Abstract
BACKGROUND AND PURPOSE:
Children with shunted hydrocephalus have been undergoing surveillance neuroimaging, generally in the form of head CT, for evaluation of ventricular size. As the life expectancy of these children has improved due to better shunt technology and medical care, risks related to the ionizing radiation incurred during multiple head CT examinations that they are expected to undergo throughout their lifetime have become a concern. The purpose of this study is to estimate the LAR of developing fatal cancer due to head CT for ventricular size assessment in children with shunted hydrocephalus and to assess the impact of instituting a rapid brain MR imaging protocol in reducing radiation exposure.
MATERIALS AND METHODS:
Retrospective review of medical records yielded 182 patients who underwent neuroimaging for assessment of ventricular size. Available neuroimaging studies (head CT and rapid brain MR) were counted and annual neuroimaging frequency was calculated. It was assumed that these patients undergo a similar number of neuroimaging studies annually through 20 years of age. A risk estimate was calculated based on the BEIR VII report and effective doses obtained using the International Commission on Radiologic Protection Report 103 organ weighting factors.
RESULTS:
The mean annual neuroimaging study frequency was 2.1. Based on the average age of 1.89 years, it was assumed neuroimaging surveillance commences in the second year of life. LAR was calculated assuming that a patient undergoes neuroimaging in the form of head CT at this frequency (2/year) through 20 years of age. Assuming 2 scans are performed per year and the low-dose head CT protocol is used, approximately 1 excess lifetime fatal cancer would be generated per 230 patients; with standard head CT, there would be 1 excess lifetime fatal cancer per 97 patients.
CONCLUSIONS:
Children with shunted hydrocephalus are at increased risk of developing fatal cancer if they are to undergo surveillance using head CT. Implementation of a rapid brain MR imaging protocol with no radiation detriment will reduce this risk.
With increasing use of CT in the diagnosis of both pediatric and adult disease, there is a growing awareness, in the medical community and public, of increased cancer risk caused by ionizing radiation. The pediatric radiology community has been on the forefront in recognition of long-term risks of ionizing radiation and implementing changes, which resulted in decreased radiation doses incurred during CT examinations. In the span of 5 years, the main determinants of radiation dose (peak kV and effective mAs) used for pediatric multidetector body CT examinations have significantly decreased, presumably due to the efforts to increase awareness of the risks of radiation.1 The Image Gently campaign is considered to have been instrumental in helping to reduce the radiation dose incurred by children during diagnostic imaging procedures.2 Although the radiation dose of a single CT examination can be reduced by decreasing the peak kilovoltage and effective mAs settings, if the patient undergoes multiple CT examinations, the cumulative absorbed radiation dose may still be high.
Children with shunted hydrocephalus constitute a unique group of patients because they are expected to undergo surveillance neuroimaging, generally in the form of head CT, throughout their lives. Moreover, assessment of ventricular size commences at a very early age, usually in the neonatal period. With CSF diversion techniques and advances in care of patients with shunted hydrocephalus, more patients reach adulthood compared with earlier decades. To avoid ionizing radiation exposure, rapid brain MR imaging has been proposed as a satisfactory alternative to head CT in the assessment of ventricular size, which is an integral part of shunt function evaluation.
To our knowledge, the LAR of developing fatal cancer in children who undergo neuroimaging surveillance using CT was not studied before. We undertook this retrospective review to calculate the annual frequency of neuroimaging (CT or MR) in children with shunted hydrocephalus at a single institution. We attempted to calculate the LAR of developing fatal cancer if only CT is used for surveillance of ventricular size in children with hydrocephalus.
Materials and Methods
This study was approved by our institutional review board and was compliant with the Health Insurance Portability and Accountability Act.
