Abstract
BACKGROUND AND PURPOSE:
A limitation in postoperative monitoring of patients with glioblastoma is the lack of objective measures to quantify residual and recurrent disease. Automated computer-assisted volumetric analysis of contrast-enhancing tissue represents a potential tool to aid the radiologist in following these patients. In this study, we hypothesize that computer-assisted volumetry will show increased precision and speed over conventional 1D and 2D techniques in assessing residual and/or recurrent tumor.
MATERIALS AND METHODS:
This retrospective study included patients with native glioblastomas with MR imaging performed at 24–48 hours following resection and 2–4 months postoperatively. 1D and 2D measurements were performed by 2 neuroradiologists with Certificates of Added Qualification. Volumetry was performed by using manual segmentation and computer-assisted volumetry, which combines region-based active contours and a level set approach. Tumor response was assessed by using established 1D, 2D, and volumetric standards. Manual and computer-assisted volumetry segmentation times were compared. Interobserver correlation was determined among 1D, 2D, and volumetric techniques.
RESULTS:
Twenty-nine patients were analyzed. Discrepancy in disease status between 1D and 2D compared with computer-assisted volumetry was 10.3% (3/29) and 17.2% (5/29), respectively. The mean time for segmentation between manual and computer-assisted volumetry techniques was 9.7 minutes and <1 minute, respectively (P < .01). Interobserver correlation was highest for volumetric measurements (0.995; 95% CI, 0.990–0.997) compared with 1D (0.826; 95% CI, 0.695–0.904) and 2D (0.905; 95% CI, 0.828–0.948) measurements.
CONCLUSIONS:
Computer-assisted volumetry provides a reproducible and faster volumetric assessment of enhancing tumor burden, which has implications for monitoring disease progression and quantification of tumor burden in treatment trials.
Glioblastoma multiforme (GBM) is an invasive and highly aggressive tumor with a median patient survival of 14.6 months with combined radiation therapy and temozolomide.1 New therapies are being developed to treat GBM, which may decrease morbidity and lengthen the period of progression-free survival. However, to fully use new therapies in the treatment of GBM, quantitative MR imaging metrics are needed to guide therapy, risk stratify patients undergoing therapy, and prognosticate outcome.2,3 A major limitation is this lack of prognostic imaging parameters.4 Simple radiographic monitoring with freehand measurements of the amount of contrast-enhancing tumor in 2 or 3 planes is commonly used for assessing response to different therapies, which is used to guide treatment strategies.5,6 Commonly used techniques include the Response Evaluation Criteria in Solid Tumors and the MacDonald criteria, which use unidimensional and bidimensional measurements, respectively.7–9 However, the postsurgical cavity tends to be highly irregular in shape, which may increase the difficulty in obtaining accurate and reproducible measurements. In particular, single-dimensional techniques may be inaccurate, given the propensity of high-grade gliomas to grow in an eccentric and nodular fashion, and may not be reflective of change in actual tumor burden.10
Recently, the Response Assessment in Neuro-Oncology (RANO) Working Group proposed new recommendations for assessing response criteria for high-grade gliomas, which included a modification to the MacDonald criteria.11 While the RANO criterion used 2D measurements, the Working Group suggested that volumetric analysis could provide more accurate measurements with respect to bidimensional techniques.11 Outside the central nervous system, volumetric assessment has been proved superior to unidimensional measurements when used to assess treatment response in hepatic, pulmonary, and pancreatic malignancies.12,13
Despite the potential advantages of volumetric assessment, this technique requires manual outlining of the contrast-enhancing border, which can be both time-consuming and technically challenging.5,10 This technique may be further limited in cases with irregular enhancement and subependymal extension.10 For these reasons, computer-aided volumetry techniques applied to the contrast-enhancing tissue represent a potential tool to aid the radiologist in following these patients. Such techniques may increase both the accuracy and reproducibility in assessing GBM recurrence. Thus far, such automation has been explored in the assessment of advanced lung cancer with promising results.14 Application of computer-assisted volumetry (CAV) has been explored for the evaluation of gliomas; however, these studies have dealt with native nontreated disease and have not been validated against other measurement techniques.15–17
In this study, we describe a novel CAV technique for assessment of tumor burden in the patient with GBM. Specifically, we describe the reliability and feasibility of this technique compared with traditional linear-based measurements in the patient with postresection GBM.
