SUMMARY:
Fetal and neonatal MR imaging is increasingly used as a complementary diagnostic tool to sonography. MR imaging is an ideal technique for imaging fetuses and neonates because of the absence of ionizing radiation, the superior contrast of soft tissues compared with sonography, the availability of different contrast options, and the increased FOV. Motion in the normally mobile fetus and the unsettled, sleeping, or sedated neonate during a long acquisition will decrease image quality in the form of motion artifacts, hamper image interpretation, and often necessitate a repeat MR imaging to establish a diagnosis. This article reviews current techniques of motion compensation in fetal and neonatal MR imaging, including the following: 1) motion-prevention strategies (such as adequate patient preparation, patient coaching, and sedation, when required), 2) motion-artifacts minimization methods (such as fast imaging protocols, data undersampling, and motion-resistant sequences), and 3) motion-detection/correction schemes (such as navigators and self-navigated sequences, external motion-tracking devices, and postprocessing approaches) and their application in fetal and neonatal brain MR imaging. Additionally some background on the repertoire of motion of the fetal and neonatal patient and the resulting artifacts will be presented, as well as insights into future developments and emerging techniques of motion compensation.
MR imaging is an ideal diagnostic technique for the evaluation of infants and fetuses1–7 because of the absence of ionizing radiation, the superior contrast of soft tissues compared with sonography, and the availability of different contrast options (T1-weighted, T2-weighted, and diffusion-weighted imaging, Fig 1) to improve characterization of both anatomy and pathology. However MR imaging remains a relatively slow technique, with scanning times for most applications in the order of seconds to minutes, leaving them susceptible to motion artifacts. The normally mobile fetus and the unsettled neonate present a major difficulty because the presence of motion during a long acquisition will decrease image quality in the form of motion artifacts (Fig 2), hamper accurate image interpretation, and often necessitate a repeat MR imaging to establish a diagnosis. This may have major emotional implications for parents and can stress the tight budgets of health care providers.
Fig 1.
T1-weighted (A), T2-weighted (B), and diffusion-weighted (C) axial MR brain images of a 5-day-old full-term neonate without motion artifacts acquired at 1.5T.
Fig 2.
T1-weighted (A), T2-weighted (B), and diffusion-weighted (C) axial MR brain images of a 14-day-old full-term neonate acquired at 1.5T. Motion artifacts in the form of high-signal-intensity ghosts can be seen.
Introduction and Scope
In pediatric, neonatal, and fetal MR imaging examinations, image quality is governed mainly by the SNR and the presence of motion artifacts: the lower the SNR and the more prominent the motion artifacts, the lower the quality of MR images will be. With appropriate modern hardware and optimized sequences, SNR should no longer be an issue and control of motion remains the main determinant of image quality. Clinical demand for MR imaging of both the neonatal and fetal brain is on the increase. Mild hypothermia has become standard practice for neonates with hypoxic-ischemic encephalopathy in many countries, and MR imaging is recommended to assess the extent of brain injury posttreatment.8 Additionally, ongoing studies9 are assessing the potential role of brain MR imaging in the routine evaluation of the preterm infant at term-equivalent age. Numerous studies have shown that MR imaging provides complementary information to sonography scans of the fetal brain.10–12 All these potential applications herald further demand for neonatal and fetal brain MR imaging, making motion compensation in the MR imaging of this population a priority.
The purpose of this article is to review currently available motion-compensation techniques including different approaches such as motion prevention, motion-artifacts minimization, and motion-correction schemes and to put these into the context of fetal and neonatal brain MR imaging. All motion-compensation strategies in this review assume rigid body motion during imaging acquisition. Additionally, some background on fetal and neonatal patient motion and artifacts will be provided as well as insights into future developments and emerging techniques.
Patient Motion
Motion
Motion relating to clinical MR imaging can be classified under 2 main categories: macroscopic and microscopic motion. Microscopic motion, including blood flow and water diffusion, is actually exploited in different clinical applications, namely MR angiography and diffusion MR imaging, respectively. Macroscopic motion may either be bulk (gross) patient motion, which is the focus of this review, relating to movement of the imaging object as a whole (whether this involves extremities, head, or torso), or physiologic motion, relating to motion induced by normal body functions (respiratory motion, cardiac motion, blood flow, peristalsis in the genitourinary/gastrointestinal systems, and so forth).
Motion in the fetal and neonatal MR imaging context may be predictable (eg, maternal respiratory motion, Fig 3), but in most cases, it is random and unpredictable (ie, fetal movements in utero or neonatal head movements ex utero). In general, motion may be continuous throughout the MR imaging acquisition (eg, an unsettled neonate), periodic (eg, respiratory motion), or intermittent (eg, provoked by MR imaging scanner acoustic noise). In neonatal and fetal brain imaging, motion can be assumed to be rigid body motion, with minimal or no deformation and with all dimensions of the imaging target being preserved.13 Motion can be restricted in a 2D field, involving rotation and translation within an acquired imaging section or it may be 3D, including also through-plane motion, with rotations and translations potentially spanning different sections; through-plane motion is one of the most difficult to compensate for. Most important, both fetal and neonatal motion (with amplitudes in the range of a few centimeters14) can be relatively greater than that of adults (with amplitudes in the range of a few millimeters) and also of a scale greater than the actual dimensions of the anatomy of interest; therefore, its effects on image quality are often detrimental.
Fig 3.
T1-weighted gradient-echo axial brain images at 1.5T (TR, 142 ms; TE, 6 ms; section thickness, 4 mm; scanning time, 16 seconds) of a 28-week-old fetus without motion artifacts after a successful breath-hold (A) and with motion artifacts after an incomplete breath-hold (B). Breathing artifacts appear in the form of high-signal ghosts in the operator-selected phase-encoding direction and severely degrade image quality.
Fetal Motion
Recent cine MR imaging studies, in which an enlarged FOV allows full coverage of the fetus, confirm that rotations, flexions, and extensions in all the main anatomic regions (upper limbs, lower limbs, head, and trunk) can be observed during intrauterine life. Less frequent were yawns and other mouthing movements including swallowing. Eye and paradoxic breathing movements could also be observed at all ages, as well as kicking, brief twitches, and startles (Fig 4).15
Fig 4.
Successive snapshots of a cine bFFE acquisition obtained at 1.5T (TR, 3.21 ms; TE, 1.59 ms; slab thickness, 30–40 mm; scanning duration, 30 seconds for 100 dynamic scans) of a 25-week-old fetus moving inside the uterus. The range and direction of movement of the fetal head (red star) and body and legs (dotted lines) at 6 (A), 12 (B), 18 (C), 24 (D), and 30 (E) seconds can be appreciated.
