologic studies (polytomography and CT examinations). SCC abnormalities were the most frequent finding, with hypoplastic cochleae also observed. None of these studies were conducted in conjunction with molecular analysis, however, and most did not account for WS types. We and others recently reported isolated cases of inner ear abnormalities in patients with SOX10 mutations. In this article, we report a series of imaging results associated with molecular analysis (SOX10 mutations) in WS. In addition to confirming SCC defects and cochlear abnormalities, this series allows further conclusions to be drawn regarding this genetic subtype of WS.
Even though there is an inclusion bias in that only patients with reported abnormalities of the temporal bone were included, this study allowed analysis of the different patterns and their relative prevalence. Cochlear and vestibular morphologic abnormalities were similar in frequency to each other (Table 1: cochlear abnormalities 93%, vestibular abnormalities 100%). Although all SCCs showed a comparable frequency of abnormalities, the agenesis pattern had a more specific distribution. Posterior SCC agenesis was seen with the highest frequency and was followed by agenesis of the superior SCC and lateral SCC, respectively.
Generally, posterior and superior SCC abnormalities are rare, and lateral SCC abnormalities are frequent, possibly due to the later formation of the lateral SCC during embryogenesis.22 The frequency of posterior and superior SCC agenesis found in patients with SOX10 mutations does not follow the hypothesis of Jackler et al,23 which stipulates that radiographically detectable malformations correlate with arrested inner ear organogenesis at different stages. Agenesis of posterior and superior SCCs being rare findings, they may be more evocative of a SOX10 mutation than agenesis of the lateral SCC.
Total absence of the SCCs is found in coloboma, heart malformation, choanal atresia, growth retardation, genital hypoplasia, and ear abnormalities (CHARGE) syndrome, often with a hypoplastic vestibule and variable cochlear deformities, including abnormal partitioning; moreover, cochlear nerve canal atresia with cochlear nerve aplasia is common.10,24 While absence of all the SCCs is more common, absence of only 1 or 2 has also been described in CHARGE.25 Nevertheless, abnormalities of the middle ear structures, such as ossicular malformations, facial canal malposition, and oval window aplasia, are usually associated,24 and the clinical spectrum in CHARGE is different from that in WS. Total or partial absence of SCCs has also been described in Noonan and Alagille syndromes. Noonan syndrome shows a dysmorphic external ear and may also present with middle ear abnormalities.26 Histopathologic27 and CT28 studies of Alagille syndrome have shown partial agenesis of the posterior SCC, a hypoplastic superior SSC, and a normal lateral SCC.
We found inner ear abnormalities in patients with both WS2 and WS4, which, in some cases, also presented neurologic symptoms (eg, PCWH). This finding correlates with the hypothesis of Oysu et al9 that inner ear malformations are often present in WS2 but not in WS1, because 15% of WS2 cases (and no WS1 cases) are caused by SOX10 mutations.2 The malformations we describe in this article have not been associated with other WS genes and may represent pathognomonic features of SOX10 molecular defects. However, within SOX10-linked WS, we did not find a correlation between the type of WS (WS2, WS4, or PCWH) and the type or severity of the inner ear malformations observed.
SOX10 is a regulator of neural crest development.29 It is particular among WS genes for its early expression in inner ear development: in the otic placode and the otic vesicle, then in the developing epitheliums of the cochlea and vestibule before being restricted to supporting cells of the neurosensory epithelium. SOX10 has been shown to promote the survival of cochlear progenitors during otocyst formation and the establishment of the organ of Corti.30 It is also expressed persistently in the cochleovestibular ganglia and plays a role in glial development.30–34
In Sox10 homozygous mutant mice, the differentiation and cellular organization of the organ of Corti and the vestibule appear normal. However, the cochlear duct is shortened.30 In humans, where SOX10 mutations are heterozygous, vestibular abnormalities are much more apparent than those in mice. Other differences have been observed across species: In Sox10 mutant zebrafish, the otic vesicle is smaller or distended, semicircular canals have a delayed development and are thinner than normal, and the sensory epithelia are incorrectly patterned.35 These expression and functional studies, in combination with our findings, suggest that cochleosaccular degeneration is not the only mechanism leading to SNHL in patients carrying SOX10 mutations.
