Abstract
BACKGROUND AND PURPOSE:
Stent-retriever devices play an increasing role in the interventional treatment of acute stroke patients, because fast recanalization can be achieved. The purpose of this study was to evaluate the feasibility of stent-retriever recanalization in patients with wake-up stroke in the anterior circulation.
MATERIALS AND METHODS:
We retrospectively analyzed clinical and angiographic data of 19 consecutive patients with wake-up stroke who were treated with stent-retriever devices between 2009 and October 2011. Recanalization was assessed by using the Thrombolysis in Cerebral Infarction score. Clinical outcome was evaluated at discharge and after 90 days by using the modified Rankin Scale.
RESULTS:
Median NIHSS score at admission was 17 (IQR, 15–20). Before the procedure, the TICI score was 0 in 18 patients and 1 in 1 patient. Recanalization with stent-retriever devices was successful (TICI ≥ 2) in 94.7%. Mean time to flow restoration was 36.7 minutes and to complete recanalization 83.7 minutes. Symptomatic intracranial hemorrhage occurred in 4 patients (21.1%). Eight patients had an NIHSS improvement of ≥4 points between admission and discharge. After 90 days, 2 (10.5%) of our patients presented with mRS 0–2; seven (36.8%) died.
CONCLUSIONS:
Despite successful and rapid recanalization with stent-retriever devices, good clinical outcome in patients with wake-up stroke is achieved in a minority of patients. Clinical outcome remains poor. Bleeding rates were higher compared with recanalization procedures within 6 hours after stroke onset.
Approximately one-quarter of patients with ischemic stroke awake or are found with their neurologic deficits1,2; thus, the time of stroke onset is uncertain. However, it has been shown that thrombolysis in patients with wake-up stroke is safe in the absence of extensive early ischemia2 and that these patients experience a better outcome than nontreated patients with wake-up stroke. Because early recanalization of the occluded vessel is known to be among the most important predictors of favorable clinical outcome,3,4 it is crucial to identify those patients with salvageable brain tissue and to re-establish flow within the occluded vessel segment as fast as possible.
A variety of endovascular recanalization techniques have been developed during recent years, most recently recanalization with stent-retriever devices.5,6 The thrombus formation is entrapped between the stent strut and the vessel wall, and rapid antegrade flow restoration providing oxygen supply to the brain tissue is achieved. In addition, thrombectomy is performed by pulling back the deployed stent into the guiding catheter with the stent struts engaging the thrombus material.
In this report, we describe our experiences with stent-retriever recanalization in patients with wake-up stroke and occlusion in the anterior circulation.
Materials and Methods
Approval for collection of interventional and clinical data was given by the institutional review board.
We retrospectively analyzed the angiographic and clinical data of 19 patients with wake-up stroke and occlusion in the anterior circulation who were treated with stent-retriever recanalization between 2009 and October 2011.
At admission, all patients were examined by a stroke neurologist. Thirteen patients underwent stroke MR imaging, including MRA and diffusion/perfusion imaging to demonstrate vessel occlusion and to assess the extent of salvageable brain tissue; 6 patients underwent CT/CTA and perfusion CT imaging.
Inclusion for interventional stroke treatment was based on the following criteria:
1) Severe neurologic deficit (eg, NIHSS ≥10); 2) Major-vessel occlusion (ICA/M1/2) on CTA or MRA; 3) Diffusion/perfusion mismatch of >50% and DWI lesion of less than one-third of the MCA territory on stroke MR imaging or CBV/TTP mismatch of >50% on CTP; 4) Absence of early signs of a major infarction (more than one-third of the MCA territory) with simultaneously reduced perfusion in the territory of the occluded vessel on CT.
Follow-up CT or MR imaging was performed 20–36 hours after the intervention or whenever significant neurologic worsening occurred. Symptomatic intracerebral hemorrhage was defined as neurologic deterioration of at least 4 points on the NIHSS or death, together with blood at any site in the brain.7 Neurologic status was quantified by NIHSS at admission and discharge, and the modified Rankin Scale was assessed at discharge and at day 90 by a certified neurologist. Good outcome at 90 days was defined as mRS 0–2.
Patients were assessed for the following parameters: site of occlusion (carotid bifurcation/MCA or MCA alone), time to flow restoration (defined as the time interval between the first diagnostic angiogram and the first angiogram with evidence of perfusion within the occluded vessel segment), time to complete revascularization (defined as the time interval between the first diagnostic angiogram and the completion angiogram), number of stent passes to achieve recanalization, hemorrhagic transformation according to the ECASS classification,7 and clinically significant procedure-related complications. Furthermore, additional application of IV or intra-arterial thrombolytic drugs was noted.
