SUMMARY:
Reversible cerebral vasoconstriction syndrome is a clinical and radiologic syndrome that represents a common presentation of a diverse group of disorders. The syndrome is characterized by thunderclap headache and reversible vasoconstriction of cerebral arteries, which can either be spontaneous or related to an exogenous trigger. The pathophysiology of reversible cerebral vasoconstriction syndrome is unknown, though alterations in cerebral vascular tone are thought to be a key underlying mechanism. The syndrome typically follows a benign course; however, reversible cerebral vasoconstriction syndrome may result in permanent disability or death in a small minority of patients secondary to complications such as ischemic stroke or intracranial hemorrhage.
Reversible cerebral vasoconstriction syndrome (RCVS) is a clinical and radiologic syndrome whose primary features include the hyperacute onset of severe headache and segmental vasoconstriction of cerebral arteries that resolves by 3 months.1–5 RCVS is not a single disease entity but should be considered a common presentation of multiple disorders characterized by reversible vasoconstriction of the cerebral vasculature.3,6–8 The term “RCVS” now encompasses what was previously thought to be a group of distinct clinical entities, including Call-Fleming syndrome, thunderclap headache, and postpartum angiopathy.4–6,8–11
Timely and accurate diagnosis of RCVS is essential to ensuring appropriate patient care and avoiding unnecessary diagnostic tests. However, the diagnosis can be challenging because its signs and symptoms can overlap those of better known disorders of the central nervous system, including aneurysmal subarachnoid hemorrhage and primary angiitis of the CNS.1,2,6,12–14 Furthermore, a key feature of RCVS, segmental arterial vasoconstriction, may be absent early in the course of the disease.1,2,4,5,14 Consequently, both the clinician and radiologist must maintain a high level of suspicion for this entity in patients presenting with characteristic features.
The first part of this article will review the history of RCVS, including the previously described clinical entities that it is now thought to include. We will then discuss the epidemiology, diagnostic criteria, and clinical presentations of this disorder and explore the association of RCVS with posterior reversible encephalopathy syndrome (PRES). In the second part, we will review the imaging features of RCVS, including more recent work exploring associated imaging changes in the cerebral arterial vasculature beyond segmental vasoconstriction.
Historical Background
Reversible segmental cerebral vasoconstriction has been described in the medical literature in a diverse array of clinical settings dating back to the 1960s.15–17 The earliest clinical reports associated this finding with the postpartum state, migraine headaches, unruptured cerebral aneurysms, and the use of vasoactive medication such as ergot derivatives. Initially, patients presenting with cerebral vasoconstriction were thought to have unique disease entities, depending on the given clinical scenario and specialist treating the patient (Table 1).4–6,8–11 The common features of these cases, including clinical presentation with severe headache, reversibility of angiographic findings, and lack of histologic abnormalities on arterial biopsy, were not well appreciated or understood.
Table 1:
Prior terms for RCVS
| Prior Terms |
|---|
| Migrainous vasospasm |
| Benign angiopathy of the central nervous system |
| Postpartum angiopathy |
| Thunderclap headache with reversible vasospasm |
| Sexual headache |
| Drug-induced angiopathy |
| Call-Fleming syndrome |
In 1988, Gregory Call and Marie Fleming described a unique clinical and radiographic syndrome in a small case series of 4 patients presenting with acute headache and reversible cerebral artery vasoconstriction.18 When the authors included 12 previously published case reports of patients presenting with similar findings, comprising some associated with migraines and postpartum state, they noted distinctive features of the syndrome, such as its association with hyperacute headache, transient or permanent neurologic deficits, normal or nonspecific findings on CSF analysis, and the lack of correlation between the resolution of patient symptoms and arterial vasoconstriction. In this small series, the authors demonstrated a wide range of possible clinical outcomes, from complete resolution of symptoms to permanent disability with hemiparesis and/or cortical blindness. The eponym “Call-Fleming syndrome” was subsequently used to describe the entity.
In 2007, Calabrese et al,6 made a case for unifying the various cerebral vasoconstriction syndromes, including Call-Fleming, under the term “reversible cerebral vasoconstriction syndrome” and proposed specific diagnostic criteria (Table 2). In recent years, our understanding of possible triggers, imaging findings, and the clinical course of RCVS has greatly improved. However, a good deal remains unknown about the syndrome. Although RCVS is becoming more widely recognized in the medical community, the overlap of its features with other conditions such as primary angiitis of the CNS continues to be a challenge. Finally, the heterogeneity of clinical and radiologic manifestations of RCVS, along with the diverse clinical settings in which it is encountered, strongly suggests that the syndrome represents a common end point of numerous disease processes, as opposed to a specific disease entity.3,6–8
Table 2:
Diagnostic criteria for RCVS
| Criteria |
|---|
| Severe, acute headaches, with or without additional neurologic signs or symptoms |
| Uniphasic disease course with no new symptoms after 1 month of onset |
| No evidence for aneurysmal SAH |
| Normal or near-normal findings on CSF analysis (protein level, <80 mg/dL; leukocyte level, <10/mm3; normal glucose level) |
| Multifocal segmental cerebral artery vasoconstriction demonstrated on either catheter angiography or indirectly on CTA/MRA |
| Reversibility of angiographic abnormalities within 12 weeks after onset. If death occurs before the follow-up studies are completed, postmortem rules out such conditions as vasculitis, intracranial atherosclerosis, and aneurysmal SAH, which can also manifest with headache and stroke |
Diagnostic Criteria
The key diagnostic criteria for RCVS proposed by Calabrese et al6 have since been slightly modified by the International Headache Society (Table 2). Although these criteria have not been validated in any prospective study, they have proved very useful clinically to diagnose RCVS and to increase physician awareness of the disease.
