Abstract
BACKGROUND AND PURPOSE:
Intraplaque hemorrhage in carotid artery atherosclerotic plaque has been shown to be a marker of risk, associated with prior and future ischemic events, and has been associated with regions of intraplaque high-intensity signal on 3D-TOF MRA. We assessed the association of intraplaque high-intensity signal determined on 3D-TOF MRA with the incidence of prior ipsilateral stroke or TIA.
MATERIALS AND METHODS:
We assessed intraplaque hemorrhage by evaluating for intraplaque high-intensity signal adapting a recently validated technique on 3D-TOF source images in participants with high-grade (≥70%) extracranial carotid stenosis. Logistic regression analyses were used to assess the strength of association between the presence of intraplaque high-intensity signal on routine MRA sequences and prior stroke or TIA.
RESULTS:
Intraplaque high-intensity signal was present in 22 (41.5%) of 53 carotid arteries studied in 51 patients. Ipsilateral ischemic events occurred in 15 (68.1%) of 22 in the intraplaque high-intensity signal–positive group (10 strokes, 5 TIAs) and in 4 (12.9%) of 31 in the intraplaque high-intensity signal–negative group (3 strokes, 1 TIA). Ischemic events occurred within the 6-month period preceding imaging in 18 (94.7%) of 19 cases. The univariate odds ratio of the association of intraplaque high-intensity signal with any prior ischemic event was 14.5 (95% CI, 3.6–57.6), and the multivariate age- and sex-adjusted odds ratio was 14.2 (95% CI, 3.3–60.5). The association remained present across 1.5T and 3T magnet field strengths.
CONCLUSIONS:
Intraplaque high-intensity signal determined from MRA sequences already in place to measure luminal stenosis is strongly associated with prior ipsilateral ischemic events. Prospective validation of these findings to predict outcome in carotid artery stenosis could provide a valuable and widely accessible stroke risk stratification tool.
Measurement of luminal diameter stenosis has been the mainstay of extracranial vascular imaging, with treatment guidelines from multicenter randomized controlled trials based largely on patient stratification by stenosis severity.1–3 However, with recent developments in MR imaging technology, it is possible to assess the composition of atherosclerotic carotid lesions to determine the presence of complicated or advanced plaque elements that are at greater risk to cause ischemic symptoms.4–6 One such component of carotid plaque that has been identified as a component of advanced atherosclerotic lesions is intraplaque hemorrhage (IPH). When present in carotid atherosclerotic plaque, IPH has been associated with previous7,8 and future9 stroke and has been proposed as a possible marker of not only plaque inflammation10 but also of generalized cardiovascular risk.11
MR imaging tools have allowed IPH to be detected with high diagnostic accuracy compared with histopathologic confirmation with most of the techniques that have been studied to date.4 However, a significant barrier to the widespread use of IPH assessment as a measure of embolic stroke risk has been that most studies have relied on high-resolution imaging by using specialized, dedicated MR imaging surface carotid coils12 or black-blood coronal T1-weighted fat-suppressed MR imaging techniques.9 Neither of these techniques are part of the standard sequences routinely obtained in MRA examinations, which rely on TOF techniques to assess luminal diameter stenosis. Recent reports have suggested that routinely performed MRA techniques used to measure stenosis, including 3D-TOF imaging,13,14 can accurately predict IPH compared with histopathologic studies by the detection of intraplaque high-intensity signal (IHIS) relative to adjacent skeletal muscle. These studies, however, have been performed by use of dedicated carotid coils and not by standard quadrature neck array coils. Furthermore, it is also unknown whether IPH determined by 3D-TOF MRA neck images is associated with symptomatic carotid artery disease. The purpose or our study was to assess the association between IHIS determined on noncontrast 3D-TOF imaging and prior stroke or TIA in patients with high-grade carotid artery stenosis.
Materials and Methods
Patients
Patients were screened for this institutional review board–approved retrospective study after review of consecutive MRA neck examinations performed from August 2009 through August 2012. Inclusion criteria included 1) high-grade extracranial internal carotid artery stenosis (70%–99%) identified on noncontrast 3D-TOF MRA, 2) detailed documentation of electronic medical records of whether stroke or TIA had occurred before the MRA, and 3) detailed medical record documentation of pre-existing vascular risk factors.