It is tempting to correlate the high frequency of vestibular malformations to vestibular dysfunction. The prevalence of vestibular dysfunction in WS is not well-established but may be high.36 Results of vestibular function tests were only available for 6 of our patients. The most severe imaging pattern (agenesis of all canals) was associated with complete loss of vestibular function. However, in the case of ≥1 hypoplastic canal, the vestibular function was not constantly impaired; this finding could signify that some hypoplastic canals contain functional sensory epithelium.
The large majority of our patients were implant candidates, so assessment of the cochlear nerve was mandatory. Two patients, both with a PCWH phenotype, had bilateral cochlear nerve agenesis and CNC atresia. In addition, 1 patient with WS4 had a unilateral cochlear nerve agenesis with a patent CNC on CT, and 1 patient with PCWH had a unilaterally thin cochlear nerve compared with the contralateral nerve on the oblique sagittal plane perpendicular to the internal auditory canal.
Previous articles have described in detail white matter abnormalities associated with SOX10 mutations in patients with neurologic impairments (for examples see Pingault et al,2 Inoue et al,13 Barnett et al,17 and Inoue et al37). The presence of such defects in this cohort was correlated to the PCWH phenotype, with the notable exceptions of patients D1 and I. A more surprising finding was the very high percentage of agenesis or hypoplasia of olfactory bulbs and lacrimal and parotid glands, which existed in 79%–88% of these patients with temporal bone abnormalities (Table 2). Olfactory bulb agenesis is also commonly found in CHARGE syndrome. The high percentages found here are likely to contribute to anosmia and possibly, in parallel to the previously suspected dysautonomia,38 to alacrima and asialia, which have been reported in a limited number of patients with PCWH.11,33,39 Of note, these imaging findings were not limited to patients with neurologic impairments in the cohort presented here.
Conclusions
The inner ear abnormalities we characterized in our patients (SCC agenesis or maldevelopment, enlarged vestibule, and cochlear deformity [hypoplastic, flattened]) are suggestive and should lead radiologists to consider the possibility of WS linked to a SOX10 mutation in the proper clinical context. Other anomalies such as aplasia or hypoplasia of the cochlear nerve, myelination defects, and frequent agenesis of olfactory bulbs and lacrimal and parotid glands were also present in this patient population.
Supplementary Material
Acknowledgments
We thank Profs/Drs L. Van Maldergem, J. Amiel, D. Mowat, I. Giurgea, R. Touraine, and F. Rubin for communicating inner ear imaging of patients and/or completing clinical data. We gratefully acknowledge Dr S. Wiener-Vacher (Vestibular and Oculomotor Evaluation Unit, Otorhinolaryngology Department, Hôpital Pediatrique Robert Debré, Paris, France) who performed vestibular evaluations.
ABBREVIATIONS:
- CNC
cochlear nerve canal
- PCWH
peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, Hirschsprung disease
- SCC
semicircular canals
- SNHL
sensorineural hearing loss
- WS
Waardenburg syndrome
- WS1 to WS4
Waardenburg syndrome types 1–4
Footnotes
M.E.-B. performed MRI and CT reading and assisted in writing of the article. C.B. was responsible for clinical diagnosis, addressing samples for molecular studies, and clinical description of 5 patients, N.N.-P. performed clinical diagnosis, addressing samples for molecular studies and clinical description of 1 patient and analysis of audiometric data, A.S. was responsible for MRI and CT reading. V.C., K.D., M.W., and S.M. performed clinical diagnosis, addressing samples for molecular studies, communicating imaging, and clinical description for 1 or 2 patients. G.S. was head of the department of pediatric radiology at Hôpital Robert Debré. V.P. performed molecular analysis and interpretation, collected data and images, and assisted in writing of the article.
