Abstract
BACKGROUND AND PURPOSE:
Neurophysiological monitoring for neuroendovascular procedures typically involves EEG and SSEP monitoring via cutaneous electrodes. MEP monitoring has been used less frequently because, traditionally, this has required subdural electrode placement. With the advent of transcutaneous techniques, MEP monitoring use has increased. However, little has been published regarding the use of this technique in therapeutic neuroendovascular procedures. The purpose of this study was therefore to determine whether TcMEP monitoring is feasible and efficacious in therapeutic neuroendovascular procedures.
MATERIALS AND METHODS:
We retrospectively reviewed our data base of therapeutic neuroendovascular procedures performed with the use of TcMEP monitoring. We specifically determined the incidence of TcMEP changes compared with changes in either SSEP or EEG. We then correlated these changes to actual adverse neurologic events.
RESULTS:
Although TcMEP monitoring was technically successful in all of the 140 patients in which it was attempted, we observed significant changes in TcMEP signals in only 1 patient. This patient experienced changes involving all 3 monitoring modalities after intraprocedural aneurysm rupture. In contrast, changes in SSEP tracings alone were found in 9 patients. Of these, 2 patients were known to be moribund before their procedures and neither recovered. Among the remaining 7 patients, temporary SSEP changes tended to correlate with temporary neurologic deficits, while permanent changes were associated with permanent or long-lasting deficits.
CONCLUSIONS:
These results suggest that TcMEP monitoring is feasible in therapeutic neuroendovascular procedures. However, it appears that the addition of TcMEP monitoring provides no added benefit to SSEP and EEG monitoring alone.
Neurophysiological monitoring has become an integral intraoperative tool in the surgical management of neurovascular disease. This has carried over to the treatment of cerebrovascular disease by the endovascular route, with various groups demonstrating the usefulness of transcutaneous techniques include monitoring of SSEP, EEG, and BAER.1,2 Intraoperative monitoring of MEP has also been shown to be safe and reliable in the setting of spinal surgery and open aneurysm surgery, though this typically involves placement of subdural electrodes.3 The use of MEP is of particular interest, given reported advantages in sensitivity over other neurophysiologic monitoring techniques.4,5 However, use of MEP in neuroendovascular procedures has been limited due to the need to use transcutaneous stimulation and concerns over excessive patient movement from such stimulation. As a result, little consideration has been given to the use and efficacy of MEP monitoring in neuroendovascular procedures. A review of the medical literature found only one previously published work concerning the use of MEP monitoring in the endovascular treatment of intracranial vascular disease.6
Over the past 5 years at our institution, we have selectively employed a combination of SSEP, EEG, and TcMEP monitoring during therapeutic neuroendovascular procedures. In an effort to better understand the potential usefulness of the TcMEP technique in this setting, we reviewed our series of these patients and hereby present our experience with 140 patients.
Materials and Methods
We retrospectively reviewed our data base of neuroendovascular procedures to examine the feasibility and efficacy of TcMEP in therapeutic neuroendovascular procedures. This study was approved by our institutional review board under expedited review.
We routinely use neurophysiological monitoring in the form of SSEP and EEG for almost all therapeutic neurovascular procedures. In this report, in addition to our standard protocol, we reviewed a subset of patients monitored with TcMEP. The decision regarding whether to use TcMEP was made by the primary operator in each case, with 1 of the senior authors (P.C.K.) using TcMEP in almost all interventional cases, and the other senior author (K.M.C.) using the technique only rarely. Information regarding patient demographics, procedure type, monitoring changes, and clinical outcome was collected. Special attention was paid to the monitoring technique experiencing a change and to the sequence of these changes, if more than 1 technique was affected.
Anesthesia Protocol
All patients received a TIVA with endotracheal intubation. Short-acting neuromuscular blockage was used during induction. However, due to the inherent limitations of TcMEP, neuromuscular blockade was not used during the remainder of the procedure. Typically, patients were anesthetized using propofol infusion alone or in combination with dexmedetomidine and/or remifentanil.