Perhaps the most distinguishing characteristics of fetal head motion are that it is 3D and uncontrollable.16,17 In some centers, maternal sedation is used to try to suppress fetal motion. However, even if the fetus remains still, head motion may occur, depending on fetal life (eg, in breech presentation, where the head lies close to maternal diaphragm and maternal respiratory motion is directly transmitted to fetal head). Fetal motion decreases with gestational age, mainly in lower limb movements. Head movement, though perhaps less complex, still occurs in the mature fetus.18 Other factors that may influence fetal motion include chemical exposure through the mother (alcohol or caffeine consumption, administration of steroids or other drugs), the quality and quantity of meals before the scan, and maternal emotional stress.19 Sonography has demonstrated that pathologic conditions in the fetus can also result in a variety of deviant motor behaviors, which may be broadly classified as hypo- or hyperkinetic20 (eg, the recipient polyhydramniotic twin in twin-to-twin transfusion syndrome21 often presents with excessive motion, whereas the donor oligohydramniotic twin shows restricted movement).
Maternal Motion during Fetal MR Imaging
Maternal motion may also degrade the image quality of fetal brain MR imaging examinations. Maternal motion may be involuntary or voluntary, ranging from movement of the maternal bowel and diaphragm to body movements because of discomfort, poor communication with the imaging team, or maternal stress. A common source of motion artifacts is due to the maternal diaphragm moving during incomplete or unsuccessful maternal breath-hold. Artifacts from maternal bowel movement may be difficult to prevent, particularly if the fetal head is adjacent.
Neonatal Motion
Normally developing neonates show a repertoire of body and head motions described as general movements, similar to those seen in the fetus; though in the neonatal context, these occur in air, not in amniotic fluid. Other movements may be sporadic because neonates may startle with the acoustic noise at the start of the MR imaging examination and then settle as they familiarize themselves with the scanner sounds. Scanner vibration itself may transmit motion to neonates.
Previous studies have confirmed that patients in the neonatal period have shown a greater degree of overall motion during MR imaging compared with adult patients.22 Because of the proximity of the chest and head anatomy in neonates, respiratory motion may often be transmitted through the neck to the head. Because the average resting neonatal respiratory rate is 40 breaths/min (compared with approximately 12 breaths/min in adults),23 there is little stationary time between breaths. Furthermore, neonates commonly demonstrate irregular respiratory rates and variable breath-to-breath amplitude, often obviating respiratory gating or navigator echoes. Because of this breathing pattern, head motion often occurs through-plane. Additionally, continuous positive airway pressure used to ventilate some very sick neonates may increase the amplitude of neonatal head motion.
Other factors that may influence the amplitude, frequency, and pattern of neonatal head movements include pharmacologic sedation (discussed later); drug administration, such as anticonvulsants; coexisting neurologic abnormalities (eg, seizures); milk/fluid intake; the presence of gastroesophageal reflux (common in the preterm infants at term-equivalent age); and patient positioning/immobilization.
Motion Artifacts
Definitions
Patient motion is evident on MR images in the form of motion artifacts. The word “artifact” has a Latin origin, from the terms “artis” for “art” and “facere” meaning “to make.” Artifacts are undesired “works of art,” which refer to parts of images failing to accurately reproduce anatomy or pathology because of distortion, addition, or deletion of information.24 Motion artifacts are actually artifactual images of the source image, resulting from uncorrected data inconsistencies due to source motion.25 These can be quite widespread and overt and degrade image quality, ultimately rendering examinations nondiagnostic, or they may be more localized and inconspicuous, leaving much of the useful imaging data unaffected.
How Do Motion Artifacts Originate?
Motion during data acquisition causes data to be inconsistent. The final MR image and the raw data, or k-space data, are related by the Fourier transform so that each pixel of the image is composed of a weighted sum of every k-space point and each k-space point contains a weighted sum of signals from every point in the region of the object being imaged; therefore, any inconsistency in k-space data sampling has the potential to affect every pixel in the resulting MR image.26,27
There are essentially 2 ways in which data inconsistencies due to motion may produce artifacts24: 1) intra-view (or within view) effects are caused by motion occurring between each RF pulse excitation and echo formation, and 2) inter-view (or view-to-view) effects are caused by motion occurring between the acquisition of successive phase-encoding steps. Interview effects result in phase errors due to the inconsistent location and signal intensity of the moving spins during phase-encoding, while intra-view effects result in phase incoherence among the moving spins at the time of echo formation. When intra-view effects take place, signal loss due to dephasing or spatial misregistration may occur. When inter-view effects occur and motion is periodic (such as with respiration), ghosting artifacts appear on MR images. Similarly, when inter-view effects are present and motion is random, image blurring degrades MR images. Both blurring and ghosting artifacts are mostly evident in the phase-encoding direction, irrespective of the actual direction of motion. This review will focus on inter-view motion effects.
How Do Motion Artifacts Manifest?
Motion artifacts usually appear in the phase-encoding direction, where spatial encoding of the MR imaging signal is much slower (in the order of seconds) compared with the frequency-encoding direction (in the order of milliseconds). Spatial encoding in the frequency direction is many times faster compared with the duration of motion, so motion is effectively “frozen” for that time and motion artifacts are not pronounced; in the phase direction though, where encoding takes longer, there is more time available to “see” motion and, therefore, to represent it as motion artifacts.27
Motion artifacts may present as “blurring” (Fig 5C), “ghosting” (Fig 5J), contrast changes (Fig 5P), and even signal voids (Fig 5B) as described in the previous section. Blurring is similar to motion blur in photography, producing a marked decrease in spatial resolution; in the case of MR imaging though this is mostly evident in the phase-encoding direction, regardless of the actual direction of the original motion. Ghost images comprise lines concentric or parallel to bright imaged structures, such as subcutaneous fat on T1-weighted images, and represent full or partial replicas of the original static source. These should not be confused with the similarly appearing Gibbs ringing artifacts due to data truncation (Fig 6). Additionally, contrast changes and signal void may occur with very fast patient motion.
Fig 5.
Successive axially planned sections of a single-shot fast-spin-echo acquisition at 1.5T (TR, 1000 ms; TE, 127 ms; section thickness, 4 mm; scanning duration, 26 seconds) of a 32-week-old fetus (E) with significant fetal motion occurring during data acquisition and resulting in blurring (C, J, M), contrast changes (P), and ultimately signal void when motion is extreme (B). Please note that though sections were planned in the axial plane, fetal movement resulted in plane transposition in the produced images (A–P) (fetal brain is circumscribed in red to distinguish it from neighboring maternal tissues).
Fig 6.
Ringing artifacts (arrow) at the back of the brain of a 4-week-old full-term neonate on an axial maximum intensity projection of an optimized neonatal MR angiography protocol79 acquired at 3T. Ringing artifacts occur due to data undersampling, and should not be confused with motion-artifacts ghosts.
The appearance of ghost artifacts resulting from strictly periodic sinusoidal motion is governed by different factors summarized in the following simple formula24,28: Distance (in pixels) = TR × Phase-Encoding Steps × Number of Signal Averages × Motion Frequency. Therefore, the location of ghost artifacts is directly proportional to the TR, the matrix size in the phase-encoding direction, the NSA (assuming parallel averaging, where each k-space line is acquired NSA times before moving to the next), and the frequency (rate) of motion; the higher the rate, the bigger is the distance between the ghosts (Fig 7). Because imaging parameters may affect the appearance of ghost motion artifacts, different types of sequences and different image weightings may produce different patterns of artifacts.29 Additionally, the greater the amplitude of motion, the brighter the ghost is. The amplitude of motion also increases the trace of each ghost in the phase-encoding direction.