Endovascular Procedure
All interventions were performed by consultant neuroradiologists on a biplanar system (Artis zee biplane system; Siemens, Erlangen, Germany) with the patient under general anesthesia. Using transfemoral access, we placed an 8F guiding catheter in the common carotid artery, and a 6F Neuron catheter (Penumbra, Alameda, California) was advanced into the internal carotid artery close to the target vessel segment. The thrombus was passed by using a Rebar-18 microcatheter (ev3, Irvine, California). Angiographic runs were performed to verify that the microcatheter tip position was distal to the thrombus and to estimate the length of the occlusion. Subsequently, the stent retriever device (Solitaire, ev3; or Revive; Codman Neurovascular, Raynham, Massachusetts) was released by pulling back the microcatheter while holding the retriever device in place. Again, angiographic runs were performed to evaluate whether flow restoration was achieved. Then, the deployed device and the microcatheter were slowly retrieved under continuous aspiration through the intermediate catheter. This maneuver was repeated until recanalization was achieved.
Successful recanalization was defined as TICI ≥ 2. The Thrombolysis in Cerebral Infarction score is a 4-point scale.8 TICI 0 describes absence of perfusion beyond the occlusion site. TICI 3 is equivalent to complete perfusion. TICI scores were estimated from the angiographic runs before and after the interventional procedure in consensus by 2 neuroradiologists (S.S., S.R.).
Statistics
Continuous data are described by median and interquartile range or mean and SD. Statistical analysis was performed by GraphPad Prism 5.0 for Mac OSX (GraphPad Software, San Diego, California).
Results
Overall, 19 patients (mean age, 73.7 ± 6.7 years; range, 64–91 years; female/male, 12:7) were included. At admission, the median NIHSS score was 17 (IQR, 15–20). Eleven patients had left-sided strokes with concomitant aphasic symptoms.
Imaging revealed early signs of infarction or diffusion restriction in 17 patients. In 12 of these patients, the changes were located in the basal ganglia. In 2 patients who underwent CT/CTA, no early changes within the brain parenchyma were found. Twelve patients had an occlusion of the MCA, and 7 patients, of the carotid bifurcation and MCA. A median of 2.0 (IQR, 2–4) stent-retriever passes was necessary to achieve recanalization. Twelve patients were treated with the Revive device alone; 5 patients, with the Solitaire stent alone; and 2 patients, with both devices. In 1 patient, permanent implantation of the Solitaire stent was performed to maintain stable perfusion within the recanalized vessel segment. In 5 patients, stent placement of a proximal ICA stenosis was necessary to get access to the intracranial vasculature before thrombectomy. These patients received tirofiban for 24 hours, and postprocedural medication with aspirin, 100 mg/day, and clopidogrel, 75 mg/day, was initiated.
Mean time to flow restoration was 36.7 minutes (range, 12–91 ± 24.2 minutes) and to complete recanalization, 83.7± 34.6 minutes (range, 30–180 minutes) from the beginning of the intervention. In 12 patients intravenous and in 2 patients additional intra-arterial thrombolytic medication was applied. Before the thrombectomy procedure, the TICI score was 0 in 18 patients and 1 in 1 patient. In 1 patient, recanalization was not successful (5.3%). In this patient, long-segment occlusion of the right MCA was found. Despite several thrombectomy attempts, the occlusion persisted. Mean thrombus length was 14.5 ± 6.7 mm. After the procedure, a median TICI of 2.0 (IQR, 2.0–3.0) was calculated (without differentiation into TICI 2a and b; TICI 0, n = 1, 5.3%; TICI 2a, n = 8, 42.1%; TICI ≥ 2b, n = 10, 52.6%).
Complications
In 1 patient, vessel perforation (M2 branch) with a microwire occurred and resulted in a small subarachnoidal hemorrhage without clinical sequelae. In another patient, perforation of an M3 branch was caused by a microwire, which resulted in subarachnoidal hemorrhage. In this patient, concomitant hemorrhage in the basal ganglia (PH 2) occurred.
Overall, we found the following hemorrhagic transformations: small petechiae (HI 1) in 6 patients, hemorrhage occupying <30% of the infarcted area (PH 1) in 1 patient, and hemorrhage occupying >30% of the infarcted area (PH 2) in 4 patients. Symptomatic ICH occurred in 4 (21.1%) patients.