Monitoring Technique
A Digitimer D185 constant voltage stimulator (Digitimer, Letchworth Garden City, Hertfordshire, United Kingdom) was used to elicit a multipulse TcMEP. Stimulation parameters consisted of train counts that ranged from 3–7 pulses, with a constant duration of 50 microseconds. The interstimulus interval ranged from 1.1–4.1 milliseconds.
Four transcranial stimulation subdermal needle electrodes were used in favor of corkscrew electrodes in an effort to reduce the amount of artifact present. Three stimulation montages were used for TcMEP stimulation. A lateral C3-C4 montage, based on the International 10–20 system, was typically used for stimulation. The precise details of this technique have been previously described.5 The myogenic response from TcMEP stimulation was recorded with subdermal needle electrodes. Muscle groups selected consisted of the flexor carpi radials, abductor pollicis brevis, anterior tibialis, and adductor hallucis brevis muscles. Electromyography activity evoked from TcMEP stimulation was recorded with a filter of 10 Hz to 3 KHz and a sweep time of 10 msec/division. Amplifier gain was varied from 50–100 μsec/division.
Preincision baseline data (before insertion of femoral sheath) were obtained immediately after induction of anesthesia. If a short-acting muscle relaxant was used during intubation, then a train of 4 was obtained. Train of 4 was recorded from the gastrocnemius muscle upon stimulation of the common peroneal nerve at the popliteal fossa; 4/4 twitches were verified before TcMEP baseline data were established.
Anodal stimulation was use to evoke a myogenic response. A threshold technique was used.7,8 Contralateral myogenic recruitment from TcMEP stimulation was compared with the ipsilateral response to cathodal stimulation. Stimulation intensity was then adjusted until cathodal stimulation resulted in no ipsilateral response. The TcMEP response amplitude, utilizing the described stimulation technique, ranged from 50 μV to 1 mV.
The techniques used for SSEP and EEG monitoring have been previously described9,10 and are therefore not described in this report.
Results
A total of 758 therapeutic neuroendovascular procedures were performed in our department between January 2005 and December 2009. Of these, 140 were performed using a combination of SSEP, EEG, and TcMEP monitoring. The patient population included 85 females and 55 males. Patients were between 6 and 87 years of age (mean age 47). Most patients (111) were treated for intracranial aneurysms. Of the remainder, 18 patients had arteriovenous malformations, 5 patients had intracranial arterial stenosis, and 4 patients had dural arteriovenous fistulas. In addition, there was 1 patient with intracranial vasculitis and 1 patient who underwent balloon test occlusion before a brain tumor resection.
TcMEP monitoring was technically successful in all patients in this series. Monitoring was not abandoned because of patient movement in any procedure. A total of 11 patients had changes noted in at least 1 of their neurophysiological monitoring parameters (Table). One patient had simultaneous changes in TcMEP, EEG, and SSEP. One patient had concurrent SSEP and EEG changes. In the remaining 9 patients, only changes in SSEP were detected.