Imaging Technique
MRA neck studies were performed on either 1.5T or 3T Signa (GE Healthcare, Milwaukee, Wisconsin) scanners by use of standard quadrature neck array coils. No dedicated high-resolution surface coils were used, nor was gadolinium administered. 3D-TOF acquisition involved a 20-cm field of view centered at the carotid bifurcation, 1.4-mm section thickness, and a matrix of 320 × 192 and 320 × 224 on 1.5T and 3T, respectively. To assess the generalizability of this technique to clinical practice, we included in our analysis all studies with image quality sufficient to warrant a clinical interpretation at the time of original image acquisition.
Imaging Data Assessment
IHIS, a presumed marker for IPH, was determined by adaption of a method used by Qiao et al,13 in which hyperintense signal intensity on 3D-TOF source images in carotid plaque was assessed relative to adjacent muscle. Unlike the study by Qiao et al,13 our technique did not use data collected with a dedicated carotid coil. We used a quantitative cutoff value of signal intensity 50% greater than skeletal muscle based on region-of-interest analysis in the area of suspected IHIS.9,15 IHIS assessment was made blinded to clinical data by 2 independent board-certified neuroradiologists with disagreements resolved by a third neuroradiologist as a tie-breaker. We further analyzed the cases with discordant interpretations and specifically addressed the possibility that a decrease in image quality could be contributing to discordant IHIS interpretations by making a subjective assessment of the presence or absence of motion degradation or other MR imaging artifacts, limiting confident diagnosis of IHIS in these cases.
Stenosis was categorized as 70%–95% or > 95%–99% by use of a method adapted from a study of diagnostic accuracy of TOF MRA in high-grade carotid artery stenosis.16 We used MRA MIP images to visually estimate the degree of stenosis, taking into account maximal luminal diameter stenosis relative to the caliber of normal-appearing distal ICA on MIP images, and used axial 3D-TOF source images to confirm stenosis measurements when MIP data did not provide unequivocal assessment of stenosis. Because measurements by use of the distal ICA as the denominator for stenosis measurements might underestimate the degree of stenosis in near-occlusion, as per North American Symptomatic Carotid Endarterectomy Trial guidelines,17 NASCET-type measurements were not used in such cases.
Clinical Data Assessment
The presence of ipsilateral TIA or stroke and coexisting vascular risk factors were determined by the consensus of 2 stroke neurologists after examination of the electronic medical record. The neurologists were blinded to the MRA IHIS assessments. Stroke and TIA were defined according to American Heart Association criteria,18 with a stroke and TIA defined as a permanent or transient episode, respectively, of neurologic dysfunction caused by focal brain or retinal ischemia. Only ipsilateral ischemic events referable to the stenotic ICA were considered positive events. The specific vascular risk factors collected in the cohort included the presence or absence of diabetes, hypertension, atrial fibrillation, hyperlipidemia, coronary artery disease, smoking history, chronic obstructive pulmonary disease, and chronic kidney disease.
Statistical Analysis
Statistical analysis was performed by use of logistic regression analysis to measure the strength of association between IHIS and ischemic events measured as an OR. A multivariate analysis was also performed, with calculation of an age- and sex-adjusted OR, as well as adjustment for covariate risk factors found to be statistically significant. Subset analyses were also performed stratifying test data from 1.5T or 3T MR imaging machines. We recorded the discrepancy rate for measurement of IHIS with interobserver agreement determined both by calculation of simple percent agreement and a Cohen κ coefficient statistic. Finally, to evaluate the possibility that interobserver interpretation differences were contributing significantly to the OR calculation, sensitivity analyses were performed with logistic regression analyses in 3 scenarios: 1) only with concordant IHIS interpretations, 2) treating all discordant interpretations as positive for IHIS, and 3) treating all discordant interpretations as negative for IHIS. All P values < .05 were considered statistically significant.
Results
After the review of 4895 consecutive neck MRAs, 4648 studies were excluded because no high-grade stenosis was present. In the 247 of 4895 MRAs with high-grade stenosis or occlusion (5.0% of total MRA cases screened), after application of additional exclusion criteria, including exclusion of cases with occlusion or cases with primary vascular imaging done with a technique besides 3D-TOF, such as with contrast-enhanced MRA, our final cohort of 51 patients with 53 unique carotid arteries met inclusion criteria. Vascular risk factors were not significantly different between groups (Table 1), though more men had IHIS. IHIS was present in 22 (41.5%) of 53 carotid arteries studied (see representative case, Fig 1). There was a 77% interobserver agreement rate (agreement in 41/53 cases), resulting in a κ coefficient of 0.50, suggesting moderate to good interobserver agreement. In the 12 cases with discordant interpretations, 10 of 12 studies were judged to have at least a moderate degree of motion degradation or other MR imaging artifacts limiting confident assessment of the presence or absence of IHIS.