Epidemiology and Potential Triggers
Although the true incidence of RCVS remains uncertain, the syndrome does not appear rare on the basis of the rates of patient recruitment or presentation into prospective and retrospective studies.19 Furthermore, recent reports have suggested that the incidence of RCVS may be increasing, though it is unclear whether this reflects a true increase in the incidence of the syndrome versus a consequence of improved imaging techniques and physician awareness.20,21 Nevertheless, RCVS likely remains underdiagnosed and should be included in the differential diagnosis of young patients presenting with severe headache or cryptogenic stroke.1,5,21,22
RCVS commonly affects patients 20–50 years of age (mean, 42–45 years), though other age groups, including children and adolescents, can be affected.1,2,5,6,17,23–27 Most interesting, the mean age of men presenting with RCVS tends to be a decade younger than that of female patients (fourth decade).9,12 There is a female predominance, with an average female/male ratio from 3 large series of patients of approximately 2.4:1.2,5,9,17,23,24 RCVS does not appear to be limited to any single ethnic or racial group.19 As Ducros19 highlighted in her review of RCVS, differences in patient characteristics in large published series could reflect either intrinsic differences in RCVS among various patient populations and/or differences in patient recruitment criteria.
RCVS can occur spontaneously, without an obvious underlying cause, or can be secondary to an identifiable trigger (roughly 25%–60% of cases).2,3 The delay in exposure to an exogenous trigger and the development of RCVS can be anywhere between a few days and several months.2 In cases in which medications act as the exogenous trigger for the syndrome, patients may be taking the drug on a regular basis or infrequently, either at recommended dosages or in excess.2 For those patients without an obvious precipitant, RCVS may be induced in vulnerable individuals due to spontaneous or evoked vascular and/or neuronal discharges.6
A diverse group of possible exogenous triggers for secondary RCVS have been proposed, though the potential delay between exposure and development of the syndrome (in some cases weeks to months) and the ubiquity of some triggers (coughing, laughing, and so forth) raise the possibility that some of these associations may be coincidental (Table 1).2,3,6,11,23,28–38 However, the association of RCVS with the most commonly reported triggers is more compelling, including the use of vasoactive drugs and the postpartum state, which together account for more than half of cases in most published series (approximately 50% and 9%–10% of cases respectively).7,17,39 Sympathomimetic drugs commonly taken over the counter for upper respiratory tract infections, including phenylpropanolamine and pseudoephedrine, as well as antimigrainous medications, have historically been associated with subarachnoid hemorrhage and ischemic stroke in rare cases, which in retrospect likely reflects the sequelae of drug-induced RCVS.40,41 The association between RCVS and the postpartum state is thought to possibly reflect increased levels of both pro- and antiangiogenic factors, some of which have also been associated with eclampsia, such as placental growth factor.5 RCVS encountered in the postpartum period typically is encountered anywhere from 1 to 3 weeks following an uncomplicated pregnancy, though presentation as late as 6 weeks has been reported.42,43
RCVS is commonly associated with a history of migraine headaches (20%–40% of cases), which may, in part, be due to the known role of migraine medications as a trigger for the syndrome.1,5,17 Cervical arterial dissection has also been associated with RCVS, though it remains uncertain whether this represents a potential etiology or complication of the syndrome.7,9,44–47 In a prospective study identifying patients with RCVS or cervical arterial dissection, Mawet et al45 found that 12% of patients in the RCVS cohort (n = 173) had or developed cervical arterial dissection, while 7% of patients in the cervical dissection cohort (n = 285) developed RCVS. In rare cases, multiple cervical arterial dissections may be present.47 Finally, some published series have noted a significant association between RCVS and cannabis use.22
Pathogenesis
The pathophysiology of RCVS remains unknown. However, alterations in cerebral vascular tone leading to vasoconstriction are thought to be a key pathophysiologic mechanism underlying the development of RCVS.1,2,6,9,23,43 This hypothesis is supported by the lack of histologic changes noted in and around the cerebral vasculature in patients with RCVS who have undergone brain biopsy.1,43 Specifically, histologic and electron-microscopic analyses have failed to demonstrate evidence of active inflammation or vasculitis.1 Deregulation of cerebral vascular tone in RCVS may be induced by sympathetic overactivity, endothelial dysfunction, and oxidative stress.3,5,11,12,23,48,49 The association of RCVS with blood pressure surges, ingestion of sympathomimetic vasoactive substances, and pheochromocytoma support the role of sympathetic overactivity in its pathogenesis. On the other hand, a significant overlap between RCVS and PRES supports the importance of endothelial dysfunction, which is known to play an important pathophysiologic role in the latter. Because RCVS likely represents a common end point of a diverse group of disease processes, it is possible that the contribution of sympathetic overactivity and endothelial dysfunction to the onset of the syndrome varies depending on the incitant event in a given patient.
Various hormonal and biochemical factors have been suggested to play a role in the deregulation of cerebral vascular tone in RCVS, including estrogen, endothelin-1, serotonin, nitric oxide, and prostaglandins, some of which have been also associated with vasoconstriction following aneurysmal subarachnoid hemorrhage.5,6,11,48 For example, urine levels of 8-iso-prostaglandin F2α, a marker of oxidative stress and a potent vasoconstrictor, were found to correlate with disease severity in patients with RCVS.48 This finding suggests that oxidative stress may play a role in the pathogenesis of RCVS. It is unclear whether the vasoconstrictive properties of 8-iso-prostaglandin F2α contribute to the segmental vasoconstriction found in RCVS.48 Other factors, including placental growth factor, soluble placental growth factor receptor (soluble fms-like tyrosine kinase-1), and soluble endoglin, play a role in angiogenesis and have been implicated in the development of RCVS in the postpartum period.8
Genetic factors may influence an individual's susceptibility to developing RCVS and the severity of its subsequent clinical course. A specific genetic polymorphism (Val66Met) in the gene for brain-derived neurotrophic factor, which is important for neuronal survival, neurogenesis, and synaptic plasticity, has been associated with more severe vasoconstriction in patients with RCVS.50 Most interesting, brain-derived neurotrophic factor can also affect vascular function and has been associated with disorders of abnormal vascular tone and unstable angina.