Table:
Summary of patients demonstrating changes in neurophysiological monitoring parameters
| Patient | Age (yrs) and Sex | Diagnosis | Procedure | EEG | SSEP | TcMEP | Immediate Outcome (GOS) | Long-Term Outcome (GOS) |
|---|---|---|---|---|---|---|---|---|
| 1 | 63 F | Anterior communicating artery aneurysm | Anterior communicating artery aneurysm coilinga | + | + | 4 | 5 (25 months) | |
| 2 | 55 F | Grade 5 SAH | Right posterior inferior cerebellar artery aneurysm coiling | + | 2 | 1 (6 days) | ||
| 3 | 56 F | Grade 5 SAH | Anterior communicating artery aneurysm coiling | + | 2 | 1 (6 days) | ||
| 4 | 69 F | Grade 1 SAH | Right anterior choroidal and posterior communicating artery aneurysms | + | 4 | 5 (8 months) | ||
| 5 | 34 F | Grade 1 SAH | Anterior communicating artery aneurysm coilinga | + | + | + | 4 | 5 (7 months) |
| 6 | 62 F | Grade 1 SAH | Anterior communicating artery aneurysm coiling | + | 5 | 3 (28 months) | ||
| 7 | 52 M | Grade 1 SAH | Left pericallosal artery aneurysm coiling | + | 4 | 4 (5 months) | ||
| 8 | 67 F | Grade 3 SAH | Right middle cerebral artery aneurysm coiling | + | 3 | 5 (17 months) | ||
| 9 | 53 M | Grade 4 SAH | Diagnostic angiogram | + | 4 | 5 (5 weeks) | ||
| 10 | 71 F | Left paraclinoid region aneurysm | Precoiling stent placement | + | 5 | 5 (14 months) | ||
| 11 | 57 F | Grade 3 SAH | Anterior communicating and right posterior communicating artery aneurysm coiling | + | 3 | 5 (4 months) |
Note:—“Grade” in SAH patients is Hunt-Hess grade. A change is indicated by a “+.”
Changes were simultaneous.
Details of Monitoring Changes
TcMEP, SSEP, and EEG changes were noted to be simultaneous in 1 patient (patient 5). This was a 34-year-old woman admitted with subarachnoid hemorrhage, Hunt-Hess grade 1, secondary to a ruptured anterior communicating artery aneurysm. During endovascular coil embolization, there was an intraprocedural rupture of the aneurysm noted after placement of the second coil. Concomitant changes in TcMEP, SSEP, and EEG were evident (Fig 1A, B). All TcMEP signals were absent, there was a global decrease in the EEG to flatline, and there was a decrease in bilateral upper and lower extremity SSEP signals. A third coil was immediately deployed and blood pressure was lowered. Systemic heparinization was reversed and an EVD was inserted. After insertion of the EVD, all monitoring signals slowly returned to baseline (Fig 1C, D). The patient showed gradual improvement in her neurologic status over the next 2 weeks and was discharged home 2 weeks after her coiling procedure. She was neurologically intact. She remained free of neurologic deficits through follow-up at 7 months.
Fig 1.
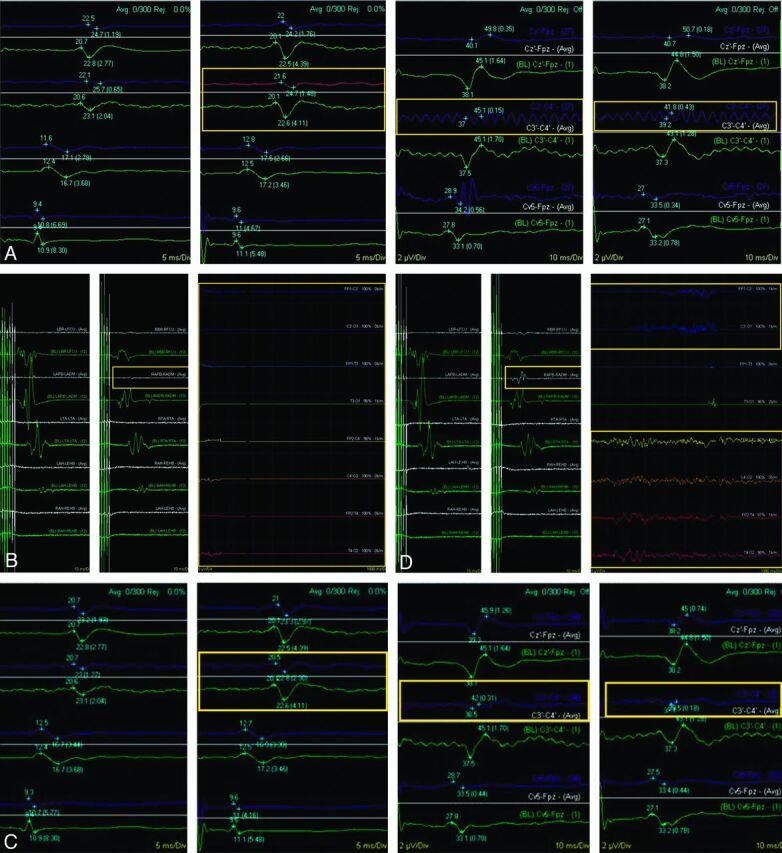
Neurophysiological monitoring tracings from patient 5 after intraoperative rupture (A), showing a decrease in right upper extremity SSEP (left yellow box) and loss of bilateral lower extremity SSEP during aneurysm coiling (center and right yellow boxes). Tracing from a similar time point (B), showing a subtle decrease in amplitude of TcMEP involving the right upper extremity (small left yellow box) and a global loss of EEG (large right yellow box). After EVD insertion (C), recovery of SSEP (yellow boxes) can be seen. Concomitantly, there is a return of the normal TcMEP signal intensity and recovery of EEG (D) (yellow boxes).