Table 1:
Vascular risk factors in patients with and without IPH defined on MRA
| Risk Factors | IPH-Positive (n = 22) | IPH-Negative (n = 31) | P Value |
|---|---|---|---|
| Age (mean years) | 76.4 ± 9.46 | 77.6 ± 9.43 | .6406 |
| Sex: male, n (%) | 13 (59) | 9 (29) | .0286 |
| Diabetes (%) | 9 (41) | 13 (42) | .9404 |
| Hypertension (%) | 18 (82) | 30 (97) | .1474 |
| Hyperlipidemia (%) | 18 (82) | 26 (84) | .8445 |
| Atrial fibrillation (%) | 4 (18) | 3 (10) | .3676 |
| Coronary artery disease (%) | 9 (41) | 14 (45) | .7583 |
| Smoking (%) | 15 (68) | 19 (61) | .6062 |
| Heart failure (%) | 3 (14) | 4 (13) | .9381 |
| COPD (%) | 2 (9) | 4 (13) | .6660 |
| Chronic kidney disease (%) | 7 (32) | 6 (19) | .2988 |
| Stenosis severity: 95%–99% (%) | 1 (5) | 3 (10) | .6332 |
Note:—COPD indicates chronic obstructive pulmonary disease.
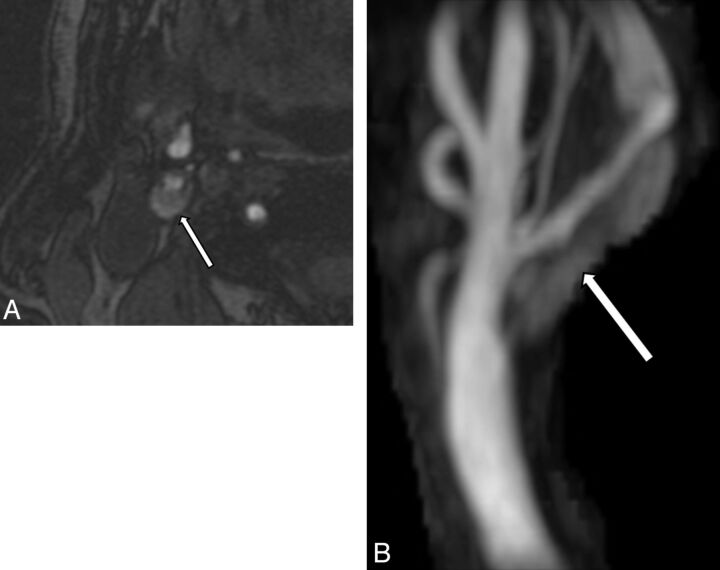
Fig 1.
A, Axial 3D-TOF source image demonstrates signal hyperintensity in the plaque (arrow) of the high-grade right internal carotid artery stenosis. B, 3D-TOF maximum-intensity projection image of the same right carotid artery bifurcation illustrates a long-segment hyperintense signal (arrow) within the plaque of the right internal carotid artery consistent with IPH.
Ipsilateral ischemic events occurred in 15 (68.1%) of 22 patients in the IHIS-positive group (10 strokes, 5 TIAs) and in 4 (12.9%) of 31 patients in the IHIS-negative group (3 strokes, 1 TIA) (Table 2). Ischemic events occurred within the 6 months preceding imaging in 18 (94.7%) of 19 cases, with the single outlier representing an ipsilateral stroke which occurred 10 years before imaging. In the univariate logistic regression analysis, the OR of the association of IHIS and prior ischemic events was 14.5 (95% CI, 3.6–57.6), whereas the age- and sex-adjusted OR was 14.2 (95% CI, 3.3–60.5). Excluding the 1 outlier ischemic event occurring 10 years before imaging, we found that the OR of the association of IPH and ischemic events within the prior 6 months was 13.5 (95% CI, 3.4–54.1). In addition, the association was preserved across magnet field strengths, as 32 arteries on a 1.5T magnet and 21 arteries on a 3T magnet had ORs of 17.8 (95% CI, 3.0–105.9) and 13.8 (95% CI, 1.2–156.6), respectively. OR data are summarized in Table 3. Finally, the strength of the association was between ischemic events, and IHIS was preserved across sensitivity analyses: 1) OR of 12.5 (95% CI, 2.53–61.8) in cases with only concordant interpretations analyzed, 2) OR of 5.46 (95% CI, 1.37–21.8) if the 12 discrepant cases were treated as IHIS negative, and 3) OR of 10.41 (95% CI, 2.73–39.8) if the 12 discrepant cases were treated as IHIS positive.