Thunderclap Headache
The thunderclap headache is a defining clinical feature of RCVS and is defined as a severe, throbbing headache that reaches peak intensity within 60 seconds of onset (Fig 1). In RCVS, the pain is often bilateral and diffuse, though it can originate in the occipital region.1,2,5,6,9,14 Thunderclap headache has been reported in 94%–100% of patients with RCVS and may be the sole presenting symptom in 70%–76% of cases.2,6,9,51,52 Often, there is significant delay between the onset of headache and patient presentation for medical care (average, 7 days).9 The thunderclap headache can be associated with other symptoms, including nausea, emesis, diplopia, elevations in blood pressure, and photosensitivity.1,2,6,9,42,44 In patients with RCVS who have migraines, the thunderclap headache is typically described as differing in location, degree, and quality from their usual migraines.13 A minority of patients with RCVS may present with a more mild or subacute headache, though the complete absence of headache is rare.2,3,19
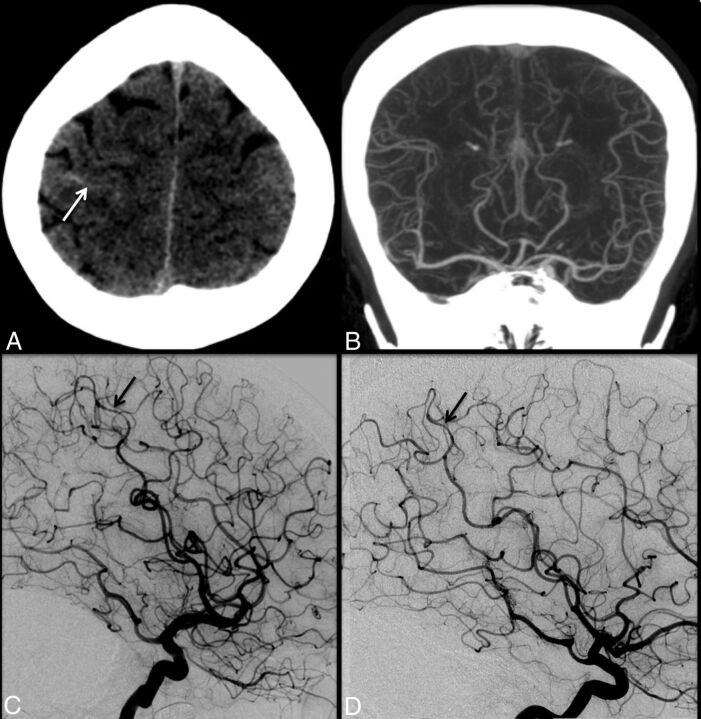
Fig 1.
A 47-year-old woman with the sudden onset of severe headache. Initial noncontrast head CT (A) demonstrates trace sulcal subarachnoid hemorrhage (white arrow) near the vertex. CT angiography performed at the same time (B) is interpreted as having unremarkable findings. Conventional angiography (C) demonstrates mild diffuse irregularity with multifocal narrowings throughout the cerebral vasculature with a beaded appearance, most pronounced in distal right middle cerebral artery cortical branches (black arrow). Findings are most consistent with RCVS. Follow-up catheter angiogram performed 1 month later (D) demonstrates complete resolution of cerebral vasoconstriction (black arrow).
Thunderclap headache is not specific for RCVS and can be associated with various other medical conditions, including aneurysmal subarachnoid hemorrhage, primary headache disorder, pituitary apoplexy, cerebral venous sinus thrombosis, unruptured cerebral aneurysm, cervical arterial dissection, and third ventricle colloid cyst, among others.53 In fact, prior reports suggest that RCVS will ultimately be diagnosed in less than half (45%) of patients presenting with a thunderclap headache.14,51 For example, Grooters et al14 found that only 8.8% of patients presenting to a single center with thunderclap headache and no evidence of aneurysmal subarachnoid hemorrhage were ultimately diagnosed with RCVS.
However, some characteristics of the thunderclap headache associated with RCVS may be more specific for the syndrome. For example, in contradistinction to patients with aneurysmal subarachnoid hemorrhage, the thunderclap headache associated with RCVS typically demonstrates a waxing and waning course, often completely resolving within 3 hours (range, minutes to days), only to recur repeatedly during 1–3 weeks.1,2,9,14,19,23,44 On average, the last episode occurs 7–8 days after symptom onset.19 In RCVS, the number of exacerbations may vary between 1 and 20 episodes and often are triggered by bathing, stress, sexual intercourse, change in position, exertion, and coughing.1,2,6,7,16,42,54,55 A more moderate headache may persist between the acute episodes.2,5,19
The exact etiology of the thunderclap headache encountered in CVS remains uncertain. Some authors have postulated that cerebral vasoconstriction may be the cause because the cerebral vasculature receives innervation from the first division of the trigeminal nerve and the dorsal ganglion of the second cervical nerve.6 However, the time course of patient symptoms such as headache and cerebral vasoconstriction argues against a causal relationship. For example, although patients typically present acutely with thunderclap headache, cerebral vasoconstriction often does not become evident for a week or more following symptom onset. Furthermore, resolution of vasoconstriction may take weeks to months in some individuals, persisting long after the resolution of patient symptomatology.56
Other Clinical Presentations and Sequelae of RCVS
Other clinical presentations, or sequelae, of RCVS include generalized seizures, encephalopathy, focal neurologic deficits, altered mental status, transient ischemic attacks, ischemic stroke, intracranial hemorrhage, cerebral edema, and PRES (Table 3).2,6,8,12,13,20,23,39,57–59 In her meta-analysis of 3 large case series of patients with RCVS, Ducros19 found that focal neurologic deficits were present in 8%–43% of patients, seizures in 1%–17%, cortical subarachnoid hemorrhage in 30%–34% (1 study had hemorrhage as an exclusion criterion and was not included), cerebral infarction in 6%–39%, and concomitant PRES in 9%–38% (Fig 2). This wide range in reported incidence of various sequelae of RCVS may reflect recruitment bias, with more ill patients being more likely to present for medical care; selection criteria; and the clinical context in which patients were encountered.19 For example, reported rates of ischemic infarct and intracranial hemorrhage in patients developing RCVS postpartum appear to be higher than those in series included by Ducros.33,60