The patient with documented SSEP and EEG changes (patient 1) was undergoing coil embolization of an unruptured anterior communicating artery aneurysm. After detachment of the final coil in this 63-year-old woman, a control angiogram demonstrated lack of flow in the right A2 segment. There was a simultaneous decrease in the amplitude of the left lower extremity SSEP as well as the EEG amplitude in the right frontal region. The patient's mean arterial pressure was increased by the anesthesia staff and abciximab (ReoPro) was administered by intravenous and intra-arterial routes. There was subsequent return of flow within the right A2 segment, and SSEP and EEG signals returned to near baseline. There was no clinical deficit noted on examination at the conclusion of the procedure.
SSEP changes were noted in the absence of TcMEP or EEG changes in 9 patients. Two of these patients were in poor clinical condition (Hunt-Hess grade V) after aneurysmal rupture (patients 2 and 3). Both patients had poor SSEP tracings at the beginning of their procedures and both experienced a global decrease in SSEP amplitude, which did not improve in either patient. Neither patient recovered neurologic function after their procedure. Both of these patients ultimately died. Of the remaining 7 patients with SSEP changes alone, 1 had temporary changes that were not associated with any clinical change (patient 8), 2 had transient changes that corresponded to temporary clinical deficits (patients 4 and 11), 2 had sustained changes without significant improvement, but no neurologic deficits (patients 6 and 10), and 2 had sustained changes that were associated with new permanent neurologic deficits (patients 7 and 9). In the single patient (patient 8) with a temporary change that was not associated with any clinical change, the decrease in SSEP amplitude was found to be related to local effects on the monitored extremity, and the changes resolved after repositioning of the extremity. In 1 of the patients with a temporary change and transient postoperative deficits (patient 11), SSEP changes were noted in association with temporary balloon occlusion during a balloon remodeling procedure for aneurysm embolization. Induced hypertension and the shortening of occlusion times led to SSEP improvement. The other patient in this category (patient 4) developed a third nerve palsy in the setting of SAH, and the coiling of tandem anterior choroidal artery and right posterior communicating artery region aneurysms. In 1 patient with an unresolved SSEP change and no subsequent clinical deficit (patient 6), a distal vessel occlusion, not apparent on the magnified images viewed during the aneurysm embolization, was found after the SSEP change, leading to early thrombolysis with IA and IV abciximab (ReoPro).