Patient Population
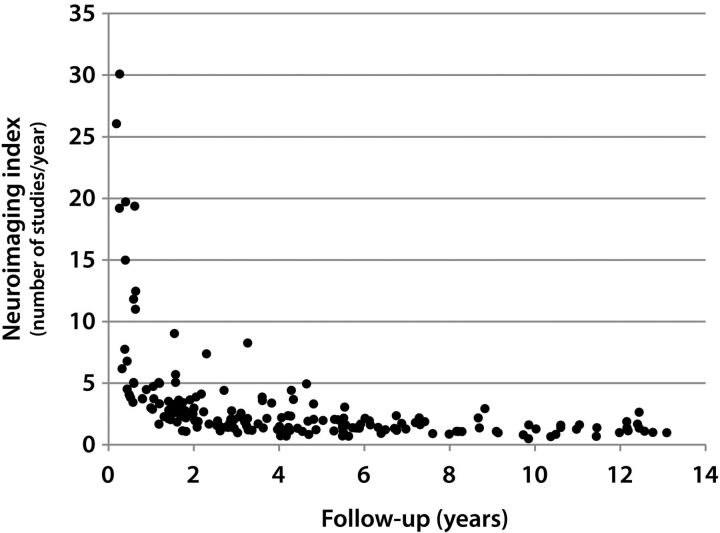
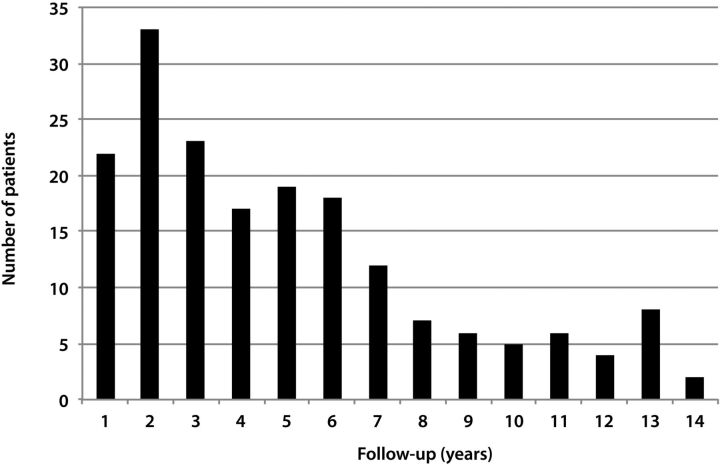
Using radiology billing records, patients who underwent rapid brain MR imaging from January 2009 through December 2010 were identified. A board-certified radiologist with certificate of additional qualification in neuroradiology (K.K.) evaluated the rapid brain MR imaging examinations on a PACS to exclude patients who had rapid brain MR for indications other than evaluation of ventricular size in shunted hydrocephalus. The rapid MR examinations were assessed for diagnostic quality. There were 182 patients included in the study, of which 90 were male. Patients with brain tumors were excluded. Using the PACS, the number of neuroimaging studies performed for evaluation of ventricular size was counted for each patient. These studies included noncontrast head CT and rapid brain MR imaging examinations. Each study and its report were evaluated by the same neuroradiologist to ascertain that the indication for the study was the assessment of ventricular size. Dates of the earliest and most recent neuroimaging studies were recorded. Studies performed during the night shift (between 9 pm and 6 am) were noted and these studies were also included in the calculation of the neuroimaging indices. During the night shift, an in-house MR technologist is not available at our institution. Twenty-two patients (8 males) whose follow-up (time between the most recent and most distant neuroimaging studies) was shorter than 1 year were excluded, as some of these patients had multiple studies in a short period, which resulted in a factitiously increased number of studies per year. For example, 1 patient underwent 8 neuroimaging studies in just over 3 months, resulting in 30.1 examinations per year, which is unrealistic (Fig 1). For the remaining 160 patients, the follow-up period was rounded to the next greatest integer. For example, if a patient had a 1.31-year follow-up, this patient was assumed to have a 2-year follow-up (Fig 2). The annual frequency of neuroimaging studies was found by dividing the total head CT and limited brain MR imaging examinations by the follow-up duration.
Fig 1.
Scatterplot shows the distribution of neuroimaging indices against follow-up in years. The patients who had follow-ups shorter than 1 year were excluded when the mean was calculated, as some of these patients had factitiously elevated neuroimaging indices that could not be extrapolated.
Fig 2.
Graph displays the number of patients in each follow-up group (eg, 2 indicates >1.00 and ≤2 years).
Assumptions and Risk Calculation
The age when a patient first underwent a neuroimaging study was taken as the patient's age. It was assumed that these patients would undergo a similar number of neuroimaging studies annually through 20 years of age. A risk estimate was calculated using the methods and data of the BEIR VII report, and effective doses were obtained using the International Commission on Radiologic Protection Report 103 organ weighting factors.3,4 Using the protocols stored in the CT scanner, we determined the CTDI delivered to the adult head phantom for the different age groups we created. The age groups were as follows: <2 years; ≥2 years and <4 years; ≥4 years and <9 years; ≥9 years and <18 years; and ≥18 years. For head scans, the scanner dose index information (CTDIvolume and DLP) is always referenced to the adult head phantom.