Materials and Methods
Subjects
After institutional review board approval of this Health Insurance Portability and Accountability Act–complaint study, a query of the neuropathology department data base at our institution from January 2011 to November 2012 was conducted. Specifically, patients who had undergone primary resection of glioblastomas were evaluated. Given that many of our patients are referred from other facilities for resections, we specifically examined patients who had undergone MR imaging evaluation at our institution where standardization of MR imaging protocols allows a better comparison of scans. A retrospective review of these medical records was conducted to determine demographic information including sex, age at time of resection, extent of resection, and duration between follow-up imaging examinations. All patients received combination radiation therapy with concurrent chemotherapy following maximal resection.
Imaging Techniques
All imaging was performed on a 3T MR imaging system (Signa; GE Healthcare, Milwaukee, Wisconsin) by using an 8-channel head-array coil (Signa HDxt; GE Healthcare). We obtained the following sequences: axial T1-weighted pre- and postcontrast, T2, and FLAIR. Volumetric acquisitions were also acquired for all postcontrast images with a T1-weighted 3D inversion recovery fast spoiled gradient-recalled sequence with the following parameters: TI, 450 ms; TR, 10.2 ms; TE, 4.2 ms; α, 13°; bandwidth, 25 KHz; FOV, 25 cm; matrix, 256 × 256; section thickness, 1.2 mm. Total scanning time was approximately 4 minutes 15 seconds. Postcontrast images were acquired by using intravenous gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy) with a weight-based dose of 0.2 mL/kg. The time between intravenous injection and postcontrast imaging was 5 minutes. All immediate postoperative imaging was performed within 2 days of resection by using the same MR imaging parameters. Follow-up imaging was performed between 2 and 4 months after surgery.
Imaging Interpretation
Two radiologists (C.G.F. and A.B.L.), who were blinded to the final calculated volume, each measured the major and minor axes for each tumor on postcontrast sequences. The major axis was defined as the longest diameter, and the minor axis was defined as the longest diameter perpendicular to the major axis. Gross total resection was defined as the absence of enhancing tissue at postoperative examination. Nonmeasurable lesions were defined as enhancing masses with lesions with maximal thickness of <4 mm (2 times the imaging section thickness) to reduce variability from volume averaging.11 Additionally, the surgical cavity, cysts, and necrosis were not included in measurements per the RANO criteria.11 In cases of multiple enhancing foci, individual diameters were measured and summed for response evaluation.10,11
Manual volumetric segmentations were performed by 1 radiologist (D.S.C.), who was blinded to the final CAV calculation, by performing manual tracing around the enhancing lesion on volumetric sequences (section thickness, 0.2 mm). All manual segmentations were performed at a dedicated workstation (Advantage Workstation, Version 4.3; GE Healthcare Europe, Buc, France). All measurements were performed by comparison with precontrast T1 images to avoid T1 shortening effects from postsurgical changes (ie, blood products). The time required to perform manual segmentation was also recorded.