REFERENCES
- 1. Waardenburg PJ. A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am J Hum Genet 1951;3:195–253 [PMC free article] [PubMed] [Google Scholar]
- 2. Pingault V, Ente D, Dastot-Le Moal F, et al. Review and update of mutations causing Waardenburg syndrome. Hum Mutat 2010;31:391–406 [DOI] [PubMed] [Google Scholar]
- 3. Merchant SN, McKenna MJ, Baldwin CT, et al. Otopathology in a case of type I Waardenburg's syndrome. Ann Otol Rhinol Laryngol 2001;110:875–82 [DOI] [PubMed] [Google Scholar]
- 4. Nakashima S, Sando I, Takahashi H, et al. Temporal bone histopathologic findings of Waardenburg's syndrome: a case report. Laryngoscope 1992;102:563–67 [DOI] [PubMed] [Google Scholar]
- 5. Rarey KE, Davis LE. Inner ear anomalies in Waardenburg's syndrome associated with Hirschsprung's disease. Int J Pediatr Otorhinolaryngol 1984;8:181–89 [DOI] [PubMed] [Google Scholar]
- 6. Higashi K, Matsuki C, Sarashina N. Aplasia of posterior semicircular canal in Waardenburg syndrome type II. J Otolaryngol 1992;21:262–64 [PubMed] [Google Scholar]
- 7. Madden C, Halsted MJ, Hopkin RJ, et al. Temporal bone abnormalities associated with hearing loss in Waardenburg syndrome. Laryngoscope 2003;113:2035–41 [DOI] [PubMed] [Google Scholar]
- 8. Marcus RE. Vestibular function and additional findings in Waardenburg's syndrome. Acta Otolaryngol 1968;229 (Suppl.):1–30 [PubMed] [Google Scholar]
- 9. Oysu C, Oysu A, Aslan I, et al. Temporal bone imaging findings in Waardenburg's syndrome. Int J Pediatr Otorhinolaryngol 2001;58:215–21 [DOI] [PubMed] [Google Scholar]
- 10. Huang BY, Zdanski C, Castillo M. Pediatric sensorineural hearing loss, Part 2: Syndromic and acquired causes. AJNR Am J Neuroradiol 2012;33:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bondurand N, Dastot-Le Moal F, Stanchina L, et al. Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am J Hum Genet 2007;81:1169–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pingault V, Bondurand N, Kuhlbrodt K, et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet 1998;18:171–73 [DOI] [PubMed] [Google Scholar]
- 13. Inoue K, Khajavi M, Ohyama T, et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet 2004;36:361–69 [DOI] [PubMed] [Google Scholar]
- 14. Pingault V, Girard M, Bondurand N, et al. SOX10 mutations in chronic intestinal pseudo-obstruction suggest a complex physiopathological mechanism. Hum Genet 2002;111:198–206 [DOI] [PubMed] [Google Scholar]
- 15. Viñuela A, Morin M, Villamar M, et al. Genetic and phenotypic heterogeneity in two novel cases of Waardenburg syndrome type IV. Am J Med Genet A 2009;149A:2296–302 [DOI] [PubMed] [Google Scholar]
- 16. Sznajer Y, Coldea C, Meire F, et al. A de novo SOX10 mutation causing severe type 4 Waardenburg syndrome without Hirschsprung disease. Am J Med Genet A 2008;146A:1038–41 [DOI] [PubMed] [Google Scholar]
- 17. Barnett CP, Mendoza-Londono R, Blaser S, et al. Aplasia of cochlear nerves and olfactory bulbs in association with SOX10 mutation. Am J Med Genet A 2009;149A:431–36 [DOI] [PubMed] [Google Scholar]
- 18. Wiener-Vacher SR. Vestibular disorders in children. Int J Audiol 2008;47:578–83 [DOI] [PubMed] [Google Scholar]
- 19. Chaoui A, Watanabe Y, Touraine RL, et al. Identification and functional analysis of SOX10 missense mutations in different subtypes of Waardenburg syndrome. Hum Mutat 2011;32:1436–49 [DOI] [PubMed] [Google Scholar]
- 20. Fatterpekar GM, Mukherji SK, Alley J, et al. Hypoplasia of the bony canal for the cochlear nerve in patients with congenital sensorineural hearing loss: initial observations. Radiology 2000;215:243–46 [DOI] [PubMed] [Google Scholar]