Fig 7.
The effect of varying imaging-acquisition parameters such as TR (A) and motion characteristics (varying speed of motion, [B] and varying amplitude of motion [C]) on motion artifacts appearance, compared with the nonmotion status (first column). Note that the longer the TR (A) (range, 25–1000 ms) and the faster the motion (measured in cycles/minute; range, 12–48 cycles/min) (B), the farther apart the ghosts appear; also the bigger the amplitude of motion (measured in centimeters; range, 1–3 cm), the brighter is the ghost and the longer, its trace (C).
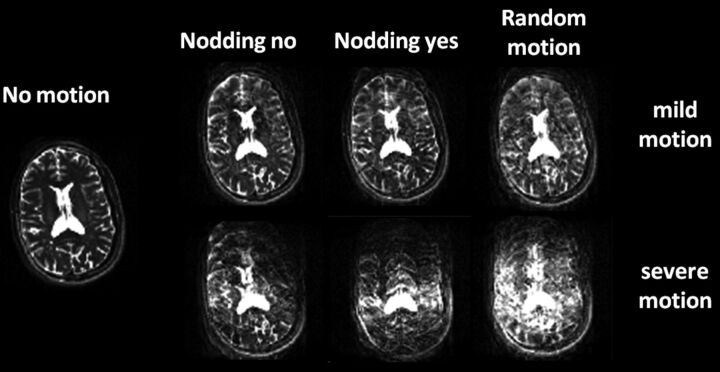
The type of motion, in-plane or through-plane, may also affect the appearance of motion artifacts, with through-plane motion being more detrimental to image quality and more difficult to compensate for. The effects of severity and plane of patient motion on the creation of motion artifacts are graphically illustrated in Fig 8. For this purpose, a healthy male adult volunteer was scanned, and we introduced the following head motion: 1) head still, 2) nodding “yes,” 3) nodding “no,” and 4) random motion under mild (low-frequency) and severe (high-frequency) amplitudes. The volunteer was scanned in a 3T scanner (Philips Healthcare, Best, the Netherlands) by using an 8-channel sensitivity encoding head coil with the standard T2-weighted FSE brain protocol, acquired in the transverse plane.
Fig 8.
The effect of in-plane motion (side-to-side head nodding or “nodding no,” first column), through-plane motion (up and down head nodding or “nodding yes,” second column), random motion (combination of in-plane and through-plane motion, third column), and different motion intensities (top row: mild motion; bottom row: severe motion) on image quality of axial T2-weighted fast spin-echo acquisitions of a healthy adult volunteer. Through-plane severe patient motion is detrimental to image quality.
Motion Compensation
For all physiologic types of motion, some remedial strategies have already been proposed and successfully applied in adults. For respiratory motion, breath-holding may be used for short acquisitions and respiratory gating or phase-encode reordering,30–32 for longer scans. Similarly for offsetting cardiac motion, cardiac gating,33 a method of synchronizing data acquisition with the cardiac cycle, is available, whereas gradient-moment nulling has been proposed34 for reducing pulsatility artifacts from flowing blood. Finally to minimize bowel peristalsis, glucagon or any other approved parenteral spasmolytics can be used to reduce motion artifacts.35
However overcoming artifacts from gross patient motion in general and in the fetal and neonatal MR imaging context in particular has proved to be more complicated, often requiring a combination of approaches to produce high-quality interpretable scans. For this review, we will be focusing on gross head movement. There are 3 different strategies to compensate for bulk motion artifacts on MR imaging: 1) prevention, 2) minimization, and 3) detection and correction (prospective/real-time and retrospective). These measures will be further explained below.
Prevention of Motion Artifacts
The first step to compensate for motion artifacts is prevention. Adequate patient preparation is vital, including patient positioning (to maximize patient comfort) and immobilization, when plausible (by using foam pads, pillows, and sandbags, with the latter often useful to decrease both bulk patient motion as well as motion from scanner vibration, particularly for very low-birth-weight infants); room temperature maintenance (to avoid cooling or heating effects on the patient); and ear protection by using headphones, earplugs, or other dedicated equipment as required (to minimize startles or discomfort by increased acoustic noise). Additionally patient coaching,36 such as giving clear instructions to pregnant women on the importance of staying still during the scan, practicing breath-holding, briefly explaining the scanning procedure to prevent anxiety or minimize the effects of claustrophobia, is useful to ensure patient compliance. Occasionally in neonatal patients with a clinical referral, sedation may be performed before the MR imaging to ensure high-quality diagnostic images. Recent data suggest that there was a more than 3-fold increase in good-quality diagnostic neonatal MR images in sedated neonatal patients (88%) compared with age-matched unsedated ones (25% of the total MR imaging scans performed during a time period) (Serena Counsell, personal communication, October 2011).
For fetal MR imaging, patient preparation refers to the mother; positioning involves the use of pillows and sandbags to make her feel comfortable and a left decubitus position is preferred to prevent inferior vena cava syndrome.1 Because of accumulation of heat during the RF pulses, the patients are advised to fully change into examination gowns and lie barefoot in the scanner for effective heat dissipation; a cooling fan in the scanner bore may also be helpful. Maternal sedation is not generally used. Some pregnant women prefer to have their partner in the room or to listen to music through the headphones to relax or overcome claustrophobia.
For neonatal MR imaging on the other hand, infants are preferably imaged supine either in natural sleep by using the “feed and wrap” method or, when necessary, after sedation with oral chloral hydrate (25–50 mg/kg, dosage depending on gestational age and age at scanning37). Severely encephalopathic neonates and those on anticonvulsants may not need extra sedation. Sedation is safe when one adheres to guidelines, with an adverse event rate ranging from 0.4% to 2.4%, and effective with a high rate of successful examinations ranging from 85% to 100%.23,38,39 All neonates should be fully monitored once sedation has been given and until fully awake postexamination; neonatally qualified staff should be present throughout. Dose should be adjusted according to the weight and neurologic condition of the child and route of administration adapted per individual case (eg, chloral hydrate may be given orally, via nasogastric tube, or rectally). Sometimes neonatal motion may occur even if the neonate is in natural sleep or sedation; molded air bags or foam placed snugly around the infant's head will keep this to a minimum. Swaddling the infant will also reduce body movements. Room temperature maintenance is very important for maximizing patient comfort and encouraging sleep, especially for very preterm infants.40,41
Motion Artifacts Minimization
In the presence of all preventative measures, motion may still occur, and the next strategy is to try to minimize the effect of motion. The faster the MR imaging, the lower is the likelihood of patient bulk motion and of motion artifacts occurring during the examination. Based on that principle, there are simple modifications to scan parameters to decrease scanning time: decrease TR, reduce matrix size in the phase-encoding direction (if resolution is not an issue), or minimize the NSA (if SNR is not an issue). For neonates and fetuses, it is also possible to decrease the number of sections because a smaller region of tissue needs to be covered. Driven by the same principle, a whole school of thought in MR imaging has invested in developing faster MR imaging techniques, either by designing faster sequences or by proposing data-undersampling methods.
