Abstract
BACKGROUND AND PURPOSE:
Imaging of bisphosphonate-induced osteonecrosis of the jaw is essential for surgical planning. We compared the extent of BONJ on contrast-enhanced MR imaging, [18F] fluoride PET/CT, and panoramic views derived from standard conebeam CT with clinical pre- and intraoperative examinations.
MATERIALS AND METHODS:
Between February 2011 and January 2012, ten subjects with written informed consent (9 women; mean, 69.6 years; range, 53–88 years) were included in this prospective ethics-board-approved study. Patients underwent CEMR imaging, [18F] fluoride PET/CT, and CBCT and were clinically examined pre- and intraoperatively. Surgery was performed, and BONJ was histologically confirmed in 9 patients. Location and extent of BONJ on different modalities/examinations were graphically compared (0 = no pathologic finding, 1 = smallest, 5 = largest extent of BONJ). Rank tests were used to assess overall and paired differences of ratings in 9 patients. A P value <.05 was considered statistically significant.
RESULTS:
Significant differences in BONJ extent among different modalities and examinations were found (P < .001). The highest median rank was seen in PET/CT (4 ± 1.12) and CEMR imaging (4 ± 1.01), followed by intraoperative examinations (3 ± 0.71), CBCT (2 ± 0.33), and preoperative examinations (1 ± 0). No significant differences were found between PET/CT and CEMR imaging (P = .23), except when comparing PET/CT to either CBCT, pre- and intraoperative examinations (all P < .05). Preoperative examinations showed significantly less extensive disease than all other modalities/examinations (all P < .05).
CONCLUSIONS:
[18F] fluoride PET/CT and CEMR imaging revealed more extensive involvement of BONJ compared with panoramic views from CBCT and clinical examinations.
Osteonecrosis of the jaw is a known complication in patients treated with bisphosphonates.1,2 The prevalence of bisphosphonate-induced osteonecrosis of the jaw depends on whether patients are receiving intravenous bisphosphonate treatment (eg, for cancer) or less powerful oral bisphosphonates (eg, for osteoporosis) and has been reported to be 1%–10% and 0.0004%–0.04%, respectively.3,4 The mandible is more often affected than the maxilla, and rarely are both bones affected at the same time.5 Although the exact pathophysiology of BONJ is not clear, potential causes include inhibition of bone turnover at sites of oral trauma (eg, tooth extraction), infection with Actinomyces israelii, soft-tissue toxic effects of bisphosphonates with resulting mucosal ulceration, direct toxic effects on bone, impaired bone remodeling, high masticatory forces, and exposure of the tooth socket to an infected environment.6 Antimicrobial rinses and systemic antibiotics are used for therapy, but sometimes surgery is required to remove necrotic bone.
In addition to clinical examinations, radiologic imaging is the key to an assessment and quantification of the extent of BONJ. Usually panoramic radiography and, more recently, conebeam CT are part of a standard preoperative imaging work-up.7 Multidetector CT or contrast-enhanced MR imaging might be performed to diagnose early stages of BONJ, rule out malignancy, diagnose pathologic fractures, monitor disease progression, or evaluate the extent of necrotic bone areas preoperatively.8 Literature is sparse regarding which imaging technique should be used, however; and little is known about the use of new imaging modalities such as [18F] fluoride PET/CT in this setting. This technique uses radioactive fluoride, a tracer with high affinity to bone, which has proved to be effective in the prediction of bone viability after trauma or reconstructive surgery and in the detection of bone changes secondary to intense sports activity.9 Dynamic CEMR imaging is less specific for the assessment of bone viability10 but does allow assessment of pathologies affecting bone, notably the medullary cavity, and adjacent soft tissues.11 Our hypothesis was that CEMR imaging and [18F] fluoride PET/CT best quantify the preoperative extent of BONJ and add value to clinical examinations and standard radiologic imaging with panoramic views of the jaw from CBCT.
Thus, the purpose of this study was to compare the extent of changes compatible with BONJ on CEMR imaging, [18F] fluoride PET/CT, and panoramic views derived from standard CBCT of the jaw with clinical preoperative and intraoperative examinations.
Materials and Methods
This prospective study was approved by our institutional ethics board, and all patients gave written informed consent for study inclusion.
