Abstract
BACKGROUND AND PURPOSE:
The prevalence and topography of small hypointense foci suggesting microbleeds on 3T SWI in various types of dementia have not been systematically investigated. The purpose of this study was to determine the prevalence and topography of SHF on 3T SWI in patients with different dementia subtypes.
MATERIALS AND METHODS:
We included 347 consecutive patients (217 women, 130 men; age range, 42–93 years; mean age, 74 years) who attended our memory clinic and underwent 3T SWI. They were divided into 6 groups: subjective complaints, MCI, AD, DLB, VaD, and FTLD. Two neuroradiologists evaluated the number and location of SHF on SWIs. Statistical analyses were performed to evaluate inter- and intragroup differences.
RESULTS:
Of the 347 patients, 160 (46.1%) exhibited at least 1 small hypointense focus. This was true in 86% with VaD, 54% with DLB, 48% with AD, 41% with MCI, 27% with FTLD, and 22% with subjective complaints. With the subjective complaints group as a reference, the odds ratio adjusted by age, sex, and arterial hypertension was 9.2 (95% CI, 2.0–43.6) for VaD; 5.4 (95% CI, 1.2–24.3) for AD; 3.1 for DLB (95% CI, 1.1–8.8); 2.0 for MCI (95% CI, 0.5–8.1); and 1.5 for FTLD (95% CI, 0.4–5.4). There was a significant lobar predilection for AD, DLB, and FTLD groups (P < .05).
CONCLUSIONS:
On 3T SWI, patients with VaD, AD, and DLB manifested a high SHF prevalence. In patients with AD, DLB, and FTLD, the SHF exhibited a lobar predilection.
Dementia is a growing medical, social, and economic problem. Approximately 24 million individuals have this disease globally, and their number is expected to double every 20 years to reach 81 million by 2040.1 Among dementias, AD is the most common primary neurodegenerative disease.2 Patients with amnesic MCI are at high risk for progression to AD.3 VaD is induced by cerebrovascular disease; it is considered the most common secondary cause of dementia.4,5 Less common but important causes of dementia are DLB and FTLD.5,6
Small hypointense foci in the brain on T2*-weighted gradient recalled-echo and SWI are thought to be microbleeds.7–9 SHF are associated with symptomatic intracerebral hemorrhage, hypertension, and advanced age.10–14 Male sex, smoking, and diabetes mellitus may be risk factors for SHF.10,14,15 On MR imaging, SHF are associated with radiologic signs of small-vessel disease, white-matter hyperintensities, and lacunar infarcts.11,14,16–18 Histologically, they represent previous extravasation of blood and are related to bleeding-prone microangiopathies of different origins (eg, lipohyalinosis, amyloid deposition).8 Deep subcortical SHF are thought to be associated with vascular risk factors,11 and lobar SHF are usually attributed to vascular β-amyloid deposits (cerebral amyloid angiopathy).11,19–21
Among patients with cognitive disorders, those with AD, MCI, and VaD tend to have SHF.22 In healthy subjects, the prevalence of SHF detected by 2D T2*-weighted GRE imaging ranged from 0% to 21%14,23,24; it increased to 32% in patients with AD,25 to 20% in patients with MCI,22 and to 85% in patients with VaD.26 Although histologic studies found a relatively high prevalence of microhemorrhages in the brains of patients with DLB at postmortem examination,27 no MR imaging studies examining SHF in patients with DLB have been reported. Although there are a few studies of SHF detected on SWI of control subjects and patients with the limited type of dementia,21,28 no cohort studies have evaluated the prevalence and topography of SHF on 3T SWI in various types of dementia. The purpose of this study was to investigate the prevalence and topography of SHF on 3T SWI in patients with different dementia subtypes.
Materials and Methods
Study Population
All procedures followed the Clinical Study Guidelines of the Ethics Committee of Kumamoto University Hospital and were approved by the internal review board. A complete description of all procedures was provided to the patients, and written informed consent was obtained from them or their caregivers.
We collected data from 592 consecutive patients who attended the Dementia Clinic of the Department of Neuropsychiatry, Kumamoto University Hospital, from January 2008 to February 2010. All patients were examined comprehensively by 2 senior neuropsychiatrists (M.I., M.H.) having sufficient experience in examining patients with dementia. Routine laboratory and standardized neuropsychological tests, such as the Mini-Mental State Examination, brain MR imaging, and single-photon emission tomography were also performed; all results were incorporated into the diagnosis. The diagnoses were made by a team of neuropsychiatrists, neuropsychologists, and radiologists. We excluded 245 patients who met the following exclusion criteria: 1) severe behavioral problems that would make MR imaging difficult; 2) evidence of focal brain lesions on MR imaging such as posttraumatic brain injury or brain tumor; 3) diagnosis of depression, posttraumatic brain injury, idiopathic normal pressure hydrocephalus, or other neurodegenerative diseases (eg, corticobasal degeneration, progressive supranuclear palsy, Parkinson disease with dementia); 4) history of serious psychiatric diseases, substance abuse, or developmental abnormalities; 5) inability to obtain informed consent; or 6) SWI with severe motion or susceptibility artifacts. Consequently, clinical and MR imaging data on 347 patients were used in this prospective study.