Fast Imaging Sequences
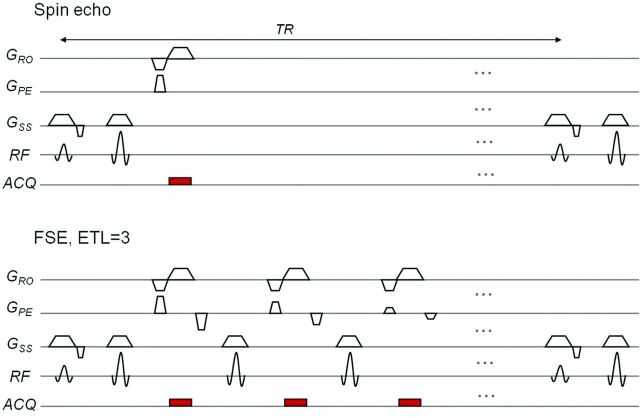
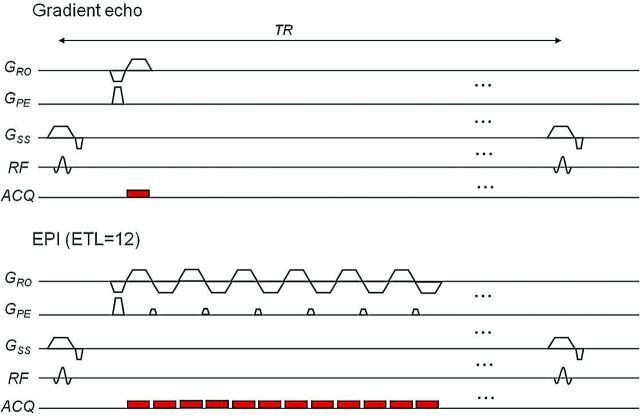
The most widely available fast sequence is FSE, also called rapid acquisition with relaxation enhancement, introduced by Hennig et al42 in 1986. This sequence uses multiple 180° refocusing pulses and thus produces multiple echoes for a single 90° excitation pulse. It is many times faster than the conventional spin-echo because more lines of k-space are filled in per excitation; the number of multiple successive refocusing RF pulses, also known as echo-train length, determines the speed-up factor of the FSE acquisition compared with the standard spin-echo (Fig 9). Although fast, image-acquisition time for FSE can still be in the order of seconds and, therefore, not immune to motion artifacts. Another multiecho fast imaging sequence is EPI, described even earlier by Sir Peter Mansfield in 197743 and still one of the fastest, with an entire image acquired in <100 ms, albeit at a low resolution. Being approximately an order of magnitude faster than FSE, EPI is correspondingly more resistant to motion artifacts. EPI owes its speed to the multiple frequency-encode gradient reversals (gradient refocusing) used instead of RF refocusing, as in FSE; the more gradient reversals (known as EPI factor), the faster the sequence (Fig 10).
Fig 9.
A sequence diagram for a spin-echo (top) and fast spin-echo sequence with an echo-train length or number of refocusing 180° pulses of 3 (bottom), which significantly decreases scanning time from 2-and-a-half minutes to 1 minute. ACQ indicates data acquisition.
Fig 10.
A sequence diagram for gradient-echo (top) and EPI sequences with an EPI factor (or number of gradient reversals) of 12 (bottom), which significantly decreases scanning time from 1 minute to 6 seconds. ACQ indicates data acquisition.
EPI remains an unchallenged technique for diffusion, perfusion, and functional MR imaging. However the benefits of the fast imaging time are not without cost; EPI is relatively demanding on the scanner hardware, in particular on gradient characteristics. It is also a noisy sequence, which may wake neonatal patients and provoke patient motion. In addition, EPI is prone to susceptibility artifacts and distortions; these are a consequence of the longer readout period used to cover multiple lines in k-space per excitation. Long readout periods can also lead to blurring (loss of resolution) in both FSE and EPI. The extreme case of both techniques is to fill the whole of the k-space after a single excitation—so called “single-shot” imaging. This is the fastest method, but results in the severest readout duration–related artifacts. Longer acquisition time can be traded for less blurring/distortion by using multiple shots instead. Single-shot T2-weighted FSE is the mainstay tool for structural imaging of fetuses because of the very fast acquisition of data, whereas multishot T2-weighted FSE is often used for scanning neonates, where motion is less extreme. In cases of extreme fetal or neonatal motion, multiple repeated imaging loops of single-shot FSE can be used in conjunction with a motion-correction algorithm, snapshot to volume reconstruction44 (discussed in the postprocessing section). Similarly, EPI is applied in diffusion-weighted and diffusion tensor studies in both neonates and fetuses.45 However due to the nature of motion and the inherently poor SNR in these populations, optimization of these sequences is advisable.
Other fast imaging sequences include FLASH,46,47 a gradient-echo acquisition that reduces scanning time by using a short TR so that it can be completed within a breath-hold. Spoiler gradients between RF pulses and RF phase cycling can be used to make images appear T1-weighted; this has been used to date as the fast T1-weighted acquisition to image the fetal brain, but image quality is often poor due to motion artifacts, poor contrast, and low SNR. A robust single-shot inversion recovery based T1-weighted alternative protocol, called snapshot inversion recovery,48 has recently been suggested, producing high-contrast fetal MR images with reduced motion artifacts and, therefore, increased anatomic delineation (Fig 11). bFFE,49 also known as true fast imaging with steady-state precession50 or balanced steady-state free precession, is another rapid gradient-echo technique with fully refocused (rather than spoiled) transverse magnetization, extensively used in cardiac MR imaging. The result is far superior SNR compared with FLASH; however, signal contrast is proportional to √(T2 / T1), making it unsuitable for all applications. Balanced fast-field echo has been recently optimized and applied to study fetal movement patterns in utero.15
Fig 11.
An axial T1-weighted gradient-echo breath-hold acquisition of the fetal brain (A) and a motion-resistant axial T1-weighted free-breathing SNAPIR (Snapshot Inversion Recovery) acquisition of the same fetal patient acquired at 34 weeks' gestation at 1.5T. (B) Sections were anatomically matched for comparison. Depiction of anatomic structures (cerebral cortex, ventricular system) is improved with the optimized SNAPIR acquisition compared with the breath-hold protocol.
Data Undersampling
Alternative approaches focus on truncation of sampled data to decrease scanning time. Parallel imaging introduced by Sodickson and Manning51 in 1997 (simultaneous acquisition of spatial harmonics) and Pruessmann et al52 in 1999 (sensitivity encoding) has revolutionized MR imaging by its ability to speed up data acquisition by using multiple receiver coils to obtain extraspatial information. This can reduce the number of phase-encode steps required to form an image and can be applied to most sequences. Scanning times can be reduced significantly on the basis of the operator-selected speed-up factor. Speed-up factors of 2 halve total scanning time, without the introduction of artifacts but with some reduction in SNR. However, dedicated phased array coils are required to implement parallel imaging techniques.