An example of significant SSEP-alone changes can be seen in patient 7, a 52-year-old man admitted with SAH, Hunt-Hess grade 1, secondary to a ruptured left pericallosal artery aneurysm. This patient underwent endovascular coil embolization of the ruptured aneurysm on postbleed day 1. During the procedure, there was thromboembolic occlusion of the left MCA immediately after placement of the guide catheter and before placement of any coils within the aneurysm (Fig 2A). There was an associated decrease in SSEP amplitude involving the right upper and lower extremities at this time (Fig 2B). Intra-arterial tPA was given, but persistent occlusion of a posterior frontal M3 division and slow flow through most other branches of the left MCA remained (Fig 2C). However, the SSEP signals improved to baseline following thrombolysis (Fig 2D). After coiling of the aneurysm, thrombosis within the parent artery was seen at the aneurysm neck (Fig 2E). There was a decrease in SSEP amplitude involving the right upper and lower extremities at this time (Fig 2F). Intra-arterial abciximab (ReoPro) was administered. Angiographic images showed resolution of the thrombus (Fig 2G). The SSEP signals improved slightly but remained decreased (below 50% of baseline amplitude) for the remainder of the procedure, with no further recovery (Fig 2H). There were no changes on TcMEP or EEG at any time during the procedure. The patient remained intubated overnight after the procedure and was extubated the next day. He was aphasic and not following commands. The patient required placement of a ventriculoperitoneal shunt later in his admission and was discharged to a rehabilitation facility 5 weeks after admission. At 5-month follow-up, the patient had a GOS of 4 (modified Rankin scale, grade 2), with minimal weakness and slight expressive aphasia.
Fig 2.
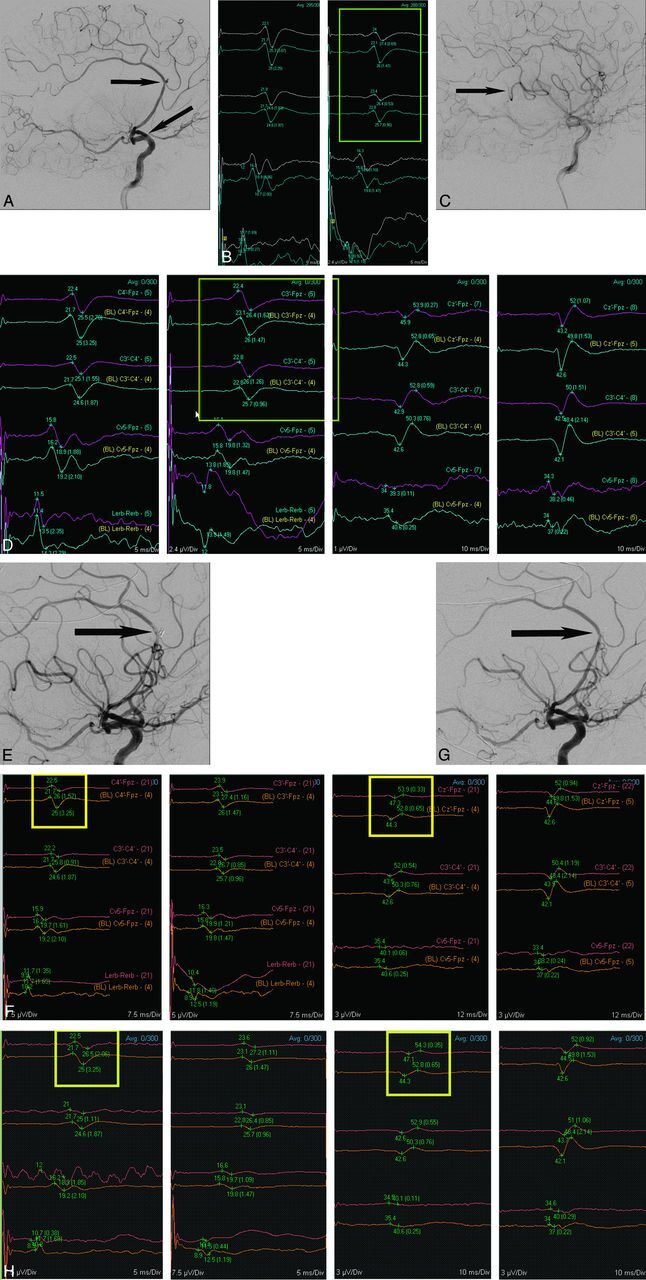
Lateral view of left ICA angiogram (A) from patient 7 shows acute left M1 occlusion (angled arrow) and pericallosal region aneurysm (horizontal arrow). Neurophysiological monitoring tracings (B) show a decrease in right upper extremity SSEP before aneurysm coiling (yellow box). After IV tPA, there is improvement in angiographic opacification of most of the left MCA territory (C), but a posterior frontal M3 branch remains occluded (arrow). In addition, after administration of IV tPA (D), recovery of right upper extremity SSEP is seen (yellow box). After coiling of the aneurysm, thrombus formation was noted at the aneurysm neck (E). This was associated with new monitoring changes (F), specifically a decrease in the left upper extremity SSEP amplitude (left yellow box) and left lower extremity SSEP amplitude (right yellow box). Intra-arterial abciximab (ReoPro) was administered and follow-up angiographic images (G) showed resolution of the thrombus (arrow). Subsequently, SSEP tracings (H) showed minimal recovery of left upper extremity amplitude (left yellow box) and left lower extremity amplitude (right yellow box) by the end of the procedure.