Inclusion criteria were clinical evidence for osteonecrosis of the jaw following bisphosphonate intake (ie, exposed necrotic bone in the maxillofacial region that has persisted for >8 weeks). Exclusion criteria were contraindications for MR imaging; pregnancy; age <18 years; extensive metallic implants of the jaw; prior radiation therapy, reconstruction, or resection of the jaw; and previous surgical treatment of BONJ. All subjects underwent CEMR imaging and PET/CT on the same day, with a maximum delay between examinations of no more than 4 hours. Clinical examination and CBCT were performed as close as possible afterward with a maximum delay of 3 weeks and in proximity to scheduled surgery.
In total, 12 study subjects fulfilling inclusion criteria were consecutively included from February 2011 to January 2012. Two patients were subsequently excluded due to extensive metallic implants and previous surgical treatment. Thus, 10 patients (9 women, 1 man; mean age, 69.6 ± 13.5 years; range, 53–88 years) were included in this study (Table). All patients underwent surgery with additional microbiologic and histologic analysis of resected specimens, except for 1 female patient (88 years of age) due to poor health. This patient was excluded from statistical analysis.
Patient demographics with disease, type, and duration of prior bisphosphonate intake, histology, and location/region of BONJ
| Patient | Sex | Age (yr) | Disease | Bisphosphonate | Duration of Treatment | Histology | Region |
|---|---|---|---|---|---|---|---|
| 1 | F | 56 | Metastases, breast Ca | Zoledronic acid (Zometa) | 54 mo | ON/OM | 23–28 |
| 2 | F | 85 | MM | Zoledronic acid (Zometa) | 48 mo | ON/OM | 44–46 |
| 3 | F | 64 | MM | Zoledronic acid (Zometa) | 48 mo | ON | 34–37 |
| 4 | F | 60 | Metastases, breast Ca | Zoledronic acid (Zometa) | 48 mo | ON | 35–36 and 45 |
| 5 | F | 59 | Metastases, mamma Ca | Zoledronic acid (Zometa) | 36 mo | ON | 14–17 |
| 6 | F | 53 | Osteoporosis | Ibandronic acid (Bondronat oral) | 12 mo | ON | 45–47 |
| 7 | F | 86 | Osteoporosis | Ibandronic acid (Bondronat oral) | Not available | ON | 45–47 |
| 8 | F | 79 | Metastases, breast Ca | Zoledronic acid (Zometa) | 7 mo | ON | 11–15 |
| 9 | M | 66 | MM | Zoledronic acid (Zometa) | 60 mo | ON | 14–15 |
| 10 | F | 88 | Metastases, breast Ca | Zoledronic acid (Zometa) | Not available | Not operated | 17–18 |
Note:—ON indicates osteonecrosis; OM, osteomyelitis; MM, multiple myeloma; Ca, cancer; Mamma, mammary.
MR Imaging
MR imaging was performed on a 1.5T scanner (Signa Excite HDxt; GE Healthcare; Milwaukee, Wisconsin), which was equipped with a high-performance gradient system (gradient amplitude, 40 mTm−1; slew rate, 200 Tm−1second−1). An 8-channel transmit-receive head coil was used. All study subjects were imaged in the supine position with the jaw at the scanner isocenter. We acquired the following imaging series: standard transaxial and coronal T1 (TR, 300–600 ms; TE, minimum full; FOV, 22 × 22 cm; section thickness, 3 mm; spacing. 0.9 mm; NEX, 2; echo-train length, 3) and T2-weighted spin-echo images (TR, 3000–6000 ms; TE, 85 ms; FOV, 22 × 22 cm; section thickness, 3 mm; spacing, 1 mm; NEX, 2; echo-train length, 13), a coronal STIR series (TR, 3500–5000 ms; TE, 30 ms; FOV, 20 × 20 cm; section thickness, 3 mm; spacing, 1 mm; NEX, 1; echo-train length, 9), an axial dynamic contrast-enhanced 3D spoiled gradient-echo series with fat-signal suppression (LAVA, GE Healthcare) (TE, minimum full; FOV, 25 × 25 cm; section thickness, 2.6 mm; NEX, 0.5; echo-train length, 9; flip angle, 10°) after injection of 0.2 mL per kg of body-weight of patient of gadolinum-based contrast agent (gadoteric-acid, 0.5 mmol gadolinium/mL, Dotarem; Guerbet, Roissy, France), followed by a transaxial T1-weighted fat-suppressed image series (TR, 300–600 ms; TE, minimum full; FOV, 22 × 22 cm; section thickness, 3 mm; spacing, 1 mm; NEX, 1; echo-train length, 3; flip angle, 10°). Images were then sent and stored on the PACS of our department (Fig 1A, -B).