Half-Fourier acquired single-shot turbo spin-echo, introduced by Margosian et al53 in 1986, is a single-shot technique combined with FSE, which measures only half the lines of k-space and makes use of the inherent theoretic symmetry of k-space to regenerate the other half, plus a few extra lines to correct imperfections. This method has been extensively applied in fetal brain imaging. There are similar techniques, such as partial (fractional) echo, in which a fraction of the generated echo is collected (the length of the readout is reduced) and the rest of the data is regenerated on the basis of echo symmetry. (Fig 12).
Fig 12.
Readout gradient diagrams and k-space sampling strategies for different data-truncation techniques such as partial echo and half-Fourier compared with full-echo. ky indicates the y-axis of the k-space; kx, the x-axis of the k-space. With partial echo or half-Fourier, scanning time can be reduced.
All the above-mentioned techniques manage to decrease total scanning time, often at a cost of image quality, and to minimize the likelihood of motion artifacts occurring during the scanning. However, they do not truly correct for motion; so motion artifacts will still appear and images will be affected in the event of patient motion during the shortened data acquisition.
Motion-Resistant Sequences
Other strategies focus on producing inherently motion-resistant sequences without overstretching scanning-time reduction. Currently, most clinical MR imaging sequences use rectilinear (Cartesian) k-space sampling (ie, the sampling points are placed on a rectangular [more often square] grid and then data are reconstructed into the MR image by using the Fourier transform). There are, however, non-Cartesian k-space trajectories,54,55 such as radial and spiral as well as hybrid k-space trajectories, such as PROPELLER56 (a Cartesian-radial hybrid, discussed further), that are considered motion-resistant because of the oversampling of the center of the k-space; this surplus of central k-space data can be used either to get information on motion patterns and correct motion artifacts (as a navigator, see next section) or to allow motion-corrupted data to be excluded by postprocessing imaging data and keeping only artifact-free information.
Other Methods to Minimize Motion Artifacts
A further approach is to reduce the effect of motion artifacts by suppressing the signal of the moving structure. This can be achieved by a number of measures: 1) correct use of surface coils57—instead of enveloping coils—by positioning the anatomic/pathologic area of interest in the center of the surface coil while keeping the unwanted moving objects in regions of low or no sensitivity; 2) using signal averaging—by increasing the NSA—to allow random signals from motion to cancel out as they add up in multiple copies and nonrandom signals, such as signal from stationary tissues, to increase their amplitude, albeit at the expense of increased scanning time (this is actually true for random mild motion, whose resulting artifacts can be treated as noise); 3) applying presaturation RF pulses (in the form of spatial saturation bands) on top of the moving tissue/structure whose signal requires suppression; and 4) using fat-saturation techniques58,59 if motion artifacts originate from this tissue (such as the ghosting artifacts from subcutaneous maternal abdominal fat in fetal imaging). Finally, because motion artifacts appear in the phase-encoding direction, a judicious choice of this parameter allows a benign localization of the artifacts outside the anatomic region of interest. Although these techniques are useful in minimizing motion artifacts in fetal and neonatal MR imaging, they are not always practical due to the extra time required for patient preparation and/or data acquisition.
Detection and Correction of Motion Artifacts
None of the above-mentioned methods actually correct for bulk motion artifacts; to achieve that, navigators, the so-called self-navigated sequences and motion-tracking devices, detect and measure motion. This information is then used to correct for motion either prospectively (adapt the imaging sequence in real-time during the scan so that the acquisition volume follows the motion of the object) or retrospectively during the reconstruction process and in conjunction with different image postprocessing schemes. Motion detection with retrospective correction requires both a detection system and a system for postprocessing the data to correct for detected motion. Retrospective methods have the disadvantage that they cannot fully correct through-plane motion because the changing position of the section with respect to the anatomy leads to different tissues “seeing” effectively different RF pulse amplitudes, causing varying signal levels throughout the scan—the so called “spin history” effect. Real-time (prospective) motion correction offers many added benefits: It requires no postprocessing because motion is monitored throughout the scan and the scanner gradients are simultaneously adjusted to “track” the object by using the knowledge of its motion, it can effectively correct for through-plane motion because it reduces spin-history effects, and the desired imaging volume is fully covered throughout the scan.60 This is very important when imaging small volumes such as in fetal and neonatal MR imaging or using 2D acquisitions. Retrospective methods are limited because parts of the moving object may leave the imaging volume or plane in the presence of a large-amplitude motion, leading to unrecoverable information loss.
Navigators
Navigators were initially developed as a short acquisition by Ehman and Felmlee in 1989,61 interleaved with the main MR image acquisition, to specifically encode information about moving subjects and improve the quality of data either prospectively or retrospectively. Their design, including RF excitation and k-space trajectory, is modified accordingly to better match the anatomic area studied. The first navigators had a linear k-space trajectory and could only detect rigid-body translation along the navigator direction. A line of k-space was repeatedly sampled during data acquisition, and then postprocessing of data revealed motion information about the target. Data acquired during motion were discarded as corrupted. Later on, developments in the navigator design used information from corrupt data to rotate and phase-correct k-space data. Simple motion can be detected with pencil-beam62 navigators. More complex patterns of motion can be detected by using orbital,63 spheric,64 or rapid cloverleaf navigators65 (named after the shape of their k-space trajectories, respectively). Prospective acquisition correction performed in real time uses a cross-sectioned navigator commonly placed on the dome of the right diaphragm for abdominal MR imaging or the head in adult patients.66,67 This technique has been applied in the fetal brain with some good results; however, it increased the scanning time of T2 single-shot FSE acquisitions from <30 seconds to approximately 7 minutes and was unable to correct more vigorous fetal movement in 2 of 20 cases.14 Also, positioning of the navigator required a bFFE pilot scan to determine motion direction and often >1 trial to get the desired results, therefore further increasing total scanning time.
Self-Navigated Sequences: Radial, Spiral, and PROPELLER
Radial and spiral sequences are inherently self-navigated because the low spatial frequencies at the center of the k-space are oversampled and this redundant information can be used to infer motion characteristics and correct for motion. PROPELLER imaging, suggested by Pipe in 1999,56 exploits this property of radial imaging to correct for bulk in-plane motion. K-space is sampled in a rotating partially overlapping fashion, with concentric rectangular strips (blades) rotating through its center. However, data acquisition with PROPELLER takes 57% longer (by a factor of π / 2) than conventional scans. Additionally, because this technique is section-selective, it remains difficult to correct for through-plane motion. Motion artifacts in PROPELLER are very different compared with conventional Cartesian acquisitions; they are radial (streak-like) artifacts, which emanate tangentially from the moving object but whose intensity close to the object is diminished. The frequency of movement determines the radius at which streak artifacts become more visible, with higher frequencies increasing the artifact-free zone. Streak artifacts are also more apparent when it comes to through-plane motion.68 Previous studies applying PROPELLER to pediatric populations concluded that though useful for correcting in-plane motion, PROPELLER is not equally successful in correcting through-plane motion, which very often degrades fetal and neonatal images.22,69 Our experience in applying PROPELLER in neonatal MR imaging confirms this finding and showed that though PROPELLER acquisitions show greater contrast than conventional single-shot images when there was neonatal through-plane motion, streak-like artifacts were detrimental to image quality.