Table 3:
Potential triggers of RCVS
| Triggers of Secondary RCVS |
|---|
| Vasoactive medications |
| Sympathomimetic drugs, bromocriptine, ergotamine, pseudoephedrine, selective serotonin-uptake inhibitors, interferon, triptans, diet pills, nonsteroidal anti-inflammatory drugs |
| Vasoactive recreational drugs |
| Alcohol, amphetamines, cannabis, cocaine, ecstasy, nicotine |
| Pregnancy and postpartum states |
| Blood products |
| Blood transfusions, erythropoietin, intravenous immunoglobulin |
| Headache disorders |
| Migraines |
| Tumors |
| Pheochromocytoma |
| Paraganglioma |
| Trauma |
| Carotid dissection, unruptured cerebral aneurysm |
| Head and neck surgery |
| Various medical conditions |
| Hemolysis, elevated liver enzymes, low platelets |
| Antiphospholipid antibody syndrome |
| Thrombotic thrombocytopenic purpura |
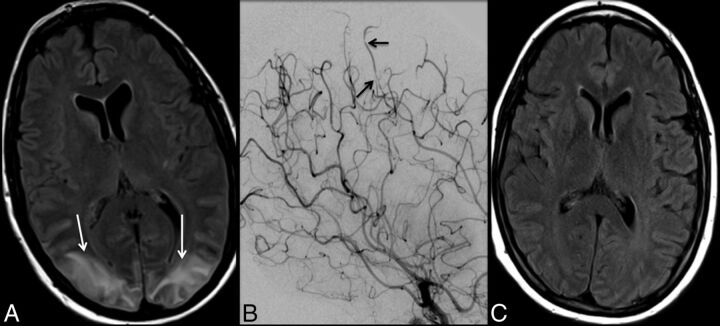
Fig 2.
A 42-year-old woman who presented with altered mental status and lethargy. FLAIR imaging (A) demonstrates signal hyperintensity involving the cortex and underlying subcortical white matter in the parietal and occipital lobes (white arrows), consistent with PRES. There is no evidence of associated diffusion restriction. Trace sulcal subarachnoid hemorrhage was also noted overlying the right frontal lobe (not shown). Note subtle irregularity and multifocal narrowings involving distal cortical branches of the bilateral middle and anterior cerebral arteries (black arrows) on cerebral angiography (B), suggestive of RCVS. The patient made a full recovery, with complete resolution of cerebral areas of abnormal FLAIR hyperintensity (C) and cerebral vasoconstriction (not shown).
Although patients with RCVS may initially present with generalized seizure, seizures rarely persist and long-term antiepileptic therapy is generally not indicated.1,42 Hypertension is commonly encountered in patients with RCVS in the acute period; however, it is unclear whether high blood pressure is from pain associated with headache, a response to cerebral vasoconstriction, or some other manifestation of the syndrome.5,13 As previously described, cervical arterial dissections may be encountered in patients with RCVS and should be excluded in patients who present with neck pain and/or territorial cerebral infract.1,2,5,19,45,46
Focal neurologic deficits encountered with RCVS include visual deficits, hemiplegia, dysarthria, aphasia, numbness, cortical blindness, or ataxia.6,42,59 Focal deficits of vision, sensory, sensation, and motor function are encountered in decreasing frequency.1,5 Focal neurologic deficits may be transient or permanent, often reflecting the sequelae of TIA or ischemic infarct resulting from severe segmental cerebral vasoconstriction, though some transient deficits may be due to a migraine-type aura phenomenon.1,2,6 Neurologic deficits lasting >24 hours are unlikely to improve and likely reflect the sequelae of ischemic infarct, which typically occur in bilateral watershed zones of the cerebral hemispheres.1,19 Cerebellar infarcts are also possible.19
Risk factors for the development of intracranial hemorrhage in patients with RCVS include a history of migraines, older age, and female sex.11,61 Subarachnoid hemorrhage, the most common hemorrhagic complication of RCVS, is most often focal and localized in superficial cerebral sulci near the cerebral convexities.1,5,8,11–13 Given this distribution, subarachnoid hemorrhage associated with RCVS may be missed on imaging and CSF analysis, and its incidence in the syndrome consequently is underestimated.11 It has been postulated that vasoconstriction of small arterioles early in the course of RCVS, along with hypertension and breakdown of autoregulatory mechanisms, may precipitate the rupture of small pial vessels with resulting subarachnoid hemorrhage.17,59,62 Other patterns of intracranial hemorrhage encountered in RCVS include intraparenchymal hemorrhage and subdural hematomas.1,8,57,59,63 Intraparenchymal hemorrhage can be see in up to 6%–20% of patients and most often is unifocal and lobar in location.1,8,19,57
The various sequelae of RCVS tend to occur at different times during the course of the syndrome.9 Hemorrhagic complications, such as subarachnoid and intraparenchymal hemorrhage and concomitant PRES and seizures, most often occur during the first week of illness.1,8,9,56 In contradistinction, ischemic events and their resulting focal neurologic deficits often arise later in RCVS, peaking between 1 and 2 weeks following patient presentation.9 Ischemic stroke can occur even later in the course of the syndrome, occasionally after resolution of symptoms such as headache, and presumably reflects the well-documented delay in resolution of cerebral vasoconstriction.44 Overall, Ducros et al9 found that ischemic events such as TIA and stroke occurred on average approximately 8 days later than hemorrhagic complications.9