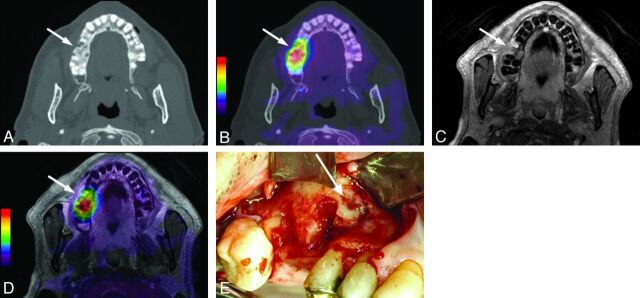
Fig 1.
Transaxial images of the upper jaw from patient 9 with BONJ in the right maxilla (regions 14–15). A, Axial CT image in a bone window depicting osteolysis and fragmentation of bone in a BONJ focus (arrow). B, CT image with fused [18F] fluoride PET signal indicating increased [18F] fluoride uptake (arrow) overlapping the morphologic BONJ focus in A. C, Axial contrast-enhanced T1-weighted MR image with fat saturation shows slightly increased contrast uptake of the BONJ focus without marked soft-tissue involvement (arrow). D, CEMR image with fused [18F] fluoride PET signal indicates slightly larger BONJ extent than expected from the CEMR image alone (arrow). E, Clinical intraoperative examination confirms BONJ in regions 14–15 of the right maxilla (white arrow).
[18F] Fluoride PET/CT Imaging
Patients were examined in the supine position on a PET/CT system (Discovery RX or Discovery STE; GE Healthcare). A dedicated thin-section CT (section thickness, 1.25 mm; FOV, 36 cm) covering the maxilla and mandible was performed (soft-tissue and bone reconstruction kernels). The same CT raw data were used for PET attenuation-correction (section thickness, 3.75 mm; FOV, 50 cm; 100–600 automatic mA; 120 kV; 0.5-second rotation time).
Static PET scanning was started 30 minutes after intravenous injection of a mean dose of 91 MBq (range, 63–112 MBq) of [18F] fluoride. 3D PET emission data were acquired for 3 minutes in 1 bed position covering the same scan range as that in CT. Attenuation-correction was performed, and images were reconstructed by using a fully 3D iterative algorithm (VUE Point HD; GE Healthcare). The acquired images were postprocessed with dedicated software (Volume Viewer PET/CT, Advantage 4.4 workstation; GE Healthcare) providing multiplanar reformatted images of PET alone, CT alone, and fused PET/CT with linked cursors (Fig 1C, -D).
Conebeam CT
A CBCT unit, KaVo 3D eXam (KaVo, Biberach, Germany) with an amorphous silicium flat panel detector (20 × 25 cm), was used. The exposure volume was set at a height of 102 mm. The voxel size was 0.4 mm. The scan was set at a high-frequency constant potential of 120 kV(peak), and the occlusal plane of each patient was set parallel to the floor base by using ear rods and a chin rest. The calculated DICOM data were transferred and evaluated by the software viewer of the system (CliniView, KaVo).
Visual Image Evaluation
Visual evaluation of images was performed by 3 independent readers. MR images were analyzed by a first reader (R.G., with 5 years of experience in head and neck imaging), while evaluation of PET/CT images was performed by a second reader (D.T.S., with 8 years of experience in nuclear medicine imaging). A third reader (P.M., with 5 years of experience in craniomaxillofacial and oral surgery) independently evaluated the location and extent of BONJ foci on panoramic views from standard preoperative CBCT images.
Findings of clinical preoperative and intraoperative examinations of the jaw (Fig 1E) were independently noted by a fourth reader (C.J., with 8 years of experience in craniomaxillofacial and oral surgery) who either performed surgery or extracted information on BONJ extent from the patient's history.