External Motion-Tracking Techniques
A more intuitive way to get information about motion models is to use external devices to track motion either prospectively or retrospectively. Different attempts with external devices include locator coils, laser detectors, deuterium crystals, sonography, infrared markers, and, most recently, optical markers.70 If used prospectively, these techniques can correct for through-plane motion; they are not time-consuming but require additional hardware and calibration of the external-device spatial coordinates to the scanner coordinates. These seem well-suited for imaging the neonatal population because of the excessive through-plane motion, but more research is required to find a safe and practical tracking device for neonates.
Prospective motion correction71 is the most recent addition to motion detection and correction techniques. It uses 3 orthogonal 2D spiral navigators interspersed within the “dead” time of standard image acquisition for flexible image-based real-time rigid-body motion tracking and correction. Additionally, it allows automatic rescanning of data acquired under significant motion. It has been clinically tested in populations of school-aged children72,73 (mean age, 10.7 years) who were advised to remain still during the scan and has successfully corrected for motion of more than a centimeter of translation and up to 15° of rotation from their original head position on T1-weighted inversion recovery volume acquisitions. It would be of interest to apply this technique in neonates and fetuses, in whom there is no patient compliance and motion can be of a scale greater than the anatomy of interest.
Postprocessing
Most postprocessing techniques require some information about motion to be able to adequately correct it. This may include different motion parameters, such as duration, amplitude, direction, and timing of motion, which may be known in advance (in the case of periodic motion); determined during data acquisition (noniterative approach) by using data oversampling including navigators, dynamic scanning, and self-navigated sequences; or derived with the use of a metric (cost function) that can identify corrupted data (iterative approach). Once this information is known, then the process can be inverted by using an algorithm to correct for motion artifacts.74
Some noniterative methods are already available on clinical scanners such as PROPELLER and are used in neonatal imaging when motion may preclude the acquisition of diagnostic images. Others, such as those used to allow the formation of 3D images from clinically acquired motion-corrupted multisection acquisitions of the fetal brain to facilitate true 3D anatomic measurements,45,75,76 though very promising, are still being developed to reduce the current long reconstruction times and are not yet suitable for clinical practice. Compressed sensing,77 a newly developed mathematic theory, states that images with an inherently sparse representation can be recovered from randomly undersampled k-space data (such as that of motion artifacts), provided an appropriate nonlinear recovery scheme is used. Initial results are promising for improved spatial resolution and accelerated acquisition for a range of imaging sequences in adult and, recently, in pediatric MR imaging.78
Conclusion and Future Directions
Both fetal and neonatal motion is unpredictable, characterized by unique patterns, perhaps the most extreme in the pediatric imaging spectrum. Artifacts from bulk head motion are destructive, may hamper diagnosis and timely intervention, and may require costly repeat scans. Although different methods of motion compensation are available for adults, there are no such techniques dedicated to neonates and fetuses. Adult-based motion-compensation strategies fail to provide satisfactory results unless adjusted to the characteristics and needs of this population.
The effort to customize these techniques is triggered by the growing clinical interest in fetal and neonatal MR imaging and its increasing use as a biomarker and a surrogate outcome measure in clinical trials. Parallel imaging, along with prospective motion-correction techniques with fast navigator echoes and time-efficient reconstruction, seems to hold promise for advancing inter-view motion correction. Compensation for intra-view motion will also be vital to facilitate diffusion tensor imaging and functional MR imaging studies. Non-Cartesian k-space trajectories also show promising results, particularly because of the low spatial-frequency oversampling in k-space in the otherwise “SNR-starved” fetal and neonatal MR images. These efforts are backed by hardware improvements, such as high-field imaging, faster and stronger gradients, advancing coil design, and transmit coil technology. Additionally, the introduction of new mathematic theories, such as compressed sensing, may help improve the efficiency of advanced postprocessing methods and make them applicable in clinical practice.
Motion compensation holds a very central role in neonatal and fetal MR imaging. Promising results rely on fine-tuning of the available methods to suit this population and often on using them in combination.
ABBREVIATIONS:
- bFFE
balanced fast-field echo
- FLASH
fast low-angle shot
- GRO
readout gradient
- NSA
number of signal averages
- PROPELLER
periodically rotated overlapping parallel lines with enhanced reconstruction
- RF
radio-frequency
Footnotes
Disclosures: Christina Malamateniou—RELATED: Grant: Biomedical Research Centre Academic Health Sciences Centre DCIM P31599, Comments: research grant support, UNRELATED: Consultancy: Philips Healthcare Hellas, Comments: research consultant. Shaihan Malik—RELATED: Engineering and Physical Science Research Council,* Comments: grant funding for the postdoctoral position under which I am employed. It is not directly related to this project, but it pays for my salary, UNRELATED: Payment for the Development of Education Presentations: ESMRMB, Comments: received honorarium for lectures on fast MRI as part of a series of lectures of the ESMRMB on advanced MRI techniques. Serena Counsell—UNRELATED: Employment: Medical Research Council, UK, Grants/Grants Pending: Medical Research Council, UK*. Joseph Hajnal—UNRELATED: Grants/Grants Pending: Lee Family Fellowship,* OTHER RELATIONSHIPS: research support from Philips Healthcare for a broad program of MRI methods development. Mary Rutherford—RELATED: Grant: Medical Research Council, UK. *Money paid to the institution.
This work was supported by research grants from the Medical Research Council, BRC Academic Health Sciences Centre, and Philips Healthcare.
Paper previously presented in part as an invited lecture at: Annual MRI course, “MRI of the Developing Brain,” March 1–2, 2011; Hammersmith Hospital Conference Centre, London, UK.
Author Contributions: data acquisition (S.J.C., J.M.A., A.K.M.), data analysis and interpretation (C.M., M.A.R., A.M.E.), manuscript drafting and manuscript revision (all authors), literature research (C.M., M.A.R.), manuscript editing (C.M., J.V.H., M.A.R.), and clinical studies (A.M.E., M.A.R.).