Association with PRES
RCVS and PRES overlap significantly in their clinical and radiographic features, and the 2 entities are frequently encountered concurrently (Fig 2).4,5,58,62,64,65 PRES is a clinical and radiographic syndrome characterized by headache, visual changes, seizure, and imaging findings, including cerebral edema affecting the cerebral cortex and underlying white matter, manifesting as areas of hyperintensity on T2 and FLAIR imaging, most often involving the occipital and posterior parietal lobes.2,62,64 However, other distribution patterns can be encountered with PRES, including involvement of the frontal and temporal lobes, basal ganglia, deep white matter, and brain stem.2,64 While the areas of cerebral edema encountered in PRES are often reversible, progression to cytotoxic edema and infarct can occur.2,62 Finally, like RCVS, the exact pathophysiologic etiology of PRES remains unknown, though a breakdown in autoregulation of the cerebral vasculature leading to hyperperfusion is 1 possible mechanism.2,62
PRES-like reversible cerebral edema is encountered in anywhere from 9% to 38% of patients with RCVS, while most patients with PRES (>85%) demonstrate some element of RCVS-like cerebral vasoconstriction when conventional angiography is performed.19 When PRES-like features are encountered in cases of RCVS, the anatomic distribution of cerebral edema is similar to PRES encountered in other settings.44 Furthermore, PRES and RCVS often arise concurrently as complications of various medical conditions, including intravenous immunoglobin therapy, Guillain-Barre syndrome, immunosuppression, stem cell transplantation, blood transfusions, and septic shock.2,7,28,54,56,66,67 Finally, both PRES and RCVS demonstrate similar clinical features, including an acute, self-limited course and symptomatology, such as headache, confusion, seizure, and transient or permanent neurologic deficits.1,2,54 Given the significant overlap between the 2 entities, it is possible that RCVS and PRES may represent a spectrum of potential clinical manifestations of a common underlying pathophysiology involving various degrees of altered cerebral vascular tone and endothelial dysfunction.
Treatment
The treatment of RCVS is based on observational data obtained from retrospective studies.1,5,21 Current treatment recommendations for RCVS include withdrawal of any suspected exogenous triggers, including vasoactive medications, and intensive care unit–level care, symptom relief with analgesics, blood pressure control, and seizure prophylaxis.1,2,5,34,68 Patients should avoid activities that are associated with the onset of symptoms/headache as much as possible. Calcium channel blockers, including nimodipine, have been administered to patients with RCVS via oral and intravenous routes and have been shown in prospective and retrospective studies to provide symptom relief, including headache.2,3,17,24,44,69–72 However, calcium channel blockers have not been shown to influence the evolution of cerebral vasoconstriction or the possible complications of RCVS, including intracranial hemorrhage and ischemic stroke.1,6,16,17
Other vasodilators, such as phosphodiesterase inhibitors, have also been used with anecdotal success in case reports.73 However, vasodilators, including calcium channel blockers, must be used with caution because drops in systolic blood pressure may impair cerebral perfusion in patients with RCVS with severe cerebral vasoconstriction.2,44 Intra-arterial administration of vasodilators and balloon angioplasty have been performed in cases of severe RCVS-related vasoconstriction, though the indications and efficacy of these treatments remain unclear (Fig 3).1,69,74,75 Although RCVS vasoconstriction has been shown to improve following intra-arterial vasodilator therapy, recurrence of arterial narrowing has been reported, sometimes necessitating multiple treatment sessions.69,76 Glucocorticoid steroids have been administered to patients with RCVS, without improvement in either patient symptoms or sequelae of the disease. Some case series have even suggested that steroid therapy may be associated with worse outcomes in RCVS.3,17,76
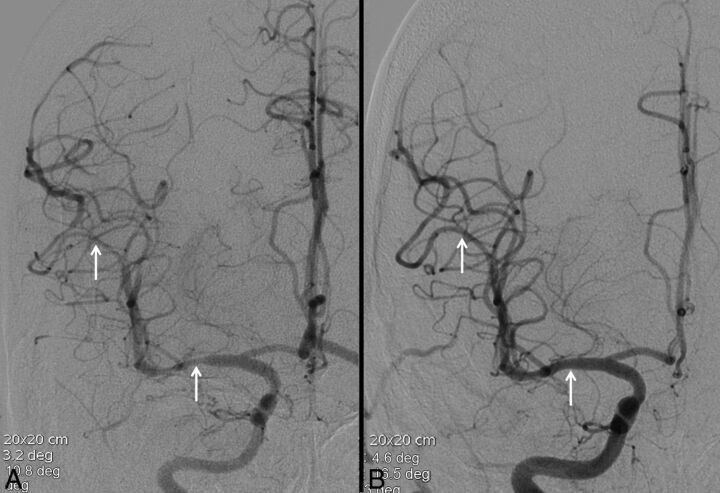
Fig 3.
A 19-year-old man with a 2-day history of recurrent headaches and prior marijuana use. Noncontrast CT was negative for acute hemorrhage (not shown). Conventional angiography (A) reveals multifocal areas of moderate narrowing and irregularity involving the cerebral vasculature (white arrows, A). These areas resolved following intra-arterial administration of verapamil (white arrows, B). Clinical course and imaging findings are consistent with RCVS.