From this, we calculated the effective doses delivered to the standard man phantom. The effective dose is dependent on the selection of the caudal limit for the scan, as there is a fairly large contribution due to scatter dose to the thyroid. There is probably 20% variability in the dose result due to the choice of the scan limit. The calculations were done with the ImPACT spreadsheet based on Monte Carlo modeling of scanner characteristics and the standard man model.5
We calculated this dose using 2 organ weighting schemes, International Commission on Radiologic Protection Reports 60 and 103.3,6 The results in this case are not highly dependent on the choice of organ weighting schemes. Corrections available with the ImPACT spreadsheet were applied to account for the fact that calculations based on the standard man adult model were being used for pediatric cases. The net result is that although the CTDIvolume values are lower for younger ages, the effective doses for a given protocol are approximately the same across age groups. Thus, the specialized low-dose ventricular shunt protocol gives an average effective dose of 1.1 mSv and the standard head protocol gives an average effective dose of 2.5 mSv for all age groups. The variability due to the thyroid dose could reduce the average effective dose to approximately 80% of these values: 0.9 mSv and 2 mSv, respectively.
The risk factors applied to these doses were obtained from BEIR VII.4 We approximated the values using the total LAR of fatal cancer and splitting this into age groups. The risk factors we used varied from 14%/Gy for the 0- to 1-year-old group to 6.6%/Gy for the ≥18 years group.
Results
The 182 patients underwent 1167 head CT examinations for evaluation of ventricular size. Approximately 83.3% (972/1167) of the head CTs were performed between 9 pm and 6 am. There were 339 rapid brain MR examinations available. There were only 2 patients in whom limited MR study was attempted, but diagnostic images could not be acquired. The mean age at which patients underwent the first neuroimaging study was 1.89 years (range 1 day–18.65 years). If the 22 patients who had follow-ups shorter than 1 year are excluded, the mean age was 1.88 year (Fig 1). Therefore, for our dose estimates, we assumed that the first neuroimaging study would be performed in the second year of life. The average number of annual neuroimaging studies was 2.1 ± 1.6 (range 1–12). Assuming a person with shunted hydrocephalus would undergo CT surveillance only, and that 2 CT scans would be performed annually until 20 years of age, we estimated that 1 excess lifetime fatal cancer would be generated per 230 patients if the low-dose CT protocol were used. If the standard head CT protocol were used for surveillance of the ventricles with the same imaging frequency (2/year), there would be 1 excess lifetime cancer per 97 patients.
Discussion
In this study, we attempted to estimate the risk of developing a fatal cancer in children with shunted hydrocephalus if they are to undergo neuroimaging using CT only. It is estimated approximately 72 million CT scans were performed in the United States in 2007.7 Although CT is used proportionately less frequently in the diagnosis of pediatric disease compared with its utilization in adults, its usage has increased more rapidly in children than in adults.7–10 While approximately 24.3% of the US population was younger than 18 years in 2009, only 7% of all the CT scans performed in 2007 were in children.7,11 In the United States, the utilization of CT in children is greater than in other countries. For example, according to a survey conducted in 2005 and 2006,12 in Germany, only 1% of all CT scans were performed in children. Even though 7% of all CT examinations were performed in the United States in 2007 were in persons younger than 18 years of age, it was calculated that approximately 15% of the projected cancers would be due to scans performed before the age of 18 years.8
In 2001, Brenner et al13 estimated that 170 additional fatal cancers will develop due to head CT examinations performed in children younger than 15 years of age in the United States in that year. In the same study, it was predicted the lifetime cancer mortality risk attributable to radiation exposure from a single head CT examination in a 1-year-old child was 1 in 1500. Although the radiation exposures caused by individual CT examinations have been brought down significantly in the last decade, the number of CT examinations has increased. The brain was once considered a comparatively radioresistant organ; however, there is evidence that it is significantly radiosensitive, particularly at very low doses, with risk increasing at younger ages.14
Head CT constitutes the most common type of CT examination in the United States, accounting for approximately 33% of all CT examinations in children and adults.8 Berrington de González et al7 estimated there will be approximately 4000 pediatric and adult additional cancers due to the head CT scans performed in 2007. They found that the mean lifetime cancer risk per 10,000 head CT scans was 4 and 5 for a 15-year-old female and male, respectively. The risk was nearly 2-fold if the age is 3 years at the time of the head CT.7
Children with shunted hydrocephalus undergo neuroimaging more frequently than any other patient group, with the possible exception of patients with brain tumors. In children who require CSF diversion, ventricular shunt placement is the most commonly used method. Aqueductal stenosis, congenital malformations (eg, Chiari II malformation, Dandy-Walker malformation), intraventricular hemorrhage, and meningitis are main causes of communicating and noncommunicating hydrocephalus.