REFERENCES
- 1. Rutherford MA. Magnetic resonance imaging of the fetal brain. Curr Opin Obstet Gynecol 2009;21:180–86 [DOI] [PubMed] [Google Scholar]
- 2. Rutherford M, Malamateniou C, Zeka J, et al. MR imaging of the neonatal brain at 3 Tesla. Eur J Paediatr Neurol 2004;8:281–89 [DOI] [PubMed] [Google Scholar]
- 3. Levine D, Hatabu H, Gaa J, et al. Fetal anatomy revealed with fast MR sequences. AJR Am J Roentgenol 1996;167:905–08 [DOI] [PubMed] [Google Scholar]
- 4. Levine D, Barnes PD, Sher S, et al. Fetal fast MR imaging: reproducibility, technical quality, and conspicuity of anatomy. Radiology 1998;206:549–54 [DOI] [PubMed] [Google Scholar]
- 5. Griffiths PD, Paley MN, Whitby EH. MR imaging of the fetal brain and spine: a maturing technology. Ann Acad Med Singapore 2003;32:483–89 [PubMed] [Google Scholar]
- 6. Prayer D. Fetal MR. Eur J Radiol 2006;57:171. [DOI] [PubMed] [Google Scholar]
- 7. Huppi PS, Inder TE. Magnetic resonance techniques in the evaluation of the perinatal brain: recent advances and future directions. Semin Neonatol 2001;6:195–210 [DOI] [PubMed] [Google Scholar]
- 8. Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011;159:851–58, e851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards AD, Azzopardi D, Rutherford MA. Evaluation of MR imaging to predict neurodevelopmental impairment in preterm infants (e-prime). National Institute for Health Research Annual Report 2009 (http://www.nihr.ac.uk/files/pdfs/400891_NIHR_AnnualReport2010−acc3.pdf). Accessed September 14, 2011
- 10. Levine D. Ultrasound versus magnetic resonance imaging in fetal evaluation. Top Magn Reson Imaging 2001;12:25–38 [DOI] [PubMed] [Google Scholar]
- 11. Frates MC, Kumar AJ, Benson CB, et al. Fetal anomalies: comparison of MR imaging and US for diagnosis. Radiology 2004;232:398–404 [DOI] [PubMed] [Google Scholar]
- 12. Whitby EH, Paley MN, Sprigg A, et al. Comparison of ultrasound and magnetic resonance imaging in 100 singleton pregnancies with suspected brain abnormalities. BJOG 2004;111:784–92 [DOI] [PubMed] [Google Scholar]
- 13. Hill DL, Batchelor PG, Holden M, et al. Medical image registration. Phys Med Biol 2001;46:R1–45 [DOI] [PubMed] [Google Scholar]
- 14. Bonel H, Frei KA, Raio L, et al. Prospective navigator-echo-based real-time triggering of fetal head movement for the reduction of artifacts. Eur Radiol 2008;18:822–29 [DOI] [PubMed] [Google Scholar]
- 15. Hayat TT, Nihat A, Martinez-Biarge M, et al. Optimization and initial experience of a multisection balanced steady-state free precession cine sequence for the assessment of fetal behavior in utero. AJNR Am J Neuroradiol 2011;32:331–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Limperopoulos C, Clouchoux C. Advancing fetal brain MRI: targets for the future. Semin Perinatol 2009;33:289–98 [DOI] [PubMed] [Google Scholar]
- 17. Rutherford M, Jiang S, Allsop J, et al. MR imaging methods for assessing fetal brain development. Dev Neurobiol 2008;68:700–11 [DOI] [PubMed] [Google Scholar]
- 18. Hayat T. Morphological and Functional Analysis of the Fetal Central Nervous System using Magnetic Resonance Imaging [thesis]. London, UK: Imaging Sciences Department, Imperial College; 2010 [Google Scholar]
- 19. Dantendorfer K, Amering M, Bankier A, et al. A study of the effects of patient anxiety, perceptions and equipment on motion artifacts in magnetic resonance imaging. Magn Reson Imaging 1997;15:301–06 [DOI] [PubMed] [Google Scholar]
- 20. de Vries JI, Fong BF. Changes in fetal motility as a result of congenital disorders: an overview. Ultrasound Obstet Gynecol 2007; 29:590–99 [DOI] [PubMed] [Google Scholar]
- 21. Glenn OA, Barkovich J. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis: part 2. AJNR Am J Neuroradiol 2006;27:1807–14 [PMC free article] [PubMed] [Google Scholar]
- 22. Vertinsky AT, Rubesova E, Krasnokutsky MV, et al. Performance of PROPELLER relative to standard FSE T2-weighted imaging in pediatric brain MRI. Pediatr Radiol 2009;39:1038–47 [DOI] [PubMed] [Google Scholar]
- 23. Chow LC. Motion correction for pediatric and MSK MRI. In: Proceedings of the International Society of Magnetic Resonance in Medicine Workshop on Current Concepts of Motion Correction for MRI & MRS, Kitzbuhel, Tyrol, Austria. February 24–28, 2010 [Google Scholar]
- 24. Arena L, Morehouse HT, Safir J. MR imaging artifacts that simulate disease: how to recognize and eliminate them. Radiographics 1995;15:1373–94 [DOI] [PubMed] [Google Scholar]
- 25. Pipe J. The impact of motion on data consistency and image quality in MRI. In: Proceedings of the International Society of Magnetic Resonance in Medicine Workshop on Current Concepts of Motion Correction for MRI & MRS. Kitzbuhel, Tyrol, Austria. February 24–28, 2010 [Google Scholar]
- 26. Atkinson D. Motion compensation strategies. In: Proceeding of the International Society of Magnetic Resonance in Medicine. Montreal, Canada. May 6–13, 2011 [Google Scholar]
- 27. Maclaren JR. Motion Detection and Correction in Magnetic Resonance Imaging [thesis]. Christchurch, New Zealand: University of Canterbury; 2008 [Google Scholar]
- 28. Schultz CL, Alfidi RJ, Nelson AD, et al. The effect of motion on two-dimensional Fourier transformation magnetic resonance images. Radiology 1984;152:117–21 [DOI] [PubMed] [Google Scholar]
- 29. Li T, Mirowitz SA. Fast T2-weighted MR imaging: impact of variation in pulse sequence parameters on image quality and artifacts. Magn Reson Imaging 2003;21:745–53 [DOI] [PubMed] [Google Scholar]
- 30. Bailes DR, Gilderdale DJ, Bydder GM, et al. Respiratory ordered phase encoding (ROPE): a method for reducing respiratory motion artefacta in MR imaging. J Comput Assist Tomogr 1985;9:835–38 [PubMed] [Google Scholar]
- 31. Haacke EM, Patrick JL. Reducing motion artifacts in two-dimensional Fourier transform imaging. Magn Reson Imaging 1986;4:359–76 [DOI] [PubMed] [Google Scholar]
- 32. Jhooti P, Wiesmann F, Taylor AM, et al. Hybrid ordered phase encoding (HOPE): an improved approach for respiratory artifact reduction. J Magn Reson Imaging 1998;8:968–80 [DOI] [PubMed] [Google Scholar]
- 33. Lanzer P, Barta C, Botvinick EH, et al. ECG-synchronized cardiac MR imaging: method and evaluation. Radiology 1985;155:681–86 [DOI] [PubMed] [Google Scholar]
- 34. Bernstein M, King K, Zhou X. Handbook of MRI Pulse Sequences. Boston: Elsevier Academic Press; 2004 [Google Scholar]
- 35. Chernish SM, Maglinte DD. Glucagon: common untoward reactions–review and recommendations. Radiology 1990; 177:145–46 [DOI] [PubMed] [Google Scholar]
- 36. Edwards AD, Arthurs OJ. Paediatric MRI under sedation: is it necessary? What is the evidence for the alternatives? Pediatr Radiol 2011;41:1353–64 [DOI] [PubMed] [Google Scholar]