Time Requirements and Cost
The overall duration of cases was not felt to be relevant because many variables impact case length, and patients were not randomly assigned to monitoring techniques. Monitoring setup time was not directly tracked. However, the subjective impression was that the addition of electrodes for TcMEP monitoring, including troubleshooting problems, added approximately 5–10 minutes to the case setup time above and beyond SSEP and EEG monitoring alone. As mentioned in the Materials and Methods section, the use of TcMEP requires the use of intravenous anesthetic agents, rather than volatile inhalational agents, and neuromuscular blockade cannot be used. Although we did not find patient movement to be a problem in most cases, the use of a TIVA protocol did subjectively appear to increase the time to arousal and extubation, particularly after long procedures.
At our institution, intraoperative monitoring is performed by an independent company. The technical fee is the same for every case monitored, whereas the professional fee is dependent on the actual monitoring service performed. In the case of TcMEP monitoring, the professional fee is approximately $24.00 per extremity pair (upper versus lower). In contrast, the professional fee for EEG is $19.00, and this is the same as the per pair extremity charge for SSEP.
Discussion
The use of neuromonitoring has become well established in the realm of neurosurgical and orthopedic procedures involving intracranial neurosurgery and spinal surgery. The efficacy of specific modalities, including EEG, SSEP, and TcMEP, has been described in the literature.11 The use of TcMEP for monitoring during microsurgical management of cerebrovascular disease was recently described by Szelenyi et al12,13 and was found to be useful in a treatment group of 119 patients. Kang et al4 reported the clinical utility of TcMEP monitoring in detecting motor dysfunction in a small cohort of patients undergoing surgery for either lesions adjacent to the brain stem or intracranial aneurysms. When it comes to neuroendovascular procedures, reports have tended to focus on SSEP, EEG, and BAER monitoring. Lui et al1 were the first to report a series of 35 patients monitored with SSEP, EEG, and BAER while undergoing endovascular therapy for the treatment of cerebral aneurysms. Nine patients experienced significant changes, and in 2 cases these led to alterations in intraoperative management. In a more recent larger series of 63 patients monitored in a similar fashion, Chen and colleagues2 noted significant monitoring changes in 3 patients, all of which led to procedural changes. Both publications concluded that these techniques were useful in detecting intraprocedural ischemia, but neither addressed the monitoring of motor pathways.
After using TcMEP intermittently over the past few years, we decided to analyze our results to determine whether the addition of TcMEP increased our ability to detect adverse neurologic events in our patients undergoing therapeutic neuroendovascular procedures.