Clinical Outcome
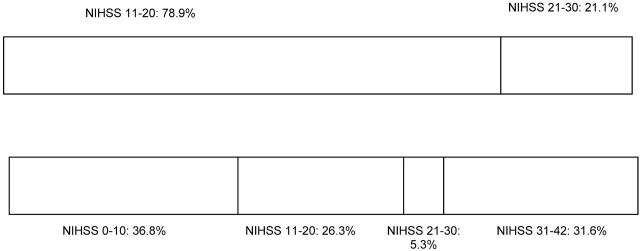
Eight patients (42.1%) had an NIHSS improvement of ≥4 points between admission and discharge (Figure). Seven patients died within the hospital stay due to extensive brain infarction. In 4, additional space-occupying hemorrhage occurred. Overall, after 90 days, median mRS was 4 (IQR, 3–6; mRS 1, n = 2; mRS 3, n = 5; mRS 4, n = 5; mRS 6, n = 7).
Figure.
NIHSS change between admission (first row) and discharge (second row).
Discussion
Recently, stent-retriever devices have been developed and play an increasing role in endovascular recanalization procedures. With these devices, high recanalization rates (89%–100%) and rapid recanalization with a good clinical outcome in a substantial number of patients can be achieved.6,9 It has been shown that stent-retriever devices outperform conventional recanalization devices. The Solitaire with the Intention for Thrombectomy (SWIFT) trial, presented at the International Stroke Conference in New Orleans in February 2012,10 compared the angiographic and clinical results of patients with acute stroke with a symptom onset of <8 hours, who were treated with either the Merci retriever (Concentric Medical, Mountain View, California) or the Solitaire stent. The SWIFT investigators demonstrated that with the Solitaire stent, a higher vessel recanalization rate and a better functional outcome of the treated patients could be achieved.
To our knowledge, all publications describing stent-retriever recanalization include only patients with known short time windows, but no publication about the use of stent-retriever devices in patients with wake-up stroke exists.
The time interval for intravenous rtPA has been shifted from 3 to 4.5 hours after stroke onset.11–13 However, there is evidence that patients with salvageable brain tissue benefit from thrombolytic treatment up to 9 hours after stroke onset14,15 and that thrombolysis is safe in patients with wake-up stroke.2 Correspondingly, patients with wake-up stroke who have MR imaging–proved diffusion/perfusion mismatch may be candidates for endovascular mechanical interventions.
In the literature, there are only a few reports of patients who underwent endovascular acute stroke treatment within an uncertain or extended time window. Natarajan et al16 performed a retrospective review of 30 patients who underwent endovascular recanalization >8 hours after stroke onset, including wake-up stroke. In this study, endovascular intervention included various techniques such as intra-arterial thrombolysis, mechanical thrombectomy by using the Merci device or intracranial stents (no stent-retriever devices), and angioplasty, with 20% of patients presenting with mRS 0–2 at 3 months. Findings in our study are similar, with unsatisfactory outcome in most patients despite high recanalization rates.
Compared with studies evaluating thrombectomy patients with a known time of symptom onset,6 mRS at 90 days is less favorable in patients with wake-up stroke. This can be explained with time-dependent progression of brain cell destruction within the infarction rim zone.
However, 36.8% of our patients had an acceptable 3-month outcome (mRS 0–3), and 8 patients had an NIHSS improvement of ≥4 points (42.1%). Recanalization with stent-retriever devices was technically successful in 94.7%, which corresponds to the results of previous stent-retriever studies with a symptom onset of ≤6 hours (89%–100%6,9). However, this recanalization rate is considerably higher than that described by Natarajan et al,16 (66.7%) who used various recanalization techniques but no stent-retriever devices for endovascular recanalization.
In our study, bleeding rates were higher than those in other interventional stroke series: Four patients presented with hemorrhage classified as sICH according to ECASS (21.1%).
As an obvious limitation of our study, the number of patients was small (n = 19), a retrospective data analysis was performed, and there was no control group.
In this series of patients with wake-up-stroke, mechanical thrombectomy with stent-retriever devices was technically successful in >90%. If one keeps in mind the severity of strokes of this cohort predominantly with CTA- or MRA-proved mainstem occlusions, favorable clinical outcome (mRS 0–2) could be achieved in 10.5%; 42.1% of patients had an NIHSS improvement of ≥4 points. Bleeding complications were higher compared with those in other interventional stroke series (21.1%).
However, our results raise the question of an improved patient selection for invasive stroke treatment, in particular for patients beyond the 4.5-hour time window. In this study, patients with wake-up stroke were referred for mechanical thrombectomy if they had a diffusion lesion of less than one-third within the territory of the middle cerebral artery and a perfusion/diffusion mismatch of >50%. These criteria also included patients with a substantial MCA infarction at the time of imaging and may explain, in part, the relatively unsatisfactory clinical outcome. The evaluation of diffusion and perfusion maps was based on visual estimation only. Furthermore, collateral supply, which probably influences the infarction extent as well, was not evaluated.