- 37. Cowan FM. Sedation for magnetic sedation for magnetic resonance scanning of infants and young children. In: Whitwam JG, McCloy RF, eds. Principles and Practice of Sedation, 2nd ed. London, UK: Blackwell Healthcare; 1998:206–13 [Google Scholar]
- 38. Bluemke DA, Breiter SN. Sedation procedures in MR imaging: safety, effectiveness, and nursing effect on examinations. Radiology 2000;216:645–52 [DOI] [PubMed] [Google Scholar]
- 39. Bisset GS, Ball WS. Preparation, sedation, and monitoring of the pediatric patient in the magnetic resonance suite. Semin Ultrasound CT MR 1991;12:376–78 [PubMed] [Google Scholar]
- 40. Rutherford MA. MRI of the Neonatal Brain. London: WB Saunders; 2002:17–21 [Google Scholar]
- 41. Merchant N, Groves A, Larkman DJ, et al. A patient care system for early 3.0 Tesla magnetic resonance imaging of very low birth weight infants. Early Hum Dev 2009;85:779–83 [DOI] [PubMed] [Google Scholar]
- 42. Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med 1986;3:823–33 [DOI] [PubMed] [Google Scholar]
- 43. Mansfield P. Multi-planar image-formation using NMR spin echoes. J Phys C Solid State Phys 1977;10:L55–58 [Google Scholar]
- 44. Jiang S, Xue H, Glover A, et al. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): application to fetal, neonatal, and adult brain studies. IEEE Trans Med Imaging 2007;26:967–80 [DOI] [PubMed] [Google Scholar]
- 45. Chen Q, Levine D. Fast fetal magnetic resonance imaging techniques. Top Magn Reson Imaging 2001;12:67–79 [DOI] [PubMed] [Google Scholar]
- 46. Frahm J, Haase A, Matthaei D. Rapid NMR imaging of dynamic processes using the FLASH technique. Magn Reson Med 1986;3:321–27 [DOI] [PubMed] [Google Scholar]
- 47. Nitz WR. Fast and ultrafast non-echo-planar MR imaging techniques. Eur Radiol 2002;12:2866–82 [DOI] [PubMed] [Google Scholar]
- 48. Malamateniou C, McGuinness AK, Allsop JM, et al. Snapshot inversion recovery: an optimized single-shot T1-weighted inversion-recovery sequence for improved fetal brain anatomic delineation. Radiology 2011;258:229–35 [DOI] [PubMed] [Google Scholar]
- 49. Graumann R, Fischer H, Oppelt A. A new pulse sequence for determining T1 and T2 simultaneously. Med Phys 1986;13:644–47 [DOI] [PubMed] [Google Scholar]
- 50. Duerk JL, Lewin JS, Wendt M, et al. Remember true FISP? A high SNR, near 1-second imaging method for T2-like contrast in interventional MRI at. 2 T. J Magn Reson Imaging 1998;8:203–08 [DOI] [PubMed] [Google Scholar]
- 51. Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 1997;38:591–603 [DOI] [PubMed] [Google Scholar]
- 52. Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–62 [PubMed] [Google Scholar]
- 53. Margosian P, Schmitt F, Purdy D. Faster MR imaging: imaging with half the data. Health Care Instrum 1986:37;93–102 [Google Scholar]
- 54. McRobbie D, Moore E, Graves M, et al. MRI from Picture to Proton. New York: Cambridge University Press; 2006 [Google Scholar]
- 55. Hennig J. K-space sampling strategies. Eur Radiol 1999;9:1020–31 [DOI] [PubMed] [Google Scholar]
- 56. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999;42:963–69 [DOI] [PubMed] [Google Scholar]
- 57. Larkman DJ, Atkinson D, Hajnal JV. Artifact reduction using parallel imaging methods. Top Magn Reson Imaging 2004;15:267–75 [DOI] [PubMed] [Google Scholar]
- 58. Delfaut EM, Beltran J, Johnson G, et al. Fat suppression in MR imaging: techniques and pitfalls. Radiographics 1999;19:373–82 [DOI] [PubMed] [Google Scholar]
- 59. Stadler A, Schima W, Ba-Ssalamah A, et al. Artifacts in body MR imaging: their appearance and how to eliminate them. Eur Radiol 2007;17:1242–55 [DOI] [PubMed] [Google Scholar]
- 60. Speck O. Prospective motion correction. In: Proceedings of the International Society of Magnetic Resonance in Medicine Workshop on Current Concepts of Motion Correction for MRI & MRS. Kitzbuhel, Tyrol, Austria. February 24–28, 2010 [Google Scholar]
- 61. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173:255–63 [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Riederer SJ, Ehman RL. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med 1995;33:713–19 [DOI] [PubMed] [Google Scholar]
- 63. Fu ZW, Wang Y, Grimm RC, et al. Orbital navigator echoes for motion measurements in magnetic resonance imaging. Magn Reson Med 1995;34:746–53 [DOI] [PubMed] [Google Scholar]
- 64. Welch EB, Manduca A, Grimm RC, et al. Spherical navigator echoes for full 3D rigid body motion measurement in MRI. Magn Reson Med 2002;47:32–41 [DOI] [PubMed] [Google Scholar]
- 65. van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med 2006;56:1019–32 [DOI] [PubMed] [Google Scholar]
- 66. Klessen C, Asbach P, Kroencke TJ, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging 2005;21:576–82 [DOI] [PubMed] [Google Scholar]
- 67. Barnwell JD, Smith JK, Castillo M. Utility of navigator-prospective acquisition correction technique (PACE) for reducing motion in brain MR imaging studies. AJNR Am J Neuroradiol 2007;28:790–91 [PMC free article] [PubMed] [Google Scholar]
- 68. Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 1992;28:275–89 [DOI] [PubMed] [Google Scholar]
- 69. Forbes KP, Pipe JG, Karis JP, et al. Brain imaging in the unsedated pediatric patient: comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. AJNR Am J Neuroradiol 2003;24:794–98 [PMC free article] [PubMed] [Google Scholar]
- 70. Zaitsev M, Dold C, Sakas G, et al. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 2006;31:1038–50 [DOI] [PubMed] [Google Scholar]
- 71. White N, Roddey C, Shankaranarayanan A, et al. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med 2010;63:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brown TT, Kuperman JM, Erhart M, et al. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage 2010;53:139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuperman JM, Brown TT, Ahmadi ME, et al. Prospective motion correction improves diagnostic utility of pediatric MRI scans. Pediatr Radiol 2011;41:1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hedley M, Yan H. Motion artifact suppression: a review of post-processing techniques. Magn Reson Imaging 1992;10:627–35 [DOI] [PubMed] [Google Scholar]
- 75. Rousseau F, Glenn OA, Iordanova B, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Acad Radiol 2006;13:1072–81 [DOI] [PubMed] [Google Scholar]
- 76. Gholipour A, Estroff JA, Warfield SK. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE Trans Med Imaging 2010;29:1739–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58:1182–95 [DOI] [PubMed] [Google Scholar]
- 78. Vasanawala SS, Alley MT, Hargreaves BA, et al. Improved pediatric MR imaging with compressed sensing. Radiology 2010;256:607–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Malamateniou C, Counsell SJ, Allsop JM, et al. The effect of preterm birth on neonatal cerebral vasculature studied with magnetic resonance angiography at 3 Tesla. Neuroimage 2006;32:1050–59 [DOI] [PubMed] [Google Scholar]