Table 2:
Presence of symptomatic cerebrovascular ischemia with or without IPH on MRA
| IPH-Positive | IPH-Negative | P Value | |
|---|---|---|---|
| History of any prior ipsilateral symptomatic disease, n (%) | 15 (68) | 4 (13) | <.0001 |
| Median time since prior TIA/stroke, days | 0 (IQR: 0–10) | 0 (IQR: 0–7) | .7619 |
| History of prior ipsilateral stroke, n (%) | 10 (67) | 3 (75) | .7500 |
| Median time since prior stroke, days | 0 (IQR: 0–7) | 0 (IQR: 0–0) | .3605 |
| History of prior TIA, n (%) | 5 (33) | 1 (25) | .7500 |
| Median time since prior TIA, days | 0 (IQR: 0–180) | 14 (IQR: N/A) | .5338 |
Note:—IQR indicates interquartile range; N/A, not available.
Table 3:
Summary of effect size and strength of association between IHIS and stroke or TIA
| Type of Association | OR | 95% CI |
|---|---|---|
| IHIS and any prior ischemic event | 14.5 | 3.6–57.6 |
| IHIS and prior ischemic event within 6 months | 13.5 | 3.4–54.1 |
| IHIS on 1.5T and prior ischemic event | 17.8 | 3.0–105.9 |
| IHIS on 3T and prior ischemic event | 13.8 | 1.2–156.6 |
Discussion
Our study demonstrates an association between IHIS, a potential marker of IPH, determined from routinely acquired 3D-TOF MRA source images and ischemic events. Specifically, our data suggest that patients with high-grade, extracranial carotid artery stenosis and IHIS are almost 14 times more likely to have had symptomatic disease compared with those with high-grade stenosis and no IHIS. Although several studies have characterized IPH or MR imaging by using black-blood, fat-suppressed T1-weighted sequences9 and high-resolution surface carotid coils,12 these studies have thus far had limited clinical usefulness, as they have relied on specialized sequences or equipment not used in most MRA neck examinations done to characterize vessel stenosis. Previous studies have used source images from 3D-TOF studies to assess the presence of IPH,13,14,19 including a study comparing MR imaging–suspected IPH with histopathologic confirmation.8 In this study, Qiao et al8 found that source images from 3D-TOF MRA or from mask-phase images from contrast-enhanced MRA could detect IPH with high diagnostic accuracy and with excellent interobserver reliability, though the study did not measure the association of IPH from MRA images and ischemic events. In another similar study, Yamada et al14 used high signal intensity on 3D-TOF MRA MIP images as a surrogate for IPH but did not correlate these findings with the presence or absence of symptomatic carotid disease. Our study demonstrates a strong association between ischemic events and IPH, as determined on widely available, large field-of-view neck coils with a 4-minute MRA sequence (3D-TOF), which is nearly universally acquired during screening examinations of the extracranial vascular structures.
It is important to recognize that the studies reported in the prior literature assessing the association between 3D-TOF plaque high signal and IPH on histologic examinations have been performed by use of dedicated surface carotid coils.13,14,19 As such, although it is likely that IHIS detected on 3D-TOF MRA performed with a standardized quadrature neck array coil will highly correspond with IPH on histologic examination, further radiologic-pathologic correlation studies are warranted to assess the diagnostic accuracy of the specific technique used in our study.