In our series of 140 patients, we observed significant changes in TcMEP signals in only 1 patient. In this patient, changes involving all 3 monitoring modalities occurred after intraprocedural aneurysm rupture. In addition, in this case, all monitoring modalities changed simultaneously, suggesting no advantage for 1 technique over another for the early detection of neurologic compromise. In contrast, there were changes in SSEP tracings alone in 9 patients. Of these, 2 patients were known to be moribund before their procedures, and their bilateral SSEP amplitude changes were more likely due to progression of their initial injury rather than a procedural event. In the end, neither patient recovered. Among the remaining 7 patients, temporary SSEP changes tended to correlate with temporary neurologic deficits, while permanent changes were associated with permanent or long-lasting deficits. In the patients with SSEP changes, at least 3 underwent interventions or had their procedures altered as a result of monitoring concerns. Although angiographic findings were noticeable before SSEP changes in 4 of the 7 patients, 3 patients experienced SSEP changes either unrelated to an angiographic finding or before the angiographic finding being detected. One patient had an upper extremity repositioned, possibly preventing a peripheral compression neuropathy. A second patient had blood pressure increased and balloon occlusion times minimized during balloon-assisted coiling, possibly averting a permanent neurologic deficit. Finally, 1 patient's SSEP changes were found to be related to a distal embolus, which was not apparent on the magnified images being viewed during the aneurysm embolization, leading to early thrombolysis and, again, possibly preventing a permanent neurologic deficit. These results lend continued support to previous work concluding that neurophysiologic monitoring using at least SSEP is useful. However, our series does not support the conclusion that the addition of TcMEP monitoring provides any significant added benefit over SSEP alone.
The use of intraoperative neurophysiologic monitoring has steadily increased, despite a paucity of clinical data purporting the sensitivity and specificity of monitoring modalities in predicting neurologic insult. Small case series using TcMEP in neurovascular surgery have supported the use of this technique, given the relative ease of setup and safety, but actual correlation of intraoperative changes to outcome remains elusive. Quinones-Hinojosa et al14 advocated the use of TcMEP in basilar artery aneurysm surgery but provided no clear proof of actual efficacy in monitoring alerts versus clinical outcome in specific patients. In the only previous report of TcMEP use in neuroendovascular patients that we could find, Hiraishi et al6 reported a series of 7 patients with anterior choroidal artery aneurysms monitored with TcMEP while undergoing coil embolization. Three of their 7 patients experienced a transient decrease in TcMEP signals. Of these, 2 patients saw their signals improve after the removal of the coils. One of the 3 patients experienced a transient neurologic deficit. No comparison with other modalities was offered, and the very small number of patients makes it difficult to draw any conclusions relative to the overall efficacy of the technique.
With regard to the feasibility of TcMEP monitoring, we submit that, from a technical standpoint, this technique is straightforward in implementation. Review of our institutional practice found that TcMEP monitoring added little time to the setup or to the overall cost of the procedure. Although TcMEP monitoring poses no direct risk to the patient, this monitoring technique does mandate modification of anesthetic technique. Neuromuscular blockade cannot be used. This translates to increased patient movement during the endovascular procedure that may potentially interfere with the precision of treatment. This has not been a problem in our experience. However, it has been our subjective impression that the use of TIVA, necessary for TcMEP monitoring, does lead to a longer time for patients to awaken from their anesthetic, thus potentially prolonging the overall duration of the case.
Obviously, this study has its limitations. Patients were not prospectively randomized to the various monitoring methods and the physicians were not blinded to the protocol being used. The technique for TcMEP monitoring is extremely sensitive to anesthetic changes. As a result, the quality of the data obtained is likely to have been variable and similar results may not necessarily be achieved at every institution. Given the relatively small number of patients, and the even smaller number with monitoring changes, it is difficult to be certain that some benefit for TcMEP in certain subsets of the neurovascular disease population undergoing endovascular therapy might not be seen.6 Clearly, not all possible clinical scenarios were encountered in the present series and further study may be indicated.
Conclusions
TcMEP appears to be feasible in therapeutic neuroendovascular procedures. However, we found little evidence to support a clinical benefit for the routine use of TcMEP as an adjunct to neurophysiological monitoring with SSEP and EEG during such procedures.
Acknowledgment
The authors thank Lynne Hamann for her assistance in the preparation of this manuscript.