Jaw schemes (line diagrams of the teeth and mandible/maxilla) were used to semiquantitatively evaluate the location and extent of suspected BONJ. On these schemes, borders of suspected disease extent were neatly drawn by using each tooth as a reference for anteroposterior extension. For disease extension in supero-inferior direction, the alveolar border, on the one hand, and the floor of the maxillary sinus in the maxilla and the canal of the inferior alveolar nerve in the mandible, on the other hand, served as landmarks.
MR Image Evaluation
T1, T2, STIR, and contrast-enhanced MR images were visually assessed. BONJ was identified as regions within the jaw bones with decreased signal of the bone marrow on T1-weighted images; and increased signal on T2-weighted and STIR images was considered confirmation of BONJ. Contrast uptake of the suspicious bone and/or the adjacent soft tissues was additionally evaluated and, if present, was taken as evidence for BONJ, especially in doubtful cases in which intermediate or low signal on T2-weighted images was seen. However, quantification of disease extent was eventually based on precontrast T1- and T2-weighted images only.
[18F] Fluoride PET/CT Evaluation
Evaluation of [18F] fluoride PET/CT was performed on screen by using a dedicated workstation (Advantage 4.4; GE Healthcare). The extent of pathologically increased tracer uptake of the jaw as visualized on PET was anatomically assigned by using the coregistered CT and noted on the jaw scheme. Fluoride uptake had to be at least 2 times higher than physiologic uptake of healthy-appearing bone. Maximum standardized uptake values, corrected for body weight, were used to measure [18F] fluoride uptake. Depending on the extent of BONJ/increased tracer uptake, we used normal bone of the contralateral side of the jaw, the cervical vertebrae, and the skull base as the normal reference.
CBCT Evaluation
Standard preoperative CBCT with generation of panoramic views of the jaw was analyzed to delineate the extent of BONJ foci on jaw schemes. Areas of osteolysis with formations of sequestrum or thickening or fragmentation of the cortex or both, with narrowing of the marrow space surrounded by increased sclerosis of bone, were regarded as signs of BONJ on CBCT.
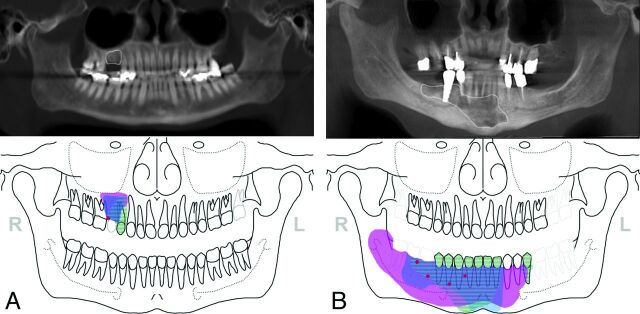
Location and extent of BONJ as marked on jaw schemes (ie, CEMR imaging, fluoride PET/CT, and preoperative clinical and intraoperative examinations) were eventually graphically assembled in 1 image and related to CBCT delineations to compare and visually rank the extent of BONJ among different modalities/examinations (0 = no pathologic finding, 1 = smallest, 5 = largest extent of BONJ) (Fig 2A, -B). Discordant findings among modalities were separately noted.
Fig 2.
Location and extent of BONJ as marked on jaw schemes from contrast-enhanced MR imaging (blue), [18F] fluoride PET/CT (pink), clinical preoperative (red), and intraoperative examinations (striated areas) were graphically assembled in 1 image and related to delineations (white contours) on panoramic views from CBCT to compare the extent of BONJ among different modalities/examinations. A, In patient 9, only a small clinically suspicious area in the right maxilla was seen. PET/CT and CEMR imaging showed slightly larger areas of suspected BONJ than CBCT and intraoperative examinations in the right maxilla (regions 14–15). BONJ was histologically proved. B, In patient 2, BONJ was suspected preoperatively in the regions 44–46 of the right mandible. The actual extent of the BONJ was markedly larger than expected clinically and on CBCT in both CEMR imaging and PET/CT. This was confirmed intraoperatively, where only parts of the necrotic bone were removed.