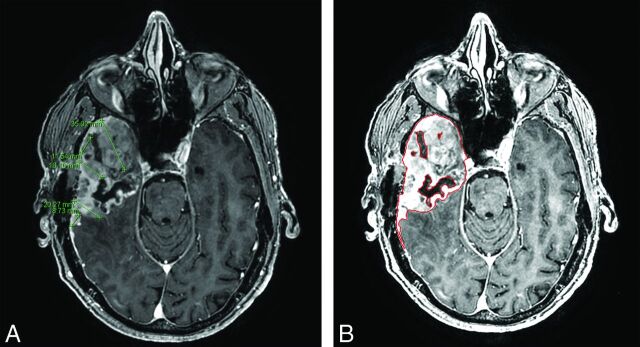
CAV measurements were performed by a separate radiologist (J.Q.) who was blinded to final manual volumetric measurements. Tumor contours were delineated on immediate and 2- to 4-month postsurgery T1-weighted MR images by using an in-house, proprietary segmentation algorithm developed to assist the computer-aided volume calculation of this project. Our CAV algorithm was originally developed for hepatic lesions and has since been adapted for different applications including brain and renal malignancies, lymphoma, and peritoneal mesothelioma.18–22 The semiautomated algorithm combines the region-based active contours and a level set approach and has the advantages of easy initialization, quick segmentation, and efficient modification. An operator manually selects a region of interest that roughly encloses the tumor on a single image. Boundary localization of the tumor and exclusion of the nonviable tissue inside the tumor are then performed automatically by the developed algorithm. Once the segmentation is completed on an image, the tumor contour is propagated to its neighboring images, serving as an initial region of interest for subsequent segmentations on the neighboring images. This process continues iteratively until all the tumor images are segmented. To ensure correct results, computer-generated tumor contours are superimposed on the original images for inspection and modification as needed by a radiologist. Once the segmentation is finalized, tumor volume is automatically calculated (Fig 1). This segmentation algorithm and a number of manual interaction functions, such as selection of a region of interest and modification of suboptimal contour results, have been integrated into a user-friendly image-viewing system developed with the Matlab (MathWorks, Natick, Massachusetts) computer language by the research group.
Fig 1.
Comparison of linear 1D measurements (A) and CAV analysis (B) in a 64-year-old man with glioblastoma multiforme 11 weeks following resection. Our readers found volumetric analysis preferable, given the irregularity of recurrence.
Definition of Response
Tumor response was assessed for each study by using established 1D, 2D and volumetric standards extrapolated from bi-dimensional standards.7–9 Cases of discrepancy between readers were re-reviewed to reach a consensus. Volumetric standards were established by using an extrapolation from the MacDonald criteria by converting orthogonal measurements to a volume assuming a spheric lesion (Table 1).10 “Complete response” was defined as complete absence of contrast-enhancing tumor for all techniques. “Partial response” was defined as >30% decrease in the sum of maximal diameters for 1D, >50% decrease in the product of orthogonal diameters for 2D, and >65% in volume for volumetric techniques. “Progression of disease” was defined as a 20% increase in the sum of maximal diameters for 1D, >25% increase in the product of orthogonal diameters for 2D, and >40% increase in volume for volumetric techniques. “Stable disease” was defined as all other changes for 1D, 2D, and volumetric techniques. Tumor response counts and discrepancy between agreements were calculated among the different measuring methods.
Table 1:
Definition of response criteria for unidimensional (RECIST), bidimensional (Macdonald), and volumetric techniques
| Criteria | CR | PR | SD | PD |
|---|---|---|---|---|
| RECIST | Resolution of all enhancement | ≥30% Decrease in sum of max dimensions | All others | ≥20% Increase in sum of max dimensions |
| Macdonald | Resolution of all enhancement | ≥50% Decrease in product of 2 orthogonal dimensions | All others | ≥25% Increase in product of 2 orthogonal dimensions |
| Volumetric | Resolution of all enhancement | ≥65% Decrease in volume | All others | ≥40% Increase in volume |
Note:—CR indicates complete response; PR, partial response; SD, stable disease; PD, progression of disease; RECIST, Response Evaluation Criteria in Solid Tumors; max, maximal.
Statistical Tests
Manual and semiautomated volumetric measurements and segmentation times were compared by using a paired t test. Pearson correlation coefficients with corresponding confidence intervals were used to assess interobserver correlations of 1D, 2D, and volumetric measurements. The inter-rater agreement statistic with corresponding confidence intervals was calculated for tumor response by using weighted κ values. κ values were interpreted as follows: 0.40–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, very good. Statistical analysis was conducted with MedCalc for Windows, Version 12.2.1 (MedCalc Software, Mariakerke, Belgium). A P value < .05 was considered statistically significant.
Results
Subjects
In total, we identified 90 patients who had a GBM resection at our institution between January 2011 and November 2012, of whom 32.2% (29/90) had undergone all standardized postoperative MR imaging at our institution. The mean age of patients was 62.2 ± 8.5 years (range, 38–81 years); of whom 55% (16/29) were men and 45% (13/29) were women. With respect to the extent of resection, 5/29 and 24/29 underwent gross total resection and subtotal resection, respectively. Demographic information is summarized in Table 2. The mean time to follow-up from baseline postoperative imaging was 12.3 ± 3 weeks (range, 6–16 weeks).