All diagnoses were based on pre-established criteria: for AD, fulfilling the criteria for probable AD of the National Institute of Neurologic Disorders and Stroke/Alzheimer Disease and Related Disorders Association29; for VaD, fulfilling the criteria for probable VaD of the National Institute of Neurologic Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences30; for MCI, fulfilling the general criteria of the International Working Group on MCI31; for DLB, fulfilling the clinical criteria of the consortium on DLB32; and for FTLD, fulfilling the Lund-Manchester criteria for behavioral variant frontotemporal dementia, semantic dementia, or progressive nonfluent aphasia.33 There were 162 patients (47%) with AD, 51 (15%) with MCI, 41 (12%) with DLB, 33 (10%) with FTLD, and 28 (8%) with VaD. When all clinical investigation results were normal, the patients were recorded as having subjective complaints (n = 32, 9%). Hypertension was judged as present when either a systolic pressure of >140 mm Hg or a diastolic pressure of >90 mm Hg was demonstrated on repeat examinations or when a history of treatment for hypertension was present.
MR Imaging Protocol
MR imaging was performed on a 3T unit (Magnetom Trio; Siemens, Erlangen, Germany). The MR imaging protocol included axial SWI (64 sections per slab, FOV = 230 mm, matrix = 256 × 256, section thickness = 2 mm, voxel size = 0.9 × 0.9 × 2 mm, TE = 20 ms, TR = 27 ms, flip angle = 15°), axial FLAIR, axial T2-weighted turbo spin-echo, 3D T1-weighted magnetization-prepared rapid acquisition of gradient echo sequences and diffusion-weighted imaging, MR spectroscopy, and MR angiography. SWI processing was with software incorporated into the MR imaging system console (Siemens) according to published methods.34 SWI was constructed by multiplying magnitude by filtered phase images to enhance the susceptibility effect, followed by 16-mm minimum intensity projection reconstruction.
Evaluation of Microbleeds and Other Findings on MR Imaging
On a PACS workstation, all SWI was independently analyzed by 2 neuroradiologists (H.U., T.H.) blinded to clinical data. They reviewed divergent evaluations to reach a consensus. They assessed the number and location of SHF on 2-mm contiguous SWI; 16-mm minimum intensity projection SWI was also used to differentiate SHF from veins. SHF suggesting microbleeds were defined as small (<10 mm diameter), homogeneous, round foci of low signal intensity (Fig 1). Symmetric hypointensities in the globi pallidi or dentate nuclei thought to reflect physiologic calcification or iron deposits, flow void artifacts of pial blood vessels, and hyposignals inside a lesion compatible with an infarct were not recorded as SHF suggesting microbleeds because they could reflect hemorrhagic transformations. Because SWI has skull base artifacts that limit the view of the base of the brain, we excluded the evaluation of SHF in that location. SHF were counted throughout the brain and categorized as SHF in the basal ganglia/thalamus (including the internal and external capsule), infratentorial (brain stem and cerebellum), and lobar (cerebral cortex and subcortical and periventricular white matter) regions. SHF in the lobar region were subgrouped as frontal, temporal, parietal, and occipital. When at least 1 small hypointense focus was detected, the region or area of the brain was defined as SHF-positive.