The recently presented preliminary results of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) 2 study17 and the Echoplanar Imaging Thrombolysis Evaluation Trial18 demonstrated that a defined target mismatch profile [PWI (time to maximum > 6 seconds) / DWI ≥1.8 and DWI < 70 mL and PWI (time to maximum > 10 seconds) < 100 mL] and a defined time to maximum threshold may predict more accurately the likelihood of cerebral ischemia and clinical outcome. Furthermore, DEFUSE 2 showed that clinicians in an emergency setting were able to analyze MR imaging profiles by using a fully automatic MR imaging analysis program (RAPID;Stanford Stroke Center, Palo Alto, California) to evaluate DWI and PWI lesions and to identify which patients have the target mismatch profile. In patients without this target mismatch profile, early reperfusion did not result in a reduction of infarct growth. In the future, such clearly predefined criteria might help to select patients who would possibly benefit from endovascular recanalization and to separate them from those patients who would only be exposed to the risks of endovascular therapy without potential improvement of their condition.
Conclusions
Recanalization with stent-retriever devices was feasible in patients with wake-up stroke. However, this study underlines the difficulty of patient selection for endovascular treatment. Although recanalization rates are similar, the outcome of severely affected patients with wake-up stroke was worse compared with studies including patients with stroke within 6 hours after onset. In the future, defined target mismatch profiles and evaluation of collateral supply might help to predict the probability of favorable outcome.
ABBREVIATIONS:
- ECASS
European Cooperative Acute Stroke Study
- IQR
interquartile range
- mRS
modified Rankin Scale
- sICH
symptomatic intracerebral hemorrhage
- TICI
Thrombolysis in Cerebral Infarction
Footnotes
Disclosures: Christian Herweh—UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: European Society of Minimally Invasive Neurological Therapy teaching course 2011, Comments: My participation at the ESMINT teaching course 2011 in Barcelona was sponsored by Codman. Martin Bendszus—RELATED: Consulting Fee or Honorarium: courses and talks for Codman, Micrus Endovascular, ev3, Payment for Lectures (including service on Speakers Bureaus): Siemens, Bayer, Novartis, Boehringer, Codman, Micrus Endovascular, ev3, Guerbet. Stefan Rohde—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Codman, Johnson & Johnson, Comments: speaker honoraria (<€1500).
References
- 1. Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002;33:988–93 [DOI] [PubMed] [Google Scholar]
- 2. Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke 2009;40:827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73:1066–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 5. Castaño C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010;41:1836–40 [DOI] [PubMed] [Google Scholar]
- 6. Rohde S, Haehnel S, Herweh C, et al. Mechanical thrombectomy in acute embolic stroke: preliminary results with the Revive device. Stroke 2011;42:2954–56 [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51 [DOI] [PubMed] [Google Scholar]
- 8. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–37 [DOI] [PubMed] [Google Scholar]
- 9. Stampfl S, Hartmann M, Ringleb PA, et al. Stent placement for flow restoration in acute ischemic stroke: a single-center experience with the Solitaire stent system. AJNR Am J Neuroradiol 2011;32:1245–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saver J. Primary results of the Solitaire TM with the Intention for Thrombectomy (SWIFT) study. In: Proceedings of the International Stroke Conference, New Orleans, Louisiana. February 1–3, 2012 [Google Scholar]
- 11. Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT(DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol 2009;8:141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29 [DOI] [PubMed] [Google Scholar]
- 13. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703 [DOI] [PubMed] [Google Scholar]
- 14. Hacke W, Albers G, Al-Rawi Y, et al. The desmoteplase in acute ischemic stroke trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73 [DOI] [PubMed] [Google Scholar]
- 15. Furlan AJ, Eyding D, Albers GW, et al. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–31 [DOI] [PubMed] [Google Scholar]
- 16. Natarajan SK, Snyder KV, Siddiqui AH, et al. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke 2009;40:3269–74 [DOI] [PubMed] [Google Scholar]
- 17. Albers G. Results of DEFUSE 2: imaging end points. In: Proceedings of the International Stroke Conference, New Orleans, Louisiana. February 1–3, 2012 [Google Scholar]
- 18. Nagakane Y, Christensen S, Ogata T, et al. Moving beyond a single perfusion threshold to define penumbra: a novel probabilistic mismatch definition. Stroke 2012;43:1548–55 [DOI] [PubMed] [Google Scholar]