Prognosis and Clinical Course
Fortunately, the prognosis for most patients with RCVS is very good. The syndrome typically follows a self-limiting, monophasic course, with resolution of symptoms by 3 weeks, and no new symptoms after 1 month.1,13,17,23,39,57,77 By definition, resolution of vasoconstriction should occur by 3 months. However, a minority of patients will demonstrate delayed clinical worsening in the first few weeks following symptoms onset, most often due to the development of an ischemic infarct.39,42,43,77 A more fulminant course of RCVS leading to permanent disability or death can be encountered in 5%–10% of patients.1,2,6,12,43,60,77 Recurrence of RCVS appears to be rare, though some patients may have chronic mild headaches and fatigue on follow-up.2,42,43,78
RCVS encountered in the postpartum period deserves special attention because it has been reported to be more likely to follow a fulminant course, with multifocal infarct, intracranial hemorrhage, extensive vasogenic edema, and death.1,39,60 When Fugate et al33 evaluated patients with postpartum angiopathy in a small retrospective series (n = 18), they found focal neurologic deficits in 50%, visual disturbances in 44%, encephalopathy in 33%, seizure in 28%, intracranial hemorrhage in 39%, vasogenic edema in 35%, and infarction in 35%. Somewhat unusual for RCVS, only slightly less than half of patients in this small series achieved a complete recovery, while the remaining patients either died or were left with significant neurologic deficits.33
Conclusions
RCVS is characterized by a thunderclap headache and reversible cerebral artery vasoconstriction on imaging. Alterations in cerebral vascular tone likely underlie development of the syndrome. Most patients with RCVS have a good outcome with no permanent sequelae, while a small minority will experience a more fulminant course culminating in permanent disability or death.
ABBREVIATIONS:
- RCVS
reversible cerebral vasoconstriction syndrome
- PRES
posterior reversible encephalopathy syndrome
References
- 1. Ducros A. L37: reversible cerebral vasoconstriction syndrome—distinction from CNS vasculitis. Presse Med 2013;42(4 pt 2):602–04 [DOI] [PubMed] [Google Scholar]
- 2. Ducros A, Bousser MG. Reversible cerebral vasoconstriction syndrome. Pract Neurol 2009;9:256–67 [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Zivadinov R, Ramasamy D, et al. Reversible cerebral vasoconstriction syndrome (RCVS) in antiphospholipid antibody syndrome (APLA): the role of centrally acting vasodilators—case series and review of literature. Clin Rheumatol 2014;33:1829–33 [DOI] [PubMed] [Google Scholar]
- 4. Marder CP, Donohue MM, Weinstein JR, et al. Multimodal imaging of reversible cerebral vasoconstriction syndrome: a series of 6 cases. AJNR Am J Neuroradiol 2012;33:1403–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheikh HU, Mathew PG. Reversible cerebral vasoconstriction syndrome: updates and new perspectives. Curr Pain Headache Rep 2014;18:414. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese LH, Dodick DW, Schwedt TJ, et al. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 2007;146:34–44 [DOI] [PubMed] [Google Scholar]
- 7. Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother 2011;11:1265–76 [DOI] [PubMed] [Google Scholar]
- 8. Stary JM, Wang BH, Moon SJ, et al. Dramatic intracerebral hemorrhagic presentations of reversible cerebral vasoconstriction syndrome: three cases and a literature review. Case Rep Neurol Med 2014;2014:782028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome: a prospective series of 67 patients. Brain 2007;130(pt 12):3091–101 [DOI] [PubMed] [Google Scholar]
- 10. Alvaro LC, Iriondo I, Villaverde FJ. Sexual headache and stroke in a heavy cannabis smoker. Headache 2002;42:224–26 [DOI] [PubMed] [Google Scholar]
- 11. Ansari SA, Rath TJ, Gandhi D. Reversible cerebral vasoconstriction syndromes presenting with subarachnoid hemorrhage: a case series. J Neurointerv Surg 2011;3:272–78 [DOI] [PubMed] [Google Scholar]
- 12. Calic Z, Choong H, Schlaphoff G, et al. Reversible cerebral vasoconstriction syndrome following indomethacin. Cephalalgia 2014;34:1181–86 [DOI] [PubMed] [Google Scholar]
- 13. Edlow BL, Kasner SE, Hurst RW, et al. Reversible cerebral vasoconstriction syndrome associated with subarachnoid hemorrhage. Neurocrit Care 2007;7:203–10 [DOI] [PubMed] [Google Scholar]
- 14. Grooters GS, Sluzewski M, Tijssen CC. How often is thunderclap headache caused by the reversible cerebral vasoconstriction syndrome? Headache 2014;54:732–35 [DOI] [PubMed] [Google Scholar]
- 15. Hajj-Ali RA, Singhal AB, Benseler S, et al. Primary angiitis of the CNS. Lancet Neurol 2011;10:561–72 [DOI] [PubMed] [Google Scholar]
- 16. Hammad TA, Hajj-Ali RA. Primary angiitis of the central nervous system and reversible cerebral vasoconstriction syndrome. Curr Atheroscler Rep 2013;15:346. [DOI] [PubMed] [Google Scholar]
- 17. Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 2011;68:1005–12 [DOI] [PubMed] [Google Scholar]
- 18. Call GK, Fleming MC, Sealfon S, et al. Reversible cerebral segmental vasoconstriction. Stroke 1988;19:1159–70 [DOI] [PubMed] [Google Scholar]
- 19. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11:906–17 [DOI] [PubMed] [Google Scholar]
- 20. Chen SP, Wang SJ. Hyperintense vessels: an early MRI marker of reversible cerebral vasoconstriction syndrome? Cephalalgia 2014;34:1038–39 [DOI] [PubMed] [Google Scholar]