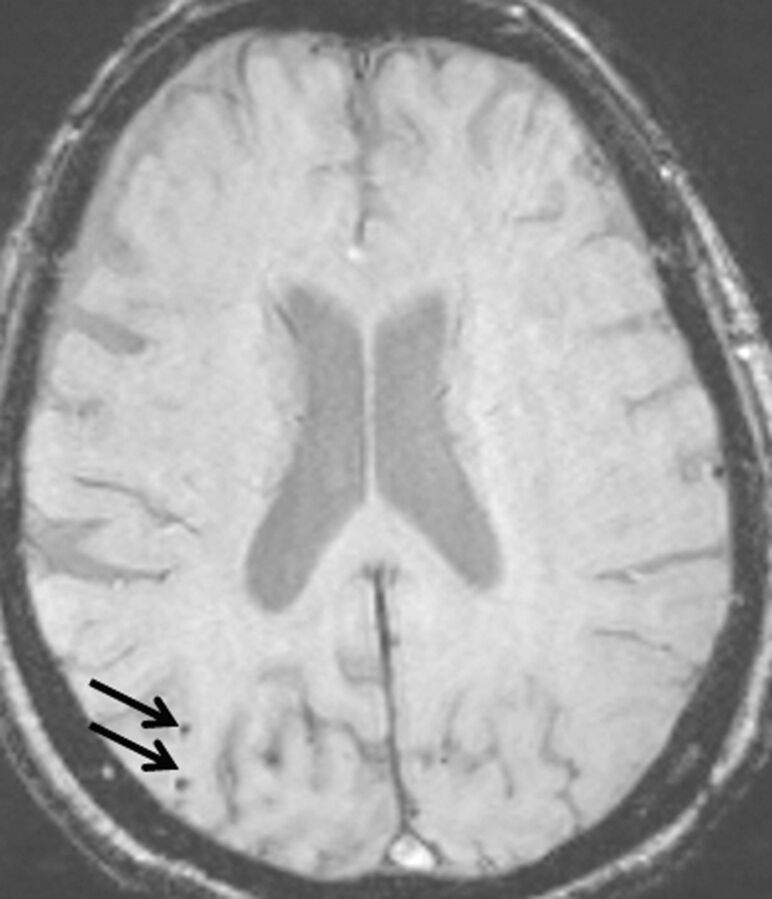
Fig 1.
A 78-year-old woman with AD without arterial hypertension. Her Mini-Mental State Examination score was 8. On a 2-mm SWI image, 2 small hypointense foci are seen in the right occipital lobe (arrows).
With regard to assessment of small-vessel disease on MR imaging, image analysis was performed in consensus by 2 radiologists (H.U., T.H.). Lacunar infarcts were defined as small round- or oval-shaped infarcts of <15 mm in diameter, with high signal intensity on T2-weighted images; low signal intensity on magnetization-prepared rapid acquisition of gradient echo and FLAIR sequences, ruling out enlarged perivascular spaces; and patchy leukoaraiosis. Lacunar infarcts were considered present or absent when there was at least 1 in the basal ganglia/thalamus or brain stem. White matter hyperintensities were graded on FLAIR sequences by using a previously described method as grades 0–3 (absent, punctuate, early confluent, or confluent abnormalities).35
Statistical Analyses
Statistical analyses were performed with the Statistical Package for the Social Sciences, Version 19 (SPSS, Chicago, Illinois). Interobserver agreement between 2 readers of SWI with respect to the number of SHF-positive regions was determined by calculating the κ coefficient (κ < 0.20, poor; κ = 0.21–0.40, fair; κ = 0.41–0.60, moderate; κ = 0.61–0.80, good; κ = 0.81–0.90, very good; and κ > 0.90, excellent agreement). Inter- and intragroup differences were assessed with the χ2, Fisher exact, or Student t test. Group comparisons with respect to the number of SHF were performed by using the Kruskal-Wallis test. Crude and adjusted odds ratios and the accompanying 95% confidence interval were calculated for every diagnostic group by using the patients with subjective complaints as the reference group. To adjust for age, sex, and arterial hypertension, we performed logistic regression analyses between the subjective complaints group and each of the other groups. Differences of P < .05 were considered statistically significant.
Results
Prevalence of Microbleeds
Interobserver agreement between 2 readers of SWI with respect to the number of SHF-positive regions was very good (κ = 0.87). Among the 347 patients, 160 (46.1%) had at least 1 small hypointense focus, 30% had one, 17% had two, 20% had 3–5, and 33% had >5 SHF. The mean patient age at the time of the MR imaging study was 74.3 ± 8.8 years; 130 (37.5%) were men. The mean Mini-Mental State Examination score was 21.0 ± 5.3. The prevalence of SHF differed significantly with age, sex, hypertension, and Mini-Mental State Examination (P < .05). Among patients 75 years or older (n = 204), 55% harbored SHF compared with 33% in patients younger than 75 years (n = 143) (χ2 = 16.27, P = .0001). Men had a higher prevalence than women (57% versus 40%) (χ2 = 9.10, P = .0026); 66% of patients with and 29% of those without hypertension manifested SHF (χ2 = 44.06, P < .0001). There was a significant difference between microbleeds and Mini-Mental State Examination (SHF-positive: mean, 20.3 ± 5.2; microbleed-negative, mean, 21.7 ± 5.4; t = 2.44, P = .015). The median number of SHF (interquartile range) for each group was subjective complaints = 1 (1–2), MCI = 2 (1–7), AD = 3 (1–7), DLB = 2 (1–5), FTLD = 3 (2–5), and VaD = 15 (2–32).