Statistical Analysis
All statistical analyses were performed by using the Statistical Package for the Social Sciences software (SPSS, Chicago, Illinois). The Friedman 2-way ANOVA test by ranks was used to test for overall differences between ratings of BONJ extent among modalities and examinations. The Wilcoxon matched-pair signed-rank test was used for paired differences of BONJ extent ratings between modalities and examinations. Intraoperative disease-extent quantification and, alternatively, clinical examination and CBCT were regarded as the reference standard. A P value <.05 was considered statistically significant.
Results
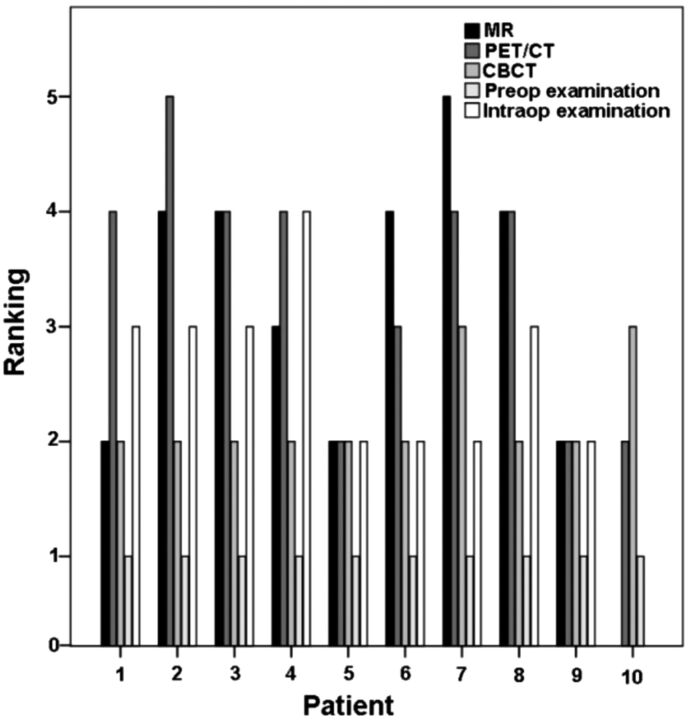
In total, 10 patients with suspected BONJ underwent CEMR imaging, fluoride PET/CT, and CBCT imaging with additional clinical correlation. Osteonecrosis was histologically confirmed in all patients with the exception of 1 female patient who could not undergo an operation due to advanced age and reduced general health, and this patient was, therefore, excluded from further statistical analysis. Of the remaining 9 different jaws affected by histologically proved BONJ, 4 had foci located in the maxilla and 5 had foci in the mandible. Individual rankings of disease extents in imaging modalities and examinations of all patients are depicted in Fig 3. Osteonecrosis was confirmed in all cases, with signs of infection in all bone specimens and the presence of Actinomyces species and/or other types of bacteria of the oral flora in 8 cases.
Fig 3.
Rankings of BONJ extent (0 = no sign of BONJ, 5 = maximal extent among modalities/examinations) in different modalities for each patient. Note in patient 10 that no abnormalities were seen in CEMR imaging due to artifacts and no intraoperative examination was performed.
On MR images, all BONJ foci showed markedly decreased signal on T1- with increased signal on T2-weighted images, except for patient 1 with intermediate signal on T2. However in this patient, contrast enhancement of the lesion was seen, and the focus was, therefore, regarded as positive for BONJ. Contrast uptake of affected bone and surrounding tissue was noted in all patients and foci of BONJ.