- 21. Koopman K, Uyttenboogaart M, Luijckx GJ, et al. Pitfalls in the diagnosis of reversible cerebral vasoconstriction syndrome and primary angiitis of the central nervous system. Eur J Neurol 2007;14:1085–87 [DOI] [PubMed] [Google Scholar]
- 22. Wolff V, Lauer V, Rouyer O, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke 2011;42:1778–80 [DOI] [PubMed] [Google Scholar]
- 23. Bain J, Segal D, Amin R, et al. Call-Fleming syndrome: headache in a 16-year-old girl. Pediatr Neurol 2013;49:130–33.e1 [DOI] [PubMed] [Google Scholar]
- 24. Hajj-Ali RA, Furlan A, Abou-Chebel A, et al. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum 2002;47:662–69 [DOI] [PubMed] [Google Scholar]
- 25. Kirton A, Diggle J, Hu W, et al. A pediatric case of reversible segmental cerebral vasoconstriction. Can J Neurol Sci 2006;33:250–53 [DOI] [PubMed] [Google Scholar]
- 26. Liu HY, Fuh JL, Lirng JF, et al. Three paediatric patients with reversible cerebral vasoconstriction syndromes. Cephalalgia 2010;30:354–59 [DOI] [PubMed] [Google Scholar]
- 27. Probert R, Saunders DE, Ganesan V. Reversible cerebral vasoconstriction syndrome: rare or underrecognized in children? Dev Med Child Neurol 2013;55:385–89 [DOI] [PubMed] [Google Scholar]
- 28. Bayer-Karpinska A, Patzig M, Adamczyk C, et al. Reversible cerebral vasoconstriction syndrome with concurrent bilateral carotid artery dissection. Cephalalgia 2013;33:491–95 [DOI] [PubMed] [Google Scholar]
- 29. Boughammoura A, Touze E, Oppenheim C, et al. Reversible angiopathy and encephalopathy after blood transfusion. J Neurol 2013;250:116–18 [DOI] [PubMed] [Google Scholar]
- 30. Comabella M, Alvarez-Sabin J, Rovira A, et al. Bromocriptine and postpartum cerebral angiopathy: a causal relationship? Neurology 1996;46:1754–56 [DOI] [PubMed] [Google Scholar]
- 31. Cvetanovich GL, Ramakrishnan P, Klein JP, et al. Reversible cerebral vasoconstriction syndrome in a patient taking citalopram and Hydroxycut: a case report. J Med Case Rep 2011;5:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dou YH, Fuh JL, Chen SP, et al. Reversible cerebral vasoconstriction syndrome after blood transfusion. Headache 2014;54:736–44 [DOI] [PubMed] [Google Scholar]
- 33. Fugate JE, Ameriso SF, Ortiz G, et al. Variable presentations of postpartum angiopathy. Stroke 2012;43:670–76 [DOI] [PubMed] [Google Scholar]
- 34. Granier I, Garcia E, Geissler A, et al. Postpartum cerebral angiopathy associated with the administration of sumatriptan and dehydroergotamine: a case report. Intensive Care Med 1999;25:532–34 [DOI] [PubMed] [Google Scholar]
- 35. Lopez-Valdes E, Chang HM, Pessin MS, et al. Cerebral vasoconstriction after carotid surgery. Neurology 1997;49:303–04 [DOI] [PubMed] [Google Scholar]
- 36. Moussavi M, Korya D, Panezai S, et al. Reversible cerebral vasoconstriction syndrome in a 35-year-old woman following hysterectomy and bilateral salpingo-oophorectomy. J Neurointerv Surg 2012;4:e35. [DOI] [PubMed] [Google Scholar]
- 37. Paliwal PR, Teoh HL, Sharma VK. Association between reversible cerebral vasoconstriction syndrome and thrombotic thrombocytopenic purpura. J Neurol Sci 2014;338:223–25 [DOI] [PubMed] [Google Scholar]
- 38. Verillaud B, Ducros A, Massiou H, et al. Reversible cerebral vasoconstriction syndrome in two patients with a carotid glomus tumour. Cephalalgia 2010;30:1271–75 [DOI] [PubMed] [Google Scholar]
- 39. Katz BS, Fugate JE, Ameriso SF, et al. Clinical worsening in reversible cerebral vasoconstriction syndrome. JAMA Neurol 2014;71:68–73 [DOI] [PubMed] [Google Scholar]
- 40. Cantu C, Arauz A, Murillo-Bonilla LM, et al. Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs. Stroke 2003;34:1667–72 [DOI] [PubMed] [Google Scholar]
- 41. Nighoghossian N, Derex L, Trouillas P. Multiple intracerebral hemorrhages and vasospasm following antimigrainous drug abuse. Headache 1998;38:478–80 [DOI] [PubMed] [Google Scholar]
- 42. Singhal AB, Bernstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care 2005;3:91–97 [DOI] [PubMed] [Google Scholar]
- 43. Williams TL, Lukovits TG, Harris BT, et al. A fatal case of postpartum cerebral angiopathy with literature review. Arch Gynecol Obstet 2007;275:67–77 [DOI] [PubMed] [Google Scholar]
- 44. Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol 2010;67:648–56 [DOI] [PubMed] [Google Scholar]
- 45. Mawet J, Boukobza M, Franc J, et al. Reversible cerebral vasoconstriction syndrome and cervical artery dissection in 20 patients. Neurology 2013;81:821–24 [DOI] [PubMed] [Google Scholar]
- 46. Mitchell LA, Santarelli JG, Singh IP, et al. Reversible cerebral vasoconstriction syndrome and bilateral vertebral artery dissection presenting in a patient after cesarean section. J Neurointerv Surg 2014;6:e5. [DOI] [PubMed] [Google Scholar]
- 47. Nouh A, Ruland S, Schneck MJ, et al. Reversible cerebral vasoconstriction syndrome with multivessel cervical artery dissections and a double aortic arch. J Stroke Cerebrovasc Dis 2014;23:e141–43 [DOI] [PubMed] [Google Scholar]
- 48. Chen SP, Chung YT, Liu TY, et al. Oxidative stress and increased formation of vasoconstricting F2-isoprostanes in patients with reversible cerebral vasoconstriction syndrome. Free Radic Biol Med 2013;61:243–48 [DOI] [PubMed] [Google Scholar]
- 49. Marra A, Vargas M, Striano P, et al. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses 2014;82:619–22 [DOI] [PubMed] [Google Scholar]