As shown in Table 1, the prevalence of SHF among the groups differed significantly (P < .0001); 86% with VaD, 54% with DLB, 48% with AD, 41% of MCI, and 27% with FTLD harbored SHF. Of our patients with subjective complaints, 22% manifested SHF. Logistic regression analysis, adjusted for age, sex, and hypertension, showed that the adjusted odds ratio (95% CI) for SHF, by using the subjective complaints group as a reference, was 9.2 (2.0–43.6) for VaD, 5.4 (1.2–24.3) for AD, 3.1 (1.1–8.8) for DLB, 2.0 (0.5–8.1) for MCI, and 1.5 (0.4–5.4) for FTLD. There was a statistically significant difference between the subjective complaints and the VaD, AD, or DLB group (P < .05).
Table 1:
Patient characteristics and SHF prevalence in each dementia subgroup
| SC (n = 32) | MCI (n = 51) | AD (n = 162) | DLB (n = 41) | FTLD (n = 33) | VaD (n = 28) | |
|---|---|---|---|---|---|---|
| Age (mean) (yr) | 71 ± 11 | 76 ± 8 | 75 ± 9 | 77 ± 6 | 68 ± 9 | 76 ± 8 |
| Men (No.) (%) | 6 (19) | 22 (43) | 54 (33) | 20 (49) | 14 (42) | 14 (50) |
| MMSE (mean) | 28 ± 2 | 25 ± 2 | 20 ± 4 | 19 ± 5 | 17 ± 7 | 19 ± 5 |
| Hypertension, (No.) (%) | 11 (35) | 28 (55) | 66 (41) | 23 (56) | 8 (24) | 24 (86) |
| SHF (No.) (%) | 7 (22) | 21 (41) | 77 (48) | 22 (54) | 9 (27) | 24 (86) |
| Odds ratio (95% CI) | 1 (ref.) | 2.5 (0.9–6.8) | 3.2 (1.3–7.9) | 4.1 (1.5–11.7) | 1.3 (0.4–4.2) | 21.4 (5.6–82.7) |
| Adjusted odds ratioa (95% CI) | 1 (ref.) | 2.0 (0.5–8.1) | 5.4 (1.2–24.3)b | 3.1 (1.1–8.8)b | 1.5 (0.4–5.4) | 9.2 (2.0–43.6)b |
Note:—ref. indicates reference; SC, subjective complaints; MMSE, Mini-Mental State Examination.
Logistic regression analyses adjusted for age, sex, and arterial hypertension were performed in the SC group and each of the dementia groups.
Statistically significant difference, P < .05.
Topography of Microbleeds
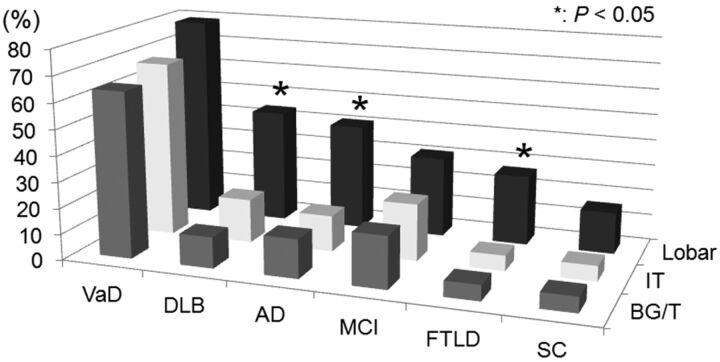
The lobar region was the most frequent site of SHF in each group (Table 2 and Fig 2); 136 (39.2%) of the 347 patients were found to have at least 1 small hypointense focus in this region. The SHF prevalence of lobar, basal ganglia/thalamus, and infratentorial regions was 41%, 15%, and 14% for patients with AD; 44%, 12%, and 17% for those with DLB; and 27%, 6%, and 6% for those with FTLD, respectively. For these 3 groups, there was a significant difference in the SHF prevalence in the lobar versus basal ganglia/thalamus or infratentorial regions (P < .05) (Fig 2). This was not the case in patients with VaD, MCI, and subjective complaints. There were no statistically significant intragroup differences with respect to involvement of the frontal, temporal, parietal, and occipital areas in any of the groups.