Two additional points regarding the MR imaging technique used for our study warrant further discussion. First, our study suggests that the association between IHIS and ischemic events is preserved across magnet field strengths. Because nearly all existing studies of IPH or IHIS have been performed on 1.5T magnets,9,11,12,20–22 and because the paramagnetic properties of IPH can potentially result in decreased signal hyperintensity on T1WI at 3T, our work supports the histopathologic correlation study by Qiao et al,8 suggesting a role for 3T imaging in IPH assessment by use of simple TOF techniques. Second, because TOF imaging has generally been used to assess for luminal diameter stenosis with limited data in the literature describing the use of this technique to assess the presence of IPH, we assessed the interobserver variability and found a κ coefficient suggesting moderate to good interobserver agreement. The logistic regression sensitivity analyses performed in our study demonstrate that our main results were robust to the interobserver variability present, and that 10 (83.3%) of 12 discrepant cases may have been related to motion-limited studies. Although we chose to analyze these motion-limited studies in our primary analysis to assess the generalizability of IHIS and IPH assessment in screening MRA studies, including studies occurring in critically ill patients or in patients unable to completely lie still for other reasons, future studies may be helpful to clarify how MR imaging quality influences the diagnostic accuracy of IPH assessment on 3D-TOF MRA vs histopathologic assessment. This is especially important regarding IPH assessment with standard field-of-view head and neck MR imaging coils because, despite the significant benefits of accessibility, speed, and ease of implementation, these coils provide less spatial resolution of the carotid artery than do multisequence, high-resolution techniques with dedicated surface carotid coils.
Our results are consistent with recent literature showing MR imaging of IPH to be associated with prior7 and future ischemic events.9,12 The magnitude of the strength of association between IHIS in our study and prior stroke (OR, approximately 14) is in line with a meta-analysis of the strength of association between IPH and future ischemic events (OR, approximately 12).9 The rate of IPH in our cohort (41.5%) is similar to that reported in recent published studies.7 We noted IPH even in cases where imaging was performed outside of the immediate 24 hours after stroke or TIA. In a recent study of IPH in symptomatic carotid disease by use of coronal T1-weighted fat-suppressed sequences to characterize IPH, Hosseini et al9 found that stroke risk with IPH was preserved for at least 5 years. The presence of IPH long after stroke and its ability to predict future stroke reinforce its potential value as a robust biomarker of plaque vulnerability in carotid atherosclerotic disease. Given the strong association between IPH and future events by use of specialized techniques, our findings raise the hope that routinely acquired imaging of the neck might play a role in predicting future events.
Our study had limitations that should be considered. First, our study was limited by a retrospective design, which could introduce significant patient heterogeneity into our analysis and the possibility that confounding vascular risk factors may be contributing to the differences present between groups. Despite this, detailed vascular risk factors were collected and were found to be similar between IHIS-positive and IHIS-negative groups, suggesting a low risk for confounding bias. In addition, we screened nearly 5000 MRA examinations to ensure a relatively homogeneous group of patients in stenosis severity (all ≥70%), in an effort to minimize the effect that differences in the degree of stenosis might have in a larger but more heterogeneous patient population. Second, a potential limitation was that IHIS could represent a combination of a lipid-rich necrotic core and IPH.14 The existing data suggest that this, however, is not a significant limitation because radiology-pathology correlation studies of 3D-TOF techniques have yielded a good diagnostic accuracy of MR imaging IPH classification compared with IPH determined from surgical specimens at carotid endarterectomy.13 Furthermore, because both a lipid-rich necrotic core and IPH are considered markers of plaque vulnerability,6 definitive differentiation may be of limited clinical relevance. Third, given the spatial resolution limits of 3D-TOF MRA by use of a quadrature neck array coil, confident differentiation of IHIS from an area of ulcerated plaque can be difficult. The effects of a quadrature neck array coil and its diagnostic accuracy in IPH determination vs histopathologic assessment are areas requiring further investigation. Fourth, our study assessed stroke or TIA that occurred before imaging, and as such, it remains unclear to what extent future events can be predicted by the use of this technique. We agree that validation of the use of 3D-TOF source images as a risk stratification tool for stroke will require a controlled prospective investigation. However, IPH detected by both coronal black-blood gradient-echo9 and dedicated carotid-coil–dependent, high-resolution techniques12 has been shown to predict future stroke or TIA in independent studies in the literature. Because 3D-TOF source images have comparable accuracy in the identification of IPH relative to these other techniques, it is reasonable to hypothesize that IHIS as determined on 3D-TOF MRA, a marker for IPH in our study, may have a similar predictive capacity.