- 50. Chen SP, Fuh JL, Wang SJ, et al. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS One 2011;6:e18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng YC, Kuo KH, Lai TH. A common cause of sudden and thunderclap headaches: reversible cerebral vasoconstriction syndrome. J Headache Pain 2014;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koopman K, Teune LK, ter Laan M, et al. An often unrecognized cause of thunderclap headache: reversible cerebral vasoconstriction syndrome. J Headache Pain 2008;9:389–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol 2006;5:621–31 [DOI] [PubMed] [Google Scholar]
- 54. Dodick DW, Eross EJ, Drazkowski JF, et al. Thunderclap headache associated with reversible vasospasm and posterior leukoencephalopathy syndrome. Cephalalgia 2003;23:994–97 [DOI] [PubMed] [Google Scholar]
- 55. Wang SJ, Fuh JL, Wu ZA, et al. Bath-related thunderclap headache: a study of 21 consecutive patients. Cephalalgia 2008;28:524–30 [DOI] [PubMed] [Google Scholar]
- 56. Chen SP, Fuh JL, Chang FC, et al. Transcranial color Doppler study for reversible cerebral vasoconstriction syndromes. Ann Neurol 2008;63:751–57 [DOI] [PubMed] [Google Scholar]
- 57. Geocadin RG, Razumovsky AY, Wityk RJ, et al. Intracerebral hemorrhage and postpartum cerebral vasculopathy. J Neurol Sci 2002;205:29–34 [DOI] [PubMed] [Google Scholar]
- 58. Noda K, Fukae J, Fujishima K, et al. Reversible cerebral vasoconstriction syndrome presenting as subarachnoid hemorrhage, reversible posterior leukoencephalopathy, and cerebral infarction. Intern Med 2011;50:1227–33 [DOI] [PubMed] [Google Scholar]
- 59. Santos E, Zhang Y, Wilkins A, et al. Reversible cerebral vasoconstriction syndrome presenting with haemorrhage. J Neurol Sci 2009;276:189–92 [DOI] [PubMed] [Google Scholar]
- 60. Fugate JE, Wijdicks EF, Parisi JE, et al. Fulminant postpartum cerebral vasoconstriction syndrome. Arch Neurol 2012;69:111–17 [DOI] [PubMed] [Google Scholar]
- 61. Ducros A, Fiedler U, Porcher R, et al. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke 2010;41:2505–11 [DOI] [PubMed] [Google Scholar]
- 62. Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 2004;61:411–16 [DOI] [PubMed] [Google Scholar]
- 63. Moskowitz SI, Calabrese LH, Weil RJ. Benign angiopathy of the central nervous system presenting with intracerebral hemorrhage. Surg Neurol 2007;67:522–27; discussion 527–28 [DOI] [PubMed] [Google Scholar]
- 64. Sadek AR, Waters RJ, Sparrow OC. Posterior reversible encephalopathy syndrome: a case following reversible cerebral vasoconstriction syndrome masquerading as subarachnoid haemorrhage. Acta Neurochir (Wien) 2012;154:413–16 [DOI] [PubMed] [Google Scholar]
- 65. Soo Y, Singhal AB, Leung T, et al. Reversible cerebral vasoconstriction syndrome with posterior leucoencephalopathy after oral contraceptive pills. Cephalalgia 2010;30:42–45 [DOI] [PubMed] [Google Scholar]
- 66. Agarwal R, Davis C, Altinok D, et al. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol 2014;50:518–21 [DOI] [PubMed] [Google Scholar]
- 67. Imataki O, Uemura M, Shintani T, et al. Reversible cerebral vasoconstriction syndrome resulted in cerebral infarction after allogeneic stem cell transplantation: a case report. Ann Hematol 2014;93:895–96 [DOI] [PubMed] [Google Scholar]
- 68. Meschia JF, Malkoff MD, Biller J. Reversible segmental cerebral arterial vasospasm and cerebral infarction: possible association with excessive use of sumatriptan and Midrin. Arch Neurol 1998;55:712–14 [DOI] [PubMed] [Google Scholar]
- 69. Elstner M, Linn J, Muller-Schunk S, et al. Reversible cerebral vasoconstriction syndrome: a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia 2009;29:677–82 [DOI] [PubMed] [Google Scholar]
- 70. Grände PO, Lundgren A, Bjartmarz H, et al. Segmental cerebral vasoconstriction: successful treatment of secondary cerebral ischaemia with intravenous prostacyclin. Cephalalgia 2010;30:890–95 [DOI] [PubMed] [Google Scholar]
- 71. Lu SR, Liao YC, Fuh JL, et al. Nimodipine for treatment of primary thunderclap headache. Neurology 2004;62:1414–16 [DOI] [PubMed] [Google Scholar]
- 72. Zuber M, Touze E, Domigo V, et al. Reversible cerebral angiopathy: efficacy of nimodipine. J Neurol 2006;253:1585–88 [DOI] [PubMed] [Google Scholar]
- 73. Bouchard M, Verreault S, Gariepy JL, et al. Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache 2009;49:142–45 [DOI] [PubMed] [Google Scholar]
- 74. John S, Donnelly M, Uchino K. Catastrophic reversible cerebral vasoconstriction syndrome associated with serotonin syndrome. Headache 2013;53:1482–87 [DOI] [PubMed] [Google Scholar]
- 75. Song JK, Fisher S, Seifert TD, et al. Postpartum cerebral angiopathy: atypical features and treatment with intracranial balloon angioplasty. Neuroradiology 2004;46:1022–26 [DOI] [PubMed] [Google Scholar]
- 76. French KF, Hoesch RE, Allred J, et al. Repetitive use of intra-arterial verapamil in the treatment of reversible cerebral vasoconstriction syndrome. J Clin Neurosci 2012;19:174–76 [DOI] [PubMed] [Google Scholar]
- 77. Robert T, Kawkabani Marchini A, Oumarou G, et al. Reversible cerebral vasoconstriction syndrome identification of prognostic factors. Clin Neurol Neurosurg 2013;115:2351–57 [DOI] [PubMed] [Google Scholar]
- 78. Ursell MR, Marras CL, Farb R, et al. Recurrent intracranial hemorrhage due to postpartum cerebral angiopathy: implications for management. Stroke 1998;29:1995–98 [DOI] [PubMed] [Google Scholar]