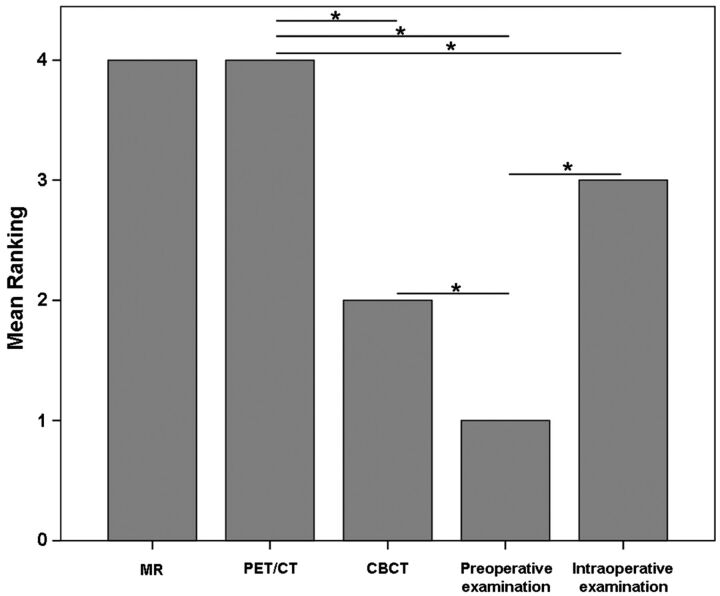
Overall, significant differences in quantification of BONJ extent among different modalities and examinations were found (P < .001). The highest median rank was found for PET/CT (4 ± 1.12) and CEMR imaging (4 ± 1.01), followed by intraoperative findings (3 ± 0.71), CBCT (2 ± 0.33), and clinical examinations (1 ± 0). No significant differences were found between PET/CT and CEMR imaging (P = .234), but there were significant differences between both modalities compared with CBCT and preoperative clinical examinations (all P < .05). Preoperative clinical examinations suggested significantly less extensive disease than all imaging modalities and intraoperative examinations (all P < .05) (Fig 4). With intraoperative examinations as the reference standard, among all imaging modalities, only PET/CT showed significantly larger disease extent (P = .023) than was determined intraoperatively.
Fig 4.
Mean rankings of BONJ extent in different modalities/examinations (0 = no sign of BONJ, 5 = maximal extent among modalities/examinations). CEMR imaging and PET/CT ranked highest and showed the largest extent of BONJ of all modalities, being significantly higher than CBCT and clinical preoperative examinations. PET/CT ranked significantly higher than intraoperative examinations. The latter showed significantly larger extent of BONJ than clinical preoperative examinations but did not significantly differ from CEMR imaging and CBCT. Horizontal lines with stars indicate significant differences among modalities (P < .05). Note that patient 10 was excluded from statistical analysis because no histologic confirmation of BONJ was obtained.
Individual comments on all patients are listed in the On-line Table. Markedly discordant findings among modalities and examinations were found in patients 2, 3, 4, 7, and 8. The actual extent of the BONJ both in CEMR imaging and PET/CT was significantly larger than expected clinically and on CBCT. CEMR and PET/CT were more specific in determining the true extent of the disease as was confirmed intraoperatively.
In patient 2, BONJ was clinically suspected superficially in the regions 44–46 of the right mandible. The actual extent of the BONJ on PET/CT and CEMR imaging was markedly larger than that expected clinically and on CBCT. PET/CT showed the largest disease extent, ranging from region 35 to the right mandibular ramus and in a craniocaudal direction from the alveolar crest to the inferior body below the inferior alveolar canal. Disease extent in CEMR imaging ranged from 32 to 47, with only central involvement of the mandibular body inferior to the alveolar canal. Both were more specific in determining the true extent of the disease because they also showed marked effect of mandibular bone on the contralateral paramedian side. This was only slightly suspected in CBCT but was confirmed intraoperatively. However, due to mechanical considerations, the necrotic bone was only subtotally removed with highly sclerotic bone remaining in the right posterior and left parasymphyseal mandibular body (Fig 2B).