Table 2:
Demographic information of included patients
| Variable | Value |
|---|---|
| Male/female (No.) | 16:13 |
| Mean age at resection (yr, SD) | 62.2 (8.5) |
| Mean follow-up (wk, SD) | 12.3 (3.0) |
| Extent of resection (No., %) | |
| GTR | 5 (17.2) |
| STR | 24 (82.8) |
Note:—GTR indicates gross total resection; STR, subtotal resection.
Disease Response
Discrepancies in disease response for 1D, 2D, and volumetric techniques were encountered in 17.2% (5/29), 10.3% (3/29), and 3.4% (1/29) of patients. Following review of discrepancies, the complete response rate was 3.4% (1/29) for all measuring techniques. The partial response rate was 10.3% (3/29), 10.3% (3/29), and 6.9% (2/29) for 1D, 2D, and CAV techniques, respectively. The stable disease rate was 10.3% (3/29), 10.3% (3/29), and 17.2% (5/29) for 1D, 2D, and CAV techniques, respectively. The progressive disease rate was 75.9% (22/29), 75.9% (22/29), and 72.4% (21/29) for 1D, 2D, and CAV techniques, respectively. These results are summarized in Table 3. Discrepancies in disease classification between 1D and 2D compared with CAV were observed in 10.3% (3/29) and 17.2% (5/29) of cases (Figs 2 and 3). The discrepancy between 1D and 2D was 6.8% (2/29).
Table 3:
Response counts of 1D, 2D, and CAV (n = 29)
| CR | PR | SD | PD | |
|---|---|---|---|---|
| 1D | 1 | 3 | 3 | 22 |
| 2D | 1 | 4 | 2 | 22 |
| CAV | 1 | 2 | 5 | 21 |
Note:—CR indicates complete response; PR, partial response; SD, stable disease; PD, progression of disease.
Fig 2.
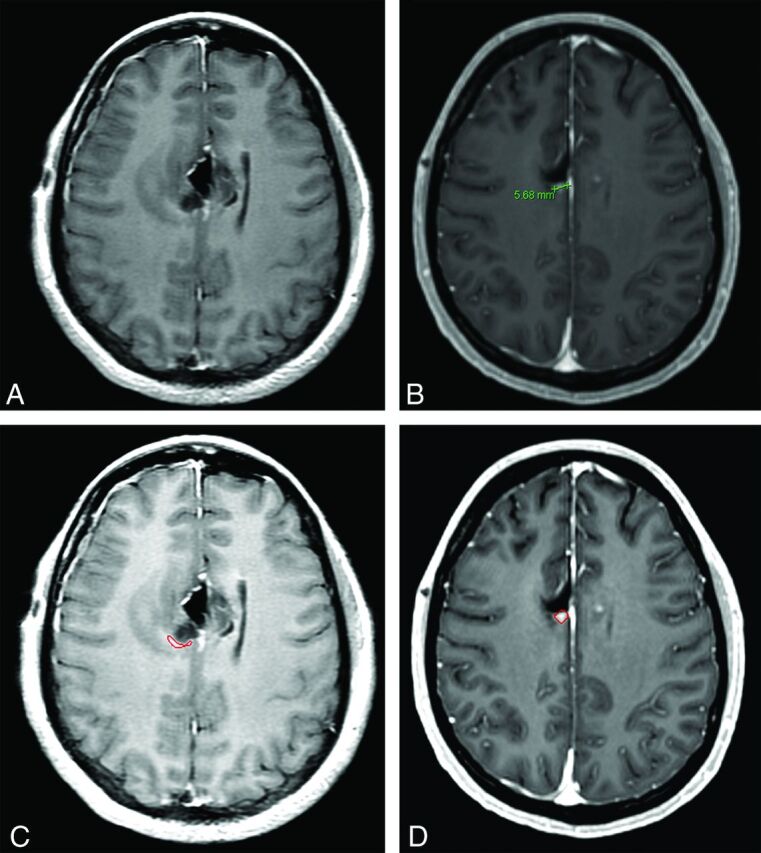
Disease status categorization in a 38-year-old woman with glioblastoma following resection, at 24 hours and at 12 weeks. 1D measurement found the contrast enhancement on initial postoperative imaging nonmeasurable (A) and subsequently labeled this case disease progression on follow-up imaging (B). CAV measurement labeled this case stable disease between baseline (C) and follow-up imaging (D).