Table 2:
Topography of SHF in each dementia subgroupa
| Total (n = 347) | SC (n = 32) | MCI (n = 51) | AD (n = 162) | DLB (n = 41) | FTLD (n = 33) | VaD (n = 28) | |
|---|---|---|---|---|---|---|---|
| Whole brain | 160 (46) | 7 (22) | 21 (41) | 77 (48) | 22 (54) | 9 (27) | 24 (86) |
| BG/T region | 62 (48) | 2 (6) | 10 (20) | 25 (15) | 5 (12) | 2 (6) | 18 (64) |
| IT region | 64 (18) | 2 (6) | 11 (22) | 23 (14) | 7 (17) | 2 (6) | 19 (68) |
| Brain stem | 32 (9) | 1 (3) | 7 (14) | 9 (6) | 2 (5) | 1 (3) | 12 (43) |
| Cerebellum | 48 (14) | 1 (3) | 7 (14) | 18 (11) | 6 (15) | 1 (3) | 15 (54) |
| Lobar region | 136 (39) | 5 (16) | 16 (31) | 66 (41) | 18 (44) | 9 (27) | 22 (79) |
| Frontal | 69 (20) | 2 (6) | 9 (18) | 30 (19) | 9 (22) | 5 (15) | 14 (50) |
| Temporal | 65 (19) | 3 (9) | 8 (16) | 27 (17) | 7 (17) | 3 (9) | 17 (61) |
| Parietal | 78 (22) | 3 (9) | 10 (20) | 31 (19) | 14 (34) | 3 (9) | 17 (61) |
| Occipital | 63 (18) | 1 (3) | 8 (16) | 32 (20) | 5 (12) | 4 (12) | 13 (46) |
Note:—SC indicates subjective complaints; BG/T, basal ganglia/thalamus; IT, infratentorial.
Data are the number of small hypointense foci–positive areas, regions, or brain, with percentages in parentheses.
Fig 2.
Graph of the topography of small hypointense foci in the different dementia subgroups. Boxes illustrate the percentage of SHF in each region. In each group, the lobar region was the most frequent site of SHF. *In patients with DLB, AD, or FTLD, the prevalence of SHF in the 3 regions of the brain was significantly different (P < .05). BG/T indicates the basal ganglia/thalamus region; IT, infratentorial region; Lobar, lobar region.
Prevalence of White Matter Hyperintensities and Lacunar Infarcts
The prevalence of white matter hyperintensities and lacunar infarcts in each dementia subgroup is shown in Table 3. Lacunar infarcts were observed in 71 of 347 (20%) patients. In patients with dementia, 82% with VaD, 26% with MCI, 17% with DLB, 16% with AD, and 6% with FTLD harbored lacunar infarcts. Our patients with subjective complaints did not have lacunar infarcts. With regard to white matter hyperintensities, confluent white matter hyperintensities were seen in 42 of 347 (12%) patients. Confluent white matter hyperintensities were observed in 54% of patients with VaD, 16% with MCI, 9% with AD, 5% with DLB, and 3% with FTLD. Of our patients with subjective complaints, 3% manifested confluent white matter hyperintensities.
Table 3:
Prevalence of white matter hypertensities and lacunar infarcts in each dementia subgroupa
| Total (n = 347) | SC (n = 32) | MCI (n = 51) | AD (n = 162) | DLB (n = 41) | FTLD (n = 33) | VaD (n = 28) | |
|---|---|---|---|---|---|---|---|
| White matter hypertensities | |||||||
| Absent | 32 (9) | 5 (16) | 3 (6) | 17 (10) | 1 (2) | 6 (18) | 0 (0) |
| Punctate | 166 (48) | 17 (53) | 28 (55) | 81 (50) | 19 (46) | 17 (52) | 4 (14) |
| Early confluent | 107 (31) | 9 (28) | 12 (24) | 49 (30) | 19 (46) | 9 (27) | 9 (32) |
| Confluent | 42 (12) | 1 (3) | 8 (16) | 15 (9) | 2 (5) | 1 (3) | 15 (54) |
| Lacunar infarcts | 71 (20) | 0 (0) | 13 (26) | 26 (16) | 7 (17) | 2 (6) | 23 (82) |
Note:—SC indicates subjective complaints; WMH, white matter hyperintensities.
Data are the number or presence of MR imaging findings, with percentages in parentheses.
Discussion
Our 3T SWI study disclosed a high prevalence of SHF among patients; 22% with subjective complaints, 27% with FTLD, 41% with MCI, 48% with AD, 54% with DLB, and 86% with VaD harbored SHF. Ours is the first study documenting the prevalence of SHF on SWI in patients with DLB and FTLD, to our knowledge. In healthy subjects or patients with subjective complaints, the prevalence of SHF depicted on 2D T2*-weighted gradient recalled-echo images ranged from 0% to 21%.14,23,24 It ranged from 18% to 32% in AD22,25 and from 65% to 85% in VaD22,26; in patients with MCI, it was reported to be 20%.22 On a 1.5T SWI study by Goos et al,28 the prevalence of SHF was 30% in subjective complaints, 39% in AD, and 44% in MCI. The sensitivity of SWI for microbleeds was reported to be 3–6 times higher than that of T2*-weighted gradient recalled-echo imaging.9,36 The depiction of microbleeds on SWI is reported to be more enhanced at 3T and 7T than at 1.5T.9,37 However, the prevalence of SHF on 3T SWI in our patients did not increase markedly compared with that on 1.5T SWI in the previous report.28 Although the exact reason is unknown, the difference in patient characteristics (eg, sex, arterial hypertension) might have affected the results.