Discussion
Clinical examination, usually in combination with panoramic views of the jaws, may be considered as the reference standard in the preoperative work-up of patients with BONJ scheduled for surgery.12 While the first may identify a suspicious superficial BONJ focus, the latter allows better delineation of the extent of bone osteonecrosis and depicts coexisting changes such as sclerosis or periosteal thickening.13 CBCT allows easy generation of panoramic views of the jaws from 3D datasets that are similar to plain radiographs but, in addition, can be reformatted by dedicated and user-friendly software.14 CBCT, furthermore, is less susceptible to metal-induced artifacts from certain endodontic prosthetic materials compared with standard multidetector CT15 and at a generally lower radiation dose.16
Necrotic foci are associated with a loss in bone mineralization and fragmentation of bone leading to decreased attenuation in CT or hyperlucent areas on panoramic views of the jaw.6,13 Associated sclerosis in the surrounding bone can often be seen and is linked to bisphosphonate-induced deranged bone homeostasis.4,5 CBCT attenuation measurements are not as reliable as in standard MDCT, however; and soft-tissue contrast is lower compared with MDCT due to basic differences in beam geometry, detector technology, and varying tube voltage with consecutive attenuation instability.17
MR imaging is free from ionizing radiation and has high soft-tissue contrast. Coincident gingival and soft-tissue involvement is highly indicative of BONJ and was, therefore, regarded as confirmation of disease in doubtful cases (ie, low signal on T1- and T2-weighted images). However, in these cases, quantification of disease extent was based only on affected bone on native T1- and T2-weighted images. Soft-tissue involvement can be detected with increased sensitivity by intravenous contrast administration, either in contrast-enhanced MDCT or in CEMR imaging.6,11,18 Although only affected bone tissue was quantified, our results showed significantly larger disease extent in CEMR imaging compared with the widely used reference CBCT and clinical examination. This finding corroborates the notion of the additive value of intravenous contrast for disease quantification. However, on CEMR images, clear-cut distinction between necrotic bone or osteomyelitis and reactive signal changes of bone marrow secondary to surrounding inflammation of soft tissue is often difficult. Hence, affected bone in BONJ might be overestimated and may not correlate well with intraoperative findings. This problem has been shown in a previous study, where MR imaging and CT provided high detectability for BONJ lesions that exceeded that of panoramic radiographs by far, but both techniques had limitations concerning the exact assessment of the extent of BONJ lesions in individual patients.18
The same observation may apply to [18F] fluoride PET/CT imaging. [18F] fluoride is a radioactive tracer that is incorporated in hydroxyapatite by osteoblasts during bone neoformation.9 Homeostasis of bone resorption and neoformation is markedly altered in patients with BONJ under bisphosphonate treatment19 and not only affects the BONJ focus itself but may lead to increased bone sclerosis or periosteal thickening beyond the clinically overt BONJ lesion.4,6 These often subtle bone changes are hardly perceivable on conventional panoramic radiographs or morphologic imaging modalities such as CT or MR imaging. However, [18F] fluoride PET/CT as a functional molecular imaging technique may allow detection of subtle foci of increased bone remodeling, considered precursors to clinically overt BONJ.20 PET images were acquired 30 minutes after the injection of the tracer in this study; hence, the detected signal originated almost entirely from radioactive fluoride incorporated into bone tissue, and direct perfusion effects of the tracer could thereby be excluded.21 Extent of BONJ detected in [18F] fluoride PET/CT significantly overlapped that of other imaging modalities and clinical preoperative and intraoperative examinations, corroborating the notion of altered bone biochemistry beyond the visible BONJ focus.
Due to mechanical considerations in certain cases, not all of the necrotic bone that was found during surgery could be resected. Thus, intraoperative quantification as a reference standard underestimated the true disease extent to some degree. When one compared all imaging modalities with intraoperative examinations, only [18F] fluoride PET/CT showed significantly larger BONJ areas than were addressed operatively, though CEMR imaging and PET/CT both depicted larger areas than CBCT or preoperative examinations. Although this finding on [18F] fluoride PET/CT may be partly caused by reactive changes in the surrounding bone, we hypothesize that these findings could nevertheless influence a surgeon's approach to BONJ. The latter essentially depends on how much bone of the jaw is affected (ie, how much will have to be resected on the basis of preoperative examination and imaging). If, for example, BONJ is clinically or, on plain radiographs, found to affect the alveolar process only, a different surgical approach is warranted compared with patients in whom the disease extends from the alveolar process into the whole hemimandible including the articular process, as was seen on [18F] fluoride PET/CT in 1 patient in this study.
We acknowledge certain limitations to this study. First, only 10 patients were included. However, 3 different imaging examinations had to be completed in all patients before surgery with ensuing histologic analysis of resected necrotic foci, which limited prospective patient acquisition. One female patient could not undergo an operation due to poor general health and, thus, lacked histologic proof of BONJ; she was, therefore, not included in the statistical analyses. Additionally, we consecutively included 9 female patients and only 1 male patient with BONJ in our study, leading to a marked female majority in our patient population. Although jaw bones of both sexes may be considered as equally susceptible to side effects from bisphosphonate treatment, subtle sex-specific differences cannot be ruled out with certainty.
Conclusions
[18F] fluoride PET/CT and CEMR imaging show more extensive changes in the setting of BONJ compared with panoramic views from CBCT and clinical examinations. This advanced imaging may be of added value in the preoperative work-up of patients and should be the focus of future studies. Awareness of differences among imaging modalities should also be considered when planning the extent of operative treatment of BONJ.