Fig 3.
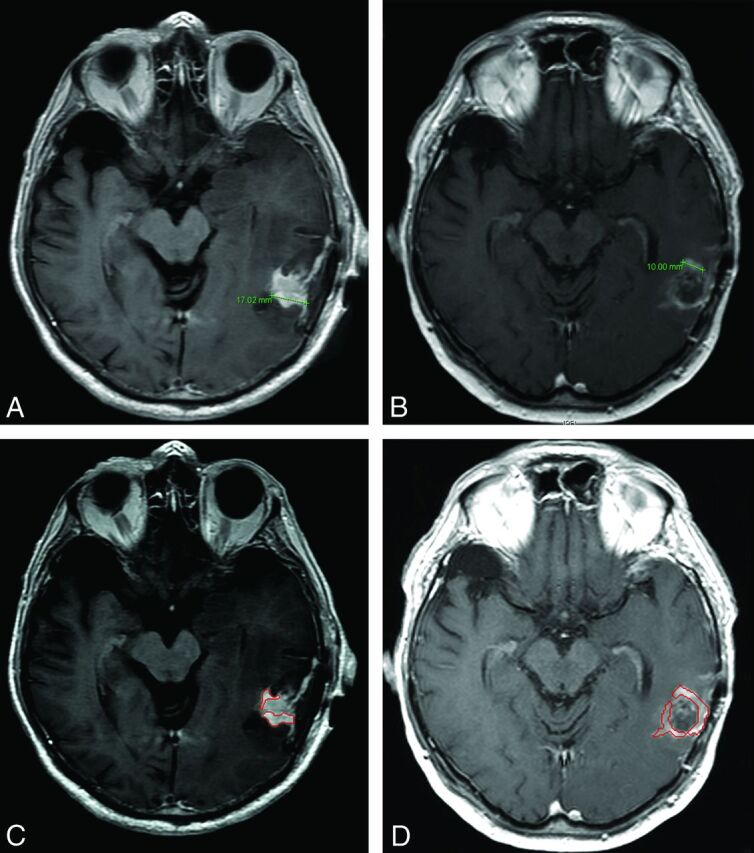
Disease status categorization in an 81-year-old man with glioblastoma following resection at 24 hours and at 12 weeks. 1D measurement labeled this case partial response between baseline (A) and follow-up imaging (B). CAV measurement labeled this case stable disease between baseline (C) and follow-up imaging (D).
Analysis of Measurement Techniques
The mean volume for manual and CAV analyses was 10.0 and 9.5 mL, respectively (P = .11). The mean time for segmentation between manual and CAV techniques was 9.7 minutes and <1 minute, respectively (P < .01). The Pearson correlation between manual and CAV analysis was 0.995 (95% CI, 0.990–0.997). This correlation was significantly higher than interobserver correlations for 1D (Pearson correlation, 0.826; 95% CI, 0.695–0.904; P < .0001) and 2D (Pearson correlation, 0.905; 95% CI, 0.828–0.948; P < .0001) measurements (Table 4). No significant difference was observed between 1D and 2D correlations (P = .12). With regard to classifying disease response, inter-reader agreement was significantly higher for volumetric techniques (κ = 0.948; 95% CI, 0.845–1.000) compared with 1D (P = .0002; κ = 0.760; 95% CI, 0.682–1.000) and 2D (P = .01; κ = 0.851; 95% CI, 0.555–0.966) measurements (Table 5). No significant difference was observed between 1D and 2D agreements (P = .25).
Table 4:
| Interobserver Pearson Correlation | 95% CI |
||
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| 1D | 0.814 | 0.686 | 0.897 |
| 2D | 0.904 | 0.825 | 0.948 |
| Volumetric | 0.995 | 0.990 | 0.997 |
Assessed with the Pearson correlation coefficient.
Manual and CAV analysis.