Ventricular shunts first became available in early 1960s and their introduction dramatically changed the management and outcome of hydrocephalus. Although many recipients of the ventricular shunts survived longer compared with the preshunt era, long-term survival was less than that of the general population, probably because of the shortcomings of the earlier shunt technology. For instance, Oakeshott et al15 reported that of the 117 consecutive patients with Chiari II malformation delivered at a single institution between 1963 and 1971, 39% were alive past their 40th birthdays. Improvements in CSF diversion techniques—and advances in urologic, respiratory, and orthopedic care—have led to increased life expectancy and better clinical outcomes of patients with shunted hydrocephalus. Currently, 75% of neonates with Chiari II malformation are expected to reach late adulthood.16
Rapid MR brain imaging allows for acquisition times generally under 30 seconds, which obviates the need for sedation or anesthesia of the pediatric patient.17 Although subtle parenchymal abnormalities cannot be excluded when using rapid brain MR imaging, the ventricles, cerebral sulci, and basal cisterns can be adequately assessed owing to the good contrast resolution of MR. Visualization of the shunt catheter is not absolutely necessary for assessment of shunt function, though its visualization can be improved by using a gradient-echo sequence.18 Instituting competitive pricing for rapid brain MR imaging may make it easier to implement the change from head CT to rapid brain MR in assessment of shunted hydrocephalus. At our institution, these 2 studies cost exactly the same amount.
We found that approximately 83.3% of the head CTs were performed between 6 am and 9 pm, during which an in-house MR technologist is available. If all the head CTs outside of these hours are replaced by rapid MR for ventricular size assessment, additional significant dose reductions can be achieved.
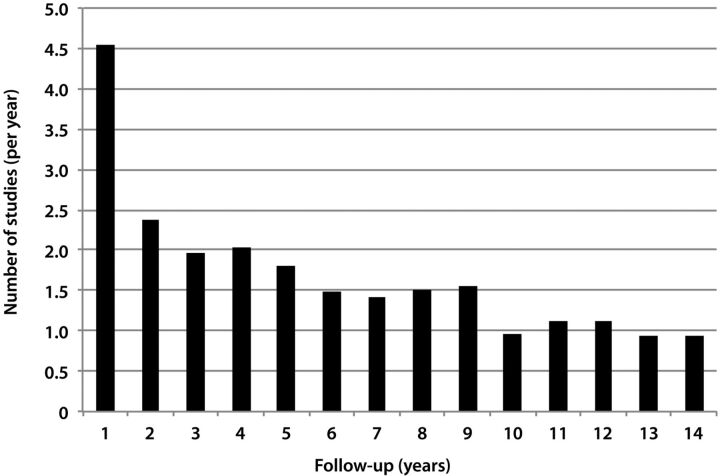
There are some limitations to our study. First, using CTDIvolume and its derivative DLP was criticized in estimating the effective dose or potential cancer risk for any individual patient, as the published K-factors used to convert DLP to effective dose assume a standard-sized patient.19 To avoid incorrect estimation, our cohort was divided into different age groups and appropriate corrections were made. Second, when estimating the fatal cancer risk, we used the linear no-threshold model, which is widely, but not universally, accepted.20,21 Third, to identify the patients with shunted hydrocephalus, we elected to find the patients who underwent rapid MR brain imaging for evaluation of shunt function. There are probably some patients who did not undergo rapid MR brain imaging during the study period. This was a deliberate choice because we wanted to include patients who successfully underwent MR imaging and therefore did not need CT scans for assessment of the ventricular size. Even with this exclusion, we were able to obtain a relatively large cohort. Although our cohort included 182 patients, the frequency of neuroimaging for evaluation of shunted hydrocephalus may be different in larger patient populations and in different clinical settings. Fourth, although we included outside CT examinations that were loaded to a PACS, not all of the outside examinations could be made available on a PACS. Therefore, there may be a small number of studies that were not taken into account. Similarly, the PACS was installed at our institution in 1996, and therefore the longest follow-up is 14 years (Fig 3). Examinations performed before this date were not included in the study. Finally, even though many of the patients in our cohort were followed for more than 3 years, potentially, a more representative annual neuroimaging index may be obtained by increasing the follow-up time.