Supplementary Material
ABBREVIATIONS:
- BONJ
bisphosphonate-induced osteonecrosis of the jaw
- CBCT
conebeam CT
- CEMR
contrast-enhanced MR
- MDCT
multidetector CT
- STIR
short τ inversion recovery
REFERENCES
- 1. Cheng A, Mavrokokki A, Carter G, et al. The dental implications of bisphosphonates and bone disease. Aust Dent J 2005;50:S4–13 [DOI] [PubMed] [Google Scholar]
- 2. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115–17 [DOI] [PubMed] [Google Scholar]
- 3. Mavrokokki T, Cheng A, Stein B, et al. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007;65:415–23 [DOI] [PubMed] [Google Scholar]
- 4. Hewitt C, Farah CS. Bisphosphonate-related osteonecrosis of the jaws: a comprehensive review. J Oral Pathol Med 2007;36:319–28 [DOI] [PubMed] [Google Scholar]
- 5. Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 2005;353:99–102, discussion 99–102 [DOI] [PubMed] [Google Scholar]
- 6. Morag Y, Morag-Hezroni M, Jamadar DA, et al. Bisphosphonate-related osteonecrosis of the jaw: a pictorial review. Radiographics 2009;29:1971–84 [DOI] [PubMed] [Google Scholar]
- 7. Tyndall DA, Rathore S. Cone-beam CT diagnostic applications: caries, periodontal bone assessment, and endodontic applications. Dent Clin North Am 2008;52:825–41, vii [DOI] [PubMed] [Google Scholar]
- 8. García-Ferrer L, Bagan JV, Martinez-Sanjuan V, et al. MRI of mandibular osteonecrosis secondary to bisphosphonates. AJR Am J Roentgenol 2008;190:949–55 [DOI] [PubMed] [Google Scholar]
- 9. Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med 2008;49:68–78 [DOI] [PubMed] [Google Scholar]
- 10. Donati OF, Zanetti M, Nagy L, et al. Is dynamic gadolinium enhancement needed in MR imaging for the preoperative assessment of scaphoidal viability in patients with scaphoid nonunion? Radiology 2011;260:808–16 [DOI] [PubMed] [Google Scholar]
- 11. Fox MG, Stephens T, Jarjour WN, et al. Contrast-enhanced magnetic resonance imaging positively impacts the management of some patients with rheumatoid arthritis or suspected RA. J Clin Rheumatol 2012;18:15–22 [DOI] [PubMed] [Google Scholar]
- 12. Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2009;67:85–95 [DOI] [PubMed] [Google Scholar]
- 13. Arce K, Assael LA, Weissman JL, et al. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg 2009;67:75–84 [DOI] [PubMed] [Google Scholar]
- 14. De Vos W, Casselman J, Swennen GR. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg 2009;38:609–25 [DOI] [PubMed] [Google Scholar]
- 15. Pauwels R, Stamatakis H, Bosmans H, et al. Quantification of metal artifacts on cone beam computed tomography images. Clin Oral Implants Res 2011. December 15. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16. Loubele M, Bogaerts R, Van Dijck E, et al. Comparison between effective radiation dose of CBCT and MSCT scanners for dentomaxillofacial applications. Eur J Radiol 2009;71:461–68 [DOI] [PubMed] [Google Scholar]
- 17. Kalender WA, Kyriakou Y. Flat-detector computed tomography (FD-CT). Eur Radiol 2007;17:2767–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stockmann P, Hinkmann FM, Lell MM, et al. Panoramic radiograph, computed tomography or magnetic resonance imaging: which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig 2010;14:311–17 [DOI] [PubMed] [Google Scholar]
- 19. Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone 2011;49:56–65 [DOI] [PubMed] [Google Scholar]
- 20. Hutchinson M, O'Ryan F, Chavez V, et al. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg 2010;68:2232–40 [DOI] [PubMed] [Google Scholar]
- 21. Frost ML, Blake GM, Park-Holohan SJ, et al. Long-term precision of 18F-fluoride PET skeletal kinetic studies in the assessment of bone metabolism. J Nucl Med 2008;49:700–07 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.