Table 5:
| Interobserver κ Correlation | 95% CI |
||
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| 1D | 0.760 | 0.555 | 0.966 |
| 2D | 0.851 | 0.682 | 1.000 |
| Volumetric | 0.948 | 0.845 | 1.000 |
Assessed with the κ coefficient.
Manual and CAV analysis.
Discussion
Surveillance and management of patients with GBM are reliant on imaging; however, measurement of residual disease can be challenging. In the present study, we examined a CAV approach to quantify residual disease and noted no significant difference compared with manual volumetric measurements, which are time-consuming and impractical in a busy clinical or academic practice. Additionally, CAV assessment was least variable compared with unidimensional and bidimensional techniques. These results are in line with other studies comparing manual volumetric tracing with diameter-based approaches, which have also noted less inter-reader and intrareader variability for volumetric techniques.23 While volumetric analysis is technically challenging and time-consuming, the CAV process that we have developed was both significantly faster and reliable, making application for routine clinical use and investigative purposes feasible. Other studies have described similar computer-aided techniques in the evaluation of gliomas; however, validation against other measurement techniques is lacking.15
Bidimensional techniques used in the MacDonald and RANO criteria are currently the most commonly used techniques for GBM assessment; however, studies have reported high interobserver and intraobserver variability, even among individuals with specialty training in neuroradiology.24,25 The increased variability in diameter-based approaches likely stems from the inherent irregularities in GBM. Specifically, GBMs are not solid ellipsoid lesions but instead typically display complex morphology with infiltrative margins and eccentric growth and demonstrate heterogeneity with areas of cystic degeneration and necrosis. Additionally, linear measurements may be affected by differences in head positioning at the time of examination and scan section techniques. These limitations are further compounded in the postoperative patient in whom blood products may be mistaken for enhancing tumor and the surgical cavity itself may have collapsed.26
Assessing residual volume in patients with GBM has been shown to be a significant and independent predictor.4,27,28 Intuitively, 3D volumetric analysis is a more accurate method for assessing tumor size compared with alternative linear-based techniques.23,26,29 Dempsey et al5 found volumetric analysis to be predictive of survival compared with diameter-based analysis, which failed to adequately assess tumor size in recurrent gliomas. Such volumetric assessment has several unique advantages over linear-based techniques, including its ability to objectively accommodate the irregular and eccentric growth of GBMs, exclude cystic and necrotic components, and account for changes in the shape of the postoperative resection cavity.10,29
Accurate assessment of tumor volume is important for clinical management and particularly in the development of new therapies and trials. This is of particular importance in the evaluation of GBM, given its exceedingly aggressive behavior.30 When assessing clinical response determined by different models, we observed a 17.2% difference in outcome classification when comparing volumetric with bidimensional techniques; however, similar studies have observed up to a 40% discrepancy.24 Additionally, disease status categorizations are defined on thresholds based on presumed ellipsoid geometry, which may, in turn, lead to increased variability in assessing GBM, given the inherent morphologic features. Such variability has been observed within 2D measurements by the same reader with a reported 14% false-positive rate for diagnosis of disease progression in otherwise stable disease.25
In this study, we subjectively observed a greater discordance between extrapolated volumes of linear-based techniques and volumetric assessment for tumors with increased eccentricity and necrotic changes. Specifically, linear measurements obtained for eccentric tumors would often be overestimated. Additionally, linear measurements obtained for cystic and necrotic tumors would often be overestimated. We also observed that extrapolated volumes for linear-based measurements of rounded nodular lesions were closer to the volumetric assessment. For reasons described previously, linear-based measurements are problematic for GBM assessment, given that these tumors are not typically solid ellipsoid lesions but display complex morphology. Such inconsistencies and potential inaccuracies may result in classifying effective treatments as ineffective or ineffective treatments as effective, which underscores the need for a reliable, reproducible, standard process of accurately determining tumor volume.6 With respect to use of computer-aided tools, researchers in body imaging have previously demonstrated improved accuracy over linear-based measurements by using a similar automated technique in the evaluation of lung, liver, and lymph node tumors.6
When one interprets the results of our study, several limitations should be kept in mind. First, this study is retrospective in design and subject to its limitations. Second, we have limited our analysis to enhancing contrast-enhancing tissue, which only represents increased blood-brain barrier permeability. While this may represent enhancing tumor, other possibilities may include inflammation, subacute ischemia, and so forth. However, there is no clear consensus on how to best assess the extent of residual and/or recurrent disease, which tends to default to the standard 1D or 2D measurement techniques, which are suboptimal. Additionally, there is no clear objective consensus on how to assess nonenhancing disease. Last, several patients were imaged within 12 weeks following resection, which is within the timeframe of pseudoprogression. It would have been optimal to repeat those studies to ensure that enhancement detected by CAV was indeed disease progression.11 However, histopathologic confirmation of recurrent disease was available for all except 1 patient imaged within the timeframe of pseudoprogression. Furthermore, the primary goal of this study was to assess the volumetric technique in comparison with other methods.