ABBREVIATIONS:
- BAER
brain stem auditory evoked response
- EEG
electroencephalogram
- EVD
external ventricular drain
- GOS
Glasgow outcome score
- MEP
motor-evoked potential
- SSEP
somatosensory-evoked potential
- TcMEP
transcranial motor-evoked potential
- TIVA
total intravenous anesthetic
Footnotes
Disclosures: Mollie Barnes—UNRELATED: Employment: Employed by Impulse Monitoring, Inc. Samuel Johnson—OTHER RELATIONSHIPS: I serve as the Director of Operations for Impulse Monitoring. Impulse is neurophysiological intraoperative monitoring company. The clinical research paper that I collaborated on with Dr. Kevin Cockroft was purely to satisfy our intellectual curiosity, and my company did not contribute nor benefit from this manuscript in any way. Kevin M. Cockroft—OTHER RELATIONSHIPS: Consultant – eV3 Endovascular (not directly related to the project/manuscript).
References
- 1. Liu AY, Lopez JR, Do HM, et al. Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol 2003; 24: 1520– 27 [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Spetzler RF, McDougal CG, et al. Detection of ischemia in endovascular therapy of cerebral aneurysms: a perspective in the era of neurophysiological monitoring. Neurosurg Rev 2011; 34: 69– 75 [DOI] [PubMed] [Google Scholar]
- 3. Neuloh G, Schramm J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg 2004; 100: 389– 99 [DOI] [PubMed] [Google Scholar]
- 4. Kang D, Wu Z, Lan Q, et al. Combined monitoring of evoked potentials during microsurgery for lesions adjacent to the brainstem and intracranial aneurysms. Chin Med J 2007; 120: 1567– 73 [PubMed] [Google Scholar]
- 5. Szelényi A, Kothbauer K, de Camargo A, et al. Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery 2005; 57: 331– 38 [DOI] [PubMed] [Google Scholar]
- 6. Hiraishi T, Fukuda M, Oishi M, et al. Usefulness of motor-evoked potential monitoring during coil embolization of anterior choriodal artery aneurysms: technical reports. Neurol Res 2011; 33: 360– 62 [DOI] [PubMed] [Google Scholar]
- 7. Novak K, de Camargo A, Neuwirth M, et al. The refractory period of fast conducting corticospinal tract axons in man and its implications for intraoperative monitoring of motor evoked potentials. Clin Neurophysiol 2004; 115: 1931– 41 [DOI] [PubMed] [Google Scholar]
- 8. Szelenyi A, Kothbauer K, Deletis V. Transcranial electric stimulation for intraoperative motor evoked potential monitoring: stimulation parameters and electrode montages. Clin Neurophysiol 2007; 118: 586– 95 [DOI] [PubMed] [Google Scholar]
- 9. Grundy B. Intraoperative monitoring of sensory-evoked potentials. Anesthesiology 1983; 58: 72– 87 [DOI] [PubMed] [Google Scholar]
- 10. Harner R. A recommendation for standard EEG montages. Am J of EEG Tech 1977; 17: 105– 14 [Google Scholar]
- 11. Neuloh G, Schramm J. Motor evoked potential monitoring for the surgery of brain tumours and vascular malformations. Adv Tech Stand Neurosurg 2004; 29: 171– 228 [DOI] [PubMed] [Google Scholar]
- 12. Szelenyi A, Langer D, Beck J, et al. Transcranial and direct cortical stimulation for motor evoked potential monitoring in intracerebral aneurysm surgery. Neurophysiol Clin 2007; 37: 391– 89 [DOI] [PubMed] [Google Scholar]
- 13. Szelenyi A, Langer D, Kothbauer K, et al. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. Neurosurgery 2006; 105: 675– 81 [DOI] [PubMed] [Google Scholar]
- 14. Quinones-Hinojosa A, Alam M, Lyon R, et al. Transcranial motor evoked potentials during basilar artery aneurysm surgery: technique application for 30 consecutive patients. Neurosurgery 2004; 54: 916– 24 [DOI] [PubMed] [Google Scholar]