Fig 3.
Graph shows mean neuroimaging indices against follow-up in years.
One important result of our study was the identification of the annual frequency of the neuroimaging studies (approximately 2) in a patient population that is expected to undergo neuroimaging surveillance throughout their lives. In addition, we calculated that even with a low-dose CT protocol—which delivers a dose of, on average, 1.1 mSv—1 of 230 patients will develop an excess fatal cancer if the neuroimaging surveillance is performed in the form of CT. If a standard-dose CT protocol is used—which, on average, delivers 2.5 mSv—1 of 93 will develop an excess fatal cancer.
Conclusions
Over a period of years, multiple head CT studies deliver a substantial amount of radiation to children with shunted hydrocephalus. Instituting a rapid brain MR imaging protocol may help significantly reduce the risk of these children developing a fatal cancer. Our study emphasizes the role of rapid brain MR imaging in markedly reducing and, if it can be universally implemented, potentially eliminating the risk of developing fatal cancer caused by diagnostic radiation exposure in children with shunted hydrocephalus.
ABBREVIATIONS:
- BEIR
Biologic Effects of Ionizing Radiation
- CTDI
CT dose index
- DLP
dose-length product
- LAR
lifetime attributable risk
Footnotes
Previously presented at: 96th Annual Meeting of the Radiological Society of North America; Chicago, Illinois; November 28–December 3, 2010.
References
- 1. Arch ME, Frush DP. Pediatric body MDCT: a 5-year follow-up survey of scanning parameters used by pediatric radiologists. AJR Am J Roentgenol 2008;191:611–17 [DOI] [PubMed] [Google Scholar]
- 2. Goske MJ, Applegate KE, Boylan J, et al. The Image Gently campaign: working together to change practice. AJR Am J Roentgenol 2008;190:273–74 [DOI] [PubMed] [Google Scholar]
- 3. International Commission on Radiological Protection. Recommendations of the ICRP. ICRP Publication 103. Ann ICRP 2008;37. [DOI] [PubMed] [Google Scholar]
- 4. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII – Phase 2. Washington, DC: National Academies Press; 2006 [PubMed] [Google Scholar]
- 5. ImPACT. ImPACT scan Web site. http://www.impactscan.org. Accessed April 15, 2011
- 6. International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 1991;21. [PubMed] [Google Scholar]
- 7. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linton OW, Mettler FA, Jr. National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 2003;181:321–29 [DOI] [PubMed] [Google Scholar]
- 10. White KS. Invited article: helical/spiral CT scanning: a pediatric radiology perspective. Pediatr Radiol 1996;26:5–14 [DOI] [PubMed] [Google Scholar]
- 11. U.S. Census Bureau. State and County QuickFacts. http://quickfacts.census.gov/qfd/states/00000.html. Accessed April 7, 2011 [Google Scholar]
- 12. Galanski M, Nagel HD, Stamm G. Paediatric CT exposure practice in the Federal Republic of Germany: results of a nationwide survey in 2005/06. Medizinische Hochschule Hannover; 2006. [Google Scholar]
- 13. Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289–96 [DOI] [PubMed] [Google Scholar]
- 14. Karlsson P, Holmberg E, Lundell M, et al. Intracranial tumors after exposure to ionizing radiation during infancy: a pooled analysis of two Swedish cohorts of 28,008 infants with skin hemangioma. Radiat Res 1998;150:357–64 [PubMed] [Google Scholar]
- 15. Oakeshott P, Hunt GM, Poulton A, et al. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol 2010;52:749–53 [DOI] [PubMed] [Google Scholar]
- 16. Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet 2004;364:1885–95 [DOI] [PubMed] [Google Scholar]
- 17. Ashley WW, Jr, McKinstry RC, Leonard JR, et al. Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. J Neurosurg 2005;103:124–30 [DOI] [PubMed] [Google Scholar]
- 18. Miller JH, Walkiewicz T, Towbin RB, et al. Improved delineation of ventricular shunt catheters using fast steady-state gradient recalled-echo sequences in a rapid brain MR imaging protocol in nonsedated pediatric patients. AJNR Am J Neuroradiol 2010;31:430–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCollough CH, Leng S, Yu L, et al. CT dose index and patient dose: they are not the same thing. Radiology 2011;259:311–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Little MP, Wakeford R, Tawn EJ, et al. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 2009;251:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tubiana M, Feinendegen LE, Yang C, et al. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 2009;251:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]