Conclusions
We have demonstrated the feasibility of a semiautomated segmentation technique to determine recurrent and/or residual tumor volume in patients with GBM, and this more reliable, reproducible, and significantly faster volumetric assessment of enhancing tumor burden has implications for the monitoring of disease progression and a potential role in therapy and novel treatment trials. Future studies should address patient outcomes with volumetric disease categorization, assess reliability among a larger number of readers, and compare reliability among different CAV algorithms.
ABBREVIATIONS:
- CAV
computer-assisted volumetry
- GBM
glioblastoma multiforme
- RANO
Response Assessment in Neuro-Oncology
Footnotes
Disclosures: Andrew B. Lassman—UNRELATED: Consultancy: Genentech, Merck Sharp & Dohme, GlaxoSmithKline, Abbott, Ludwig Institute for Cancer Research, Kyowa Hakko Kirin Pharma, Novartis, RadMD, Roche, CampusBio, Qatar National Research Fund, Italian Association for Cancer Research, Grants/Grants Pending: Abbott,* Aeterna Zentaris,* Agenus,* Amgen,* Bayer and Onyx,* Boehringer Ingelheim,* Bristol-Myers Squibb,* Celldex,* Genentech,* Keryx Biopharmaceuticals,* Medimmune,* Merck Sharp & Dohme,* Millenium,* Northwest Biotherapeutics,* Novartis,* Pfizer,* Plexxicon,* Roche,* Sigma Tau,* Theorum Clinical Research,* Payment for Lectures (including service on Speakers Bureaus): Merck Sharp & Dohme, Omniprex, American College of Radiation Oncology, Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: American Academy of Neurology, United Council for Neurologic Subspecialties. Binsheng Zhao—UNRELATED: Patents (planned, pending, or issued): A patent for our in-house segmentation algorithm is pending (no payment received). Christopher G. Filippi—UNRELATED: Consultancy: MRI Advisory Board for Guerbet LLC, Comments: I spent 1 day listening to lectures on an ionic macrocyclic contrast agent developed by this company and used primarily in Europe with no cases of nephrogenic systemic fibrosis to date. I offered advice to the company on whether such an agent would be beneficial in the United States. I received a $2000 honorarium for the day, Grants/Grants Pending: 10% effort on R01CA161404 of Dr Jeffrey N. Bruce, neurosurgeon, Comments: Phase II trial on pigs with new chemotherapy for GBM (I am responsible for the MR imaging component). *Money paid to the institution.
Author contributions: guarantors of integrity of entire study: D.S.C., C.G.F.; study concepts/study design: D.S.C., B.Z., A.L., C.G.F.; study data acquisition or data analysis/interpretation: D.S.C., J.Q., X.G., V.Z.M., A.L., B.Z., C.G.F.; manuscript drafting or manuscript revision for important intellectual content: D.S.C., J.Q., X.G., V.Z.M., F.M.I., J.N.B., A.B.L., L.H.S., A.L., B.Z., C.G.F.; manuscript editing: D.S.C., L.H.S., B.Z., C.G.F.; literature research: D.S.C., L.H.S., B.Z., C.G.F.; approval of the final version of the submitted manuscript: D.S.C., J.Q., X.G., V.Z.M., F.M.I, J.N.B, A.B.L, L.H.S., A.L., B.Z., C.G.F.