- 21. Teissier N, Van Den Abbeele T, Sebag G, et al. Computed tomography measurements of the normal and the pathologic cochlea in children. Pediatr Radiol 2010;40:275–83 [DOI] [PubMed] [Google Scholar]
- 22. Lemmerling M, Vanzieleghem B, Dhooge I, et al. CT and MRI of the semicircular canals in the normal and diseased temporal bone. Eur Radiol 2001;11:1210–19 [DOI] [PubMed] [Google Scholar]
- 23. Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 1987;97:2–14 [DOI] [PubMed] [Google Scholar]
- 24. Lemmerling M, Dhooge I, Mollet P, et al. CT of the temporal bone in the CHARGE association. Neuroradiology 1998;40:462–65 [DOI] [PubMed] [Google Scholar]
- 25. Delahaye A, Sznajer Y, Lyonnet S, et al. Familial CHARGE syndrome because of CHD7 mutation: clinical intra- and interfamilial variability. Clin Genet 2007;72:112–21 [DOI] [PubMed] [Google Scholar]
- 26. Naficy S, Shepard NT, Telian SA. Multiple temporal bone anomalies associated with Noonan syndrome. Otolaryngol Head Neck Surg 1997;116:265–67 [DOI] [PubMed] [Google Scholar]
- 27. Okuno T, Takahashi H, Shibahara Y, et al. Temporal bone histopathologic findings in Alagille's syndrome. Arch Otolaryngol Head Neck Surg 1990;116:217–20 [DOI] [PubMed] [Google Scholar]
- 28. Koch B, Goold A, Egelhoff J, et al. Partial absence of the posterior semicircular canal in Alagille syndrome: CT findings. Pediatr Radiol 2006;36:977–79 [DOI] [PubMed] [Google Scholar]
- 29. Haldin CE, LaBonne C. SoxE factors as multifunctional neural crest regulatory factors. Int J Biochem Cell Biol 2010;42:441–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Breuskin I, Bodson M, Thelen N, et al. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of Corti. Dev Biol 2009;335:327–39 [DOI] [PubMed] [Google Scholar]
- 31. Bondurand N, Kobetz A, Pingault V, et al. Expression of the SOX10 gene during human development. FEBS Lett 1998;432:168–72 [DOI] [PubMed] [Google Scholar]
- 32. Breuskin I, Bodson M, Thelen N, et al. Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J Neurochem 2010;114:1827–39 [DOI] [PubMed] [Google Scholar]
- 33. Touraine RL, Attie-Bitach T, Manceau E, et al. Neurological phenotype in Waardenburg syndrome type 4 correlates with novel SOX10 truncating mutations and expression in developing brain. Am J Hum Genet 2000;66:1496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe K, Takeda K, Katori Y, et al. Expression of the Sox10 gene during mouse inner ear development. Brain Res Mol Brain Res 2000;84:141–45 [DOI] [PubMed] [Google Scholar]
- 35. Dutton K, Abbas L, Spencer J, et al. A zebrafish model for Waardenburg syndrome type IV reveals diverse roles for Sox10 in the otic vesicle. Dis Model Mech 2009;2:68–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Black FO, Pesznecker SC, Allen K, et al. A vestibular phenotype for Waardenburg syndrome? Otol Neurotol 2001;22:188–94 [DOI] [PubMed] [Google Scholar]
- 37. Inoue K, Tanabe Y, Lupski JR. Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol 1999;46:313–18 [DOI] [PubMed] [Google Scholar]
- 38. Korsch E, Steinkuhle J, Massin M, et al. Impaired autonomic control of the heart by SOX10 mutation. Eur J Pediatr 2001;160:68–69 [DOI] [PubMed] [Google Scholar]
- 39. Unzicker A, Pingault V, Meyer T, et al. A novel SOX10 mutation in a patient with PCWH who developed hypoxic-ischemic encephalopathy after E. coli sepsis. Eur J Pediatr 2011;170:1475–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.