The relative prevalence of SHF was different among our diagnostic groups. The adjusted odds ratio for SHF by using patients with subjective complaints as the reference was 9.2 for VaD, 5.4 for AD, 3.1 for DLB, 2.0 for MCI, and 1.5 for FTLD. A statistically significant difference was found between the subjective complaints and the VaD, AD, or DLB group. Cordonnier et al22 reported the relative prevalence of SHF in a cohort of patients attending a memory clinic. Their odds ratio for microbleeds using patients with subjective complaints as the reference was 15.9 for VaD, 2.1 for AD, and 2.3 for MCI. Because there are some differences between their study and ours, it may be difficult to compare the relative prevalence of SHF between the 2 studies. They used T2*-weighted gradient recalled-echo imaging at 1T, and the mean age of their subgroups was lower than ours. In addition, they did not adjust the odds ratios for age, sex, and hypertension, and they did not perform subgroup analysis of patients with DLB and FTLD.
Carbon 11 Pittsburgh Compound B studies revealed high β-amyloid cortical binding in almost all patients with AD,38,39 in 60% of those with MCI,39 and in >50% those with DLB40,41; in 25% of patients with FTLD, binding was low.42 Among the neurodegenerative dementia groups, the order of the prevalence of β-amyloid binding was similar to that of the relative prevalence of SHF in our study. On the basis of neuropathologic studies,23,43 microbleeds are frequently observed in the brains of patients with AD and are mainly related to β-amyloid pathology (cerebral amyloid angiopathy). Moreover, a neuropathologic study showed a relatively high prevalence of β-amyloid pathology and cortical microhemorrhages in the brains of patients with DLB27,44 and a low prevalence of β-amyloid pathology in patients with FTLD.27,45 We suggest that SHF on SWI may be associated with β-amyloid pathology in these diseases.
In all of our patient groups, the lobar region was the most frequent site of SHF. Although in patients with AD, DLB, and FTLD, there was a significant predilection for the lobar region, this was not the case in patients with VaD, MCI, and subjective complaints. SHF in the basal ganglia/thalamus or infratentorial region tend to be associated with vascular risk factors.11 In patients with MCI, the distribution pattern of SHF was more similar to VaD than AD. Staekenborg et al46 demonstrated that microbleeds, lacunar infarctions, and severe white matter hyperintensities in MCI were associated with progression to non-AD dementia such as VaD. In our patients with MCI, lacunar infarctions and confluent white matter hypertensities were seen in 26% and 16% of patients, respectively. Although we did not evaluate the progression of MCI to dementia in this study, the patients with small-vessel disease may have affected the distribution pattern of the microbleeds in MCI.
We observed that in patients with VaD, their SHF were almost equally distributed among the lobar, basal ganglia/thalamus, and infratentorial regions. This SHF distribution may be explained as follows: Because cerebral amyloid angiopathy has a lobar predilection and is associated with advancing age,47 it may have coexisted with lipohyalinosis in our elderly patients with VaD. Then, lobar SHF do not include periventricular or deep white matter according to the Microbleed Anatomical Rating Scale.48 Because we defined periventricular or deep white matter SHF as a lobar region, the definition may have affected the anatomic prevalence. In addition, our observers scaled a region as SHF-positive if they identified at least 1 small hypointense focus in an area or region. This assessment method may have influenced our results.
The distribution pattern of the microbleeds in patients with FTLD was similar to that in AD. A study of postmortem MR imaging by De Reuck et al49 demonstrated that microbleeds in patients with FTLD had a lobar prevalence. Their pathologic study revealed that cerebral amyloid angiopathy does not explain all of the microbleeds in the brain. Although the exact causes of microbleeds in patients with FTLD are not known, they suggested that microbleeds were associated with disturbances of the blood-brain barrier due to the severity of neurodegeneration.
Our study confirms earlier reports that documented the lobar distribution of SHF in AD.19,22,23,25 According to Pettersen et al,19 in patients with AD, SHF are primarily found at occipital sites. Among our patients with AD, there was no significant difference in the location of SHF in frontal, temporal, parietal, and occipital areas. When our observers identified at least 1 small hypointense focus, the area was recorded as SHF-positive. Our evaluation method differed from theirs, and this discrepancy may account for the difference. We did not evaluate the degree of accumulation of SHF in a specific area of the brain.