Paper previously presented in part at: 51st Annual Meeting of the American Society of Neuroradiology, May 18–23, 2013; San Diego, California, under the title “Semi-Automated Measurement of Recurrent and Residual Disease in Glioblastoma Multiforme: A Potential New Objective Metric.”
REFERENCES
- 1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66 [DOI] [PubMed] [Google Scholar]
- 2. Reardon DA, Galanis E, DeGroot JF, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol 2011;13:353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol 2009;27:2905–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iliadis G, Kotoula V, Chatzisotiriou A, et al. Volumetric and MGMT parameters in glioblastoma patients: survival analysis. BMC Cancer 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol 2005;26:770–76 [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz LH, Ginsberg MS, DeCorato D, et al. Evaluation of tumor measurements in oncology: use of film-based and electronic techniques. J Clin Oncol 2000;18:2179–84 [DOI] [PubMed] [Google Scholar]
- 7. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16 [DOI] [PubMed] [Google Scholar]
- 8. Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80 [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 [DOI] [PubMed] [Google Scholar]
- 10. Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol 2008;29:419–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72 [DOI] [PubMed] [Google Scholar]
- 12. Welsh JL, Bodeker K, Fallon E, et al. Comparison of response evaluation criteria in solid tumors with volumetric measurements for estimation of tumor burden in pancreatic adenocarcinoma and hepatocellular carcinoma. Am J Surg 2012;204:580–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao B, Oxnard GR, Moskowitz CS, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res 2010;16:4647–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mozley PD, Bendtsen C, Zhao B, et al. Measurement of tumor volumes improves RECIST-based response assessments in advanced lung cancer. Transl Oncol 2012;5:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanaly CW, Ding D, Mehta AI, et al. A novel method for volumetric MRI response assessment of enhancing brain tumors. PLoS One 2011;6:e16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Egger J, Kapur T, Fedorov A, et al. GBM volumetry using the 3D Slicer medical image computing platform. Sci Rep 2013;3:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Y, Young GS, Xue Z, et al. Semi-automatic segmentation software for quantitative clinical brain glioblastoma evaluation. Acad Radiol 2012;19:977–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Computer-aided tumor segmentation using local region-based active contours. Columbia University Invention Report IR #2906 [Google Scholar]
- 19. Methods and Systems for Segmentation of Organs and Tumors and Objects. Patent (pending) PCT/US13/36166. April, 2013
- 20. Zaretsky J, Guo X, Fu J, et al. Novel radiologic features to predict hepatocellular carcinoma recurrence after liver transplantation: a pilot study. In: 62nd Annual Meeting of the American Association for the Study of Liver diseases (AASLD), San Francisco, California. Nov 3–8, 2011 [Google Scholar]
- 21. Abou-Alfa GK, Zhao B, Capanu M, et al. Effects of Radiologic Tumor Response of Anti-Glypican 3 GC33 and Multi Tyrosine Kinases Inhibitor Sorafenib in Hepatocellular Carcinoma. In: 24th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics. Dublin, Ireland. November 6–9, 2012 [Google Scholar]
- 22. Leinwand J, Zhao B, Guo X, et al. Quantitative CT peritoneography in malignant peritoneal mesothelioma patients receiving intraperitoneal chemotherapy. Ann Surg Oncol 2013. May 24. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 2001;19:551–57 [DOI] [PubMed] [Google Scholar]
- 24. Provenzale JM, Mancini MC. Assessment of intra-observer variability in measurement of high-grade brain tumors. J Neurooncol 2012;108:477–83 [DOI] [PubMed] [Google Scholar]
- 25. Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol 2009;193:W515–22 [DOI] [PubMed] [Google Scholar]
- 26. Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol 2008;5:634–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keles GE, Lamborn KR, Chang SM, et al. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg 2004;100:41–46 [DOI] [PubMed] [Google Scholar]
- 28. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–98 [DOI] [PubMed] [Google Scholar]
- 29. Warren KE, Patronas N, Aikin AA, et al. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst 2001;93:1401–05 [DOI] [PubMed] [Google Scholar]
- 30. Gladwish A, Koh ES, Hoisak J, et al. Evaluation of early imaging response criteria in glioblastoma multiforme. Radiat Oncol 2011;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]