Conclusions
Our study demonstrates that routinely acquired, screening MRA neck 3D-TOF source images, already widely used to measure luminal stenosis, can detect IHIS, which is strongly associated with prior ischemic events in patients with high-grade carotid artery stenosis. The prospective validation of our findings, as well as further histologic confirmation, may translate into regular reporting of IPH assessed by IHIS as a risk stratification tool to complement measures of luminal diameter stenosis on neck MRA.
ABBREVIATIONS:
- IHIS
intraplaque high-intensity signal
- IPH
intraplaque hemorrhage
Footnotes
Disclosures: Ajay Gupta—UNRELATED: Grants/Grants Pending: AUR GE Radiology Research and Academic Fellowship,* Comments: Salary support provided by this grant from 2012–2014. *Money paid to institution.
Dr. Gupta has been supported in part by the Association of University Radiologists General Electric Radiology Research Academic Fellowship.
REFERENCES
- 1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2. Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–87 [PubMed] [Google Scholar]
- 4. den Hartog AG, Bovens SM, Koning W, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013;45:7–21 [DOI] [PubMed] [Google Scholar]
- 5. Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–73 [DOI] [PubMed] [Google Scholar]
- 6. Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–74 [DOI] [PubMed] [Google Scholar]
- 7. Millon A, Mathevet JL, Boussel L, et al. High-resolution magnetic resonance imaging of carotid atherosclerosis identifies vulnerable carotid plaques. J Vasc Surg 2013;57:1046–51 [DOI] [PubMed] [Google Scholar]
- 8. Qiao Y, Etesami M, Astor BC, et al. Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. AJNR Am J Neuroradiol 2012;33:755–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosseini AA, Kandiyil N, Macsweeney ST, et al. Carotid plaque hemorrhage on MRI strongly predicts recurrent ischemia and stroke. Ann Neurol 2013;73:774–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Altaf N, Akwei S, Auer DP, et al. Magnetic resonance detected carotid plaque hemorrhage is associated with inflammatory features in symptomatic carotid plaques. Ann Vasc Surg 2013;27:655–61 [DOI] [PubMed] [Google Scholar]
- 11. Singh N, Moody AR, Rochon-Terry G, et al. Identifying a high risk cardiovascular phenotype by carotid MRI-depicted intraplaque hemorrhage. Int J Cardiovasc Imaging 2013;29:1477–83 [DOI] [PubMed] [Google Scholar]
- 12. Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke 2006;37:818–23 [DOI] [PubMed] [Google Scholar]
- 13. Qiao Y, Etesami M, Malhotra S, et al. Identification of intraplaque hemorrhage on MR angiography images: a comparison of contrast-enhanced mask and time-of-flight techniques. AJNR Am J Neuroradiol 2011;32:454–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada K, Song Y, Hippe DS, et al. Quantitative evaluation of high intensity signal on MIP images of carotid atherosclerotic plaques from routine TOF-MRA reveals elevated volumes of intraplaque hemorrhage and lipid rich necrotic core. J Cardiovasc Magn Reson 2012;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 2009;252:502–08 [DOI] [PubMed] [Google Scholar]
- 16. Babiarz LS, Romero JM, Murphy EK, et al. Contrast-enhanced MR angiography is not more accurate than unenhanced 2D time-of-flight MR angiography for determining > or = 70% internal carotid artery stenosis. AJNR Am J Neuroradiol 2009;30:761–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox AJ. How to measure carotid stenosis. Radiology 1993;186:316–18 [DOI] [PubMed] [Google Scholar]
- 18. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276–93 [DOI] [PubMed] [Google Scholar]
- 19. Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234–39 [DOI] [PubMed] [Google Scholar]
- 20. Yamada N, Higashi M, Otsubo R, et al. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. AJNR Am J Neuroradiol 2007;28:287–92 [PMC free article] [PubMed] [Google Scholar]
- 21. Kurosaki Y, Yoshida K, Endo H, et al. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery 2011;68:62–67; discussion 67 [DOI] [PubMed] [Google Scholar]
- 22. Kwee RM, van Oostenbrugge RJ, Mess WH, et al. MRI of carotid atherosclerosis to identify TIA and stroke patients who are at risk of a recurrence. J Magn Reson Imaging 2013;37:1189–94 [DOI] [PubMed] [Google Scholar]