In our study, SHF were topographically similar in patients with AD and DLB because they manifested a predilection for the lobar region. We cannot explain the apparent preference for this region in patients with DLB. Earlier neuropathologic studies showed a relatively high prevalence of AD pathology and lobar microhemorrhages, including cerebral amyloid angiopathy pathology, in individuals with DLB.27,44 These findings support ours. Because our DLB group was of the most advanced mean age, the age factor might have played a role in the induction of cerebral amyloid angiopathy pathology in this group.
Our study has some limitations. First, patients with subjective complaints served as the control. In our subjective complaints group, lacunar infarcts were not observed and the frequency of confluent white matter hyperintensities was very low. Therefore, we think that the effect of small-vessel disease in our patients with subjective complaints was small. The prevalence of lobar SHF among elderly healthy controls with a mean age of 74.6 years reported by Yates et al,21 who used 3T SWI, was similar to ours. The mean age of our patients with subjective complaints was slightly lower than theirs. Subjective memory symptoms might be related to preclinical AD and, therefore, may be artificially increasing the number of microbleeds expected in the control group. Second, the number of patients in our subgroups was relatively small, and 3T SWI studies on larger populations are needed to elucidate the prevalence and topography of SHF in patients with dementia. Third, our study had a lack of pathologic confirmation of microbleeds. Schrag et al50 reported a correlative study of 3T SWI-identified hypointense foci to tissue pathology in postmortem brains of patients with AD. The correlation showed a variety of cerebral amyloid angiopathy–related pathologies: acute microhemorrhage, hemosiderin residua of old hemorrhages, and small lacunes ringed by hemosiderin. Their study suggests that hypointense foci on SWI in patients with AD indicate a variety of cerebral amyloid angiopathy–related pathologies. Finally, all diagnoses were based on pre-established clinical criteria without biomarker support of amyloid pathology or pathologic confirmation. This may create some errors in the associations.
Conclusions
The prevalence of SHF suggesting microbleeds on 3T SWI among the groups differed significantly; 86% of those with VaD, 54% of those with DLB, 48% of those with AD, 41% of those with MCI, 27% of those with FTLD, and 22% of those with subjective complaints harbored SHF. The adjusted odds ratio for SHF by using the subjective complaints group as a reference was 9.2 for VaD, 5.4 for AD, 3.1 for DLB, 2.0 for MCI, and 1.5 for FTLD. Patients with AD, DLB, and FTLD manifested a lobar predilection. Our findings provide further evidence not only for the involvement of vascular factors in these neurodegenerative diseases but also that SHF may even relate to amyloid pathology in specific diseases. Further studies are necessary to investigate the relationship of microbleeds to the disease pathogenesis, disease progression, and prognosis.
ABBREVIATIONS:
- AD
Alzheimer disease
- CI
confidence interval
- DLB
dementia with Lewy bodies
- FTLD
frontotemporal lobar dementia
- MCI
mild cognitive impairment
- SHF
small hypointense foci
- VaD
vascular dementia
References
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matthews FE, Brayne C, Lowe J, et al. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med 2009;6:e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langbaum JB, Chen K, Lee W, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Neuroimage 2009;45:1107–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikeda M, Hokoishi K, Maki N, et al. Increased prevalence of vascular dementia in Japan: a community-based epidemiological study. Neurology 2001;57:839–44 [DOI] [PubMed] [Google Scholar]
- 5. Garre-Olmo J, Genis Batlle D, del Mar Fernandez M, et al. Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology 2010;75:1249–55 [DOI] [PubMed] [Google Scholar]
- 6. Jellinger KA, Attems J. Prevalence and pathology of dementia with Lewy bodies in the oldest old: a comparison with other dementing disorders. Dement Geriatr Cogn Disord 2011;31:309–16 [DOI] [PubMed] [Google Scholar]
- 7. Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology 1996;46:1751–54 [DOI] [PubMed] [Google Scholar]
- 8. Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42 [PMC free article] [PubMed] [Google Scholar]
- 9. Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 2009;30:338–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007;130:1988–2003 [DOI] [PubMed] [Google Scholar]
- 11. Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–14 [DOI] [PubMed] [Google Scholar]
- 12. Henskens LH, van Oostenbrugge RJ, Kroon AA, et al. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension 2008;51:62–68 [DOI] [PubMed] [Google Scholar]
- 13. Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 2004;35:1831–35 [DOI] [PubMed] [Google Scholar]
- 15. Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam Scan Study. Stroke 2010;41:S103–06 [DOI] [PubMed] [Google Scholar]
- 16. Jeong SW, Jung KH, Chu K, et al. Clinical and radiologic differences between primary intracerebral hemorrhage with and without microbleeds on gradient-echo magnetic resonance images. Arch Neurol 2004;61:905–09 [DOI] [PubMed] [Google Scholar]
- 17. Kato H, Izumiyama M, Izumiyama K, et al. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 2002;33:1536–40 [DOI] [PubMed] [Google Scholar]
- 18. Wardlaw JM, Lewis SC, Keir SL, et al. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke 2006;37:2633–36 [DOI] [PubMed] [Google Scholar]
- 19. Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook Dementia Study. Arch Neurol 2008;65:790–95 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yates PA, Sirisriro R, Villemagne VL, et al. Cerebral microhemorrhage and brain beta-amyloid in aging and Alzheimer disease. Neurology 2011;77:48–54 [DOI] [PubMed] [Google Scholar]
- 22. Cordonnier C, van der Flier WM, Sluimer JD, et al. Prevalence and severity of microbleeds in a memory clinic setting. Neurology 2006;66:1356–60 [DOI] [PubMed] [Google Scholar]
- 23. Nakata-Kudo Y, Mizuno T, Yamada K, et al. Microbleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dement Geriatr Cogn Disord 2006;22:8–14 [DOI] [PubMed] [Google Scholar]
- 24. Vernooij MW, Ikram MA, Wielopolski PA, et al. Cerebral microbleeds: accelerated 3D T2*-weighted GRE MR imaging versus conventional 2D T2*-weighted GRE MR imaging for detection. Radiology 2008;248:272–77 [DOI] [PubMed] [Google Scholar]
- 25. Hanyu H, Tanaka Y, Shimizu S, et al. Cerebral microbleeds in Alzheimer's disease. J Neurol 2003;250:1496–97 [DOI] [PubMed] [Google Scholar]
- 26. Seo S, Hwa Lee B, Kim EJ, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke 2007;38:1949–51 [DOI] [PubMed] [Google Scholar]
- 27. De Reuck J, Deramecourt V, Cordonnier C, et al. Prevalence of small cerebral bleeds in patients with a neurodegenerative dementia: a neuropathological study. J Neurol Sci 2011;300:63–66 [DOI] [PubMed] [Google Scholar]
- 28. Goos JD, van der Flier WM, Knol DL, et al. Clinical relevance of improved microbleed detection by susceptibility-weighted magnetic resonance imaging. Stroke 2011;42:1894–900 [DOI] [PubMed] [Google Scholar]
- 29. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 30. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–60 [DOI] [PubMed] [Google Scholar]
- 31. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–46 [DOI] [PubMed] [Google Scholar]
- 32. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–72 [DOI] [PubMed] [Google Scholar]
- 33. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54 [DOI] [PubMed] [Google Scholar]
- 34. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 2005;22:439–50 [DOI] [PubMed] [Google Scholar]
- 35. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–56 [DOI] [PubMed] [Google Scholar]
- 36. Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 2003;227:332–39 [DOI] [PubMed] [Google Scholar]
- 37. Theysohn JM, Kraff O, Maderwald S, et al. 7 Tesla MRI of microbleeds and white matter lesions as seen in vascular dementia. J Magn Reson Imaging 2011;33:782–91 [DOI] [PubMed] [Google Scholar]
- 38. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–19 [DOI] [PubMed] [Google Scholar]
- 39. Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68:1718–25 [DOI] [PubMed] [Google Scholar]
- 40. Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 2008;79:1331–38 [DOI] [PubMed] [Google Scholar]
- 41. Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology 2008;71:903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rabinovici GD, Furst AJ, O'Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007;68:1205–12 [DOI] [PubMed] [Google Scholar]
- 43. Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology 1996;46:1592–96 [DOI] [PubMed] [Google Scholar]
- 44. Jellinger KA, Attems J. Cerebral amyloid angiopathy in Lewy body disease. J Neural Transm 2008;115:473–82 [DOI] [PubMed] [Google Scholar]
- 45. Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 2005;57:480–88 [DOI] [PubMed] [Google Scholar]
- 46. Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 2009;40:1269–74 [DOI] [PubMed] [Google Scholar]
- 47. Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 2011;7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gregoire SM, Chaudhary UJ, Brown MM, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 2009;73:1759–66 [DOI] [PubMed] [Google Scholar]
- 49. De Reuck J, Deramecourt V, Cordonnier C, et al. Detection of microbleeds in post-mortem brains of patients with frontotemporal lobar degeneration: a 7.0-Tesla magnetic resonance imaging study with neuropathological correlates. Eur J Neurol 2012;19:1355–60 [DOI] [PubMed] [Google Scholar]
- 50. Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol 2010;119:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]