Abstract
BACKGROUND AND PURPOSE:
Local hemodynamic information may help to stratify rupture risk of cerebral aneurysms. Patient-specific modeling of cerebral hemodynamics requires accurate data on BFV in perianeurysmal arteries as boundary conditions for CFD. The aim was to compare the BFV measured with PC-MR imaging with that obtained by using intra-arterial Doppler sonography and to determine interpatient variation in intracranial BFV.
MATERIALS AND METHODS:
In 10 patients with unruptured intracranial aneurysms, BFV was measured in the cavernous ICA with PC-MR imaging in conscious patients before treatment, and measured by using an intra-arterial Doppler sonography wire when the patient was anesthetized with either propofol (6 patients) or sevoflurane (4 patients).
RESULTS:
Both techniques identified a pulsatile blood flow pattern in cerebral arteries. PSV differed >50 cm/s between patients. A mean velocity of 41.3 cm/s (95% CI, 39.3–43.3) was measured with PC-MR imaging. With intra-arterial Doppler sonography, a mean velocity of 29.3 cm/s (95% CI, 25.8–32.8) was measured with the patient under propofol-based intravenous anesthesia. In patients under sevoflurane-based inhaled anesthesia, a mean velocity of 44.9 cm/s (95% CI, 40.6–49.3) was measured.
CONCLUSIONS:
We showed large differences in BFV between patients, emphasizing the importance of using patient-specific hemodynamic boundary conditions in CFD. PC-MR imaging measurements of BFV in conscious patients were comparable with those obtained with the intra-arterial Doppler sonography when the patient was anesthetized with a sevoflurane-based inhaled anesthetic.
Acute rupture of a cerebral aneurysm resulting in SAH can be a devastating event associated with high mortality and morbidity.1 The International Study of Unruptured Intracranial Aneurysms recommended preventive treatment of unruptured aneurysms if located in the posterior circulation or if >7 mm in diameter and located in the anterior circulation.2 Reported data on ruptured aneurysms indicate, however, that many of these are small, measuring between 3 and 7 mm.3
Why some aneurysms rupture and others do not remains one of the major uncertainties in the management of intracranial aneurysms. The abiding uncertainty on the importance of aneurysm size requires better rupture risk assessment.
Hemodynamic parameters such as inflow jet size, impingement zone, and wall shear stress have all been suggested as contributing to intracranial aneurysm growth and rupture.4 CFD applied in aneurysms and surrounding vessels can predict local hemodynamics.4,5 Currently, non-patient-specific inflow boundary conditions in CFD are applied, mostly because patient-specific BFV measurements during imaging remain difficult to collect.6 The use of non-patient-specific boundary conditions has hampered the study of hemodynamics in intracranial aneurysms. Reliable patient-specific boundary conditions for CFD in patients with both ruptured and unruptured aneurysms are needed to predict local hemodynamics more accurately in intracranial aneurysms.6,7
Accurate BFV measurements in the perianeurysmal arteries in the circle of Willis are difficult to obtain, because the skull and the tortuosity of the arteries impede conventional methodologies, such as TCD. Making use of phase-contrast MR imaging enables BFV measurement at targeted arterial locations, provided that the resolution is sufficiently high.8–10 Furthermore, BFV measurements in cerebral arteries can also be performed by using an intra-arterial Doppler sensor– mounted wire.11,12 The Doppler sensor– mounted wire (ComboWire; Volcano Corporation, Rancho Cordova, California) has been validated in coronary stenosis evaluation during cardiovascular interventions.13 Measurements with the ComboWire can be performed during the endovascular treatment of intracranial aneurysms, even in patients with ruptured aneurysms.12 In these acutely ill patients, PC-MR imaging measurements are usually not feasible. Therefore, patient-specific flow measurements can be acquired, both for the ruptured as well as for the unruptured aneurysms to improve inflow-boundary conditions for CFD modeling.
The aim of this study was to compare the BFV measured with PC-MR imaging in conscious patients with that obtained by using an intra-arterial Doppler sensor wire and to determine interpatient variation in patients with unruptured intracranial aneurysms.
Materials and Methods
Patient Selection
Patients scheduled for endovascular treatment of an unruptured cerebral aneurysm between January 2008 and July 2010 were included if older than 18 and younger than 70 years of age. Patients with contraindications for MR imaging on 3T were excluded. Because of our limited experience with the ComboWire in cerebral vessels, patients with any history of vasospasm, aneurysm rupture, relevant comorbidity, or complex aneurysm geometry were also excluded. The institutional review board approved the study protocol, and written informed consent was obtained from all participants.
Phase-Contrast MR Imaging BFV measurements
Within 2 weeks before endovascular aneurysm treatment, patients underwent PC-MR imaging BFV measurement. MR images were acquired on a 3T MR imaging scanner (Intera; Phillips Healthcare, Best, the Netherlands) equipped with a sensitivity encoding 8-channel head-receive coil. The PC-MR imaging scan sequence parameters were the following: 0.65 × 0.65 mm; section thickness, 3 mm; TE/TR, 2.8/5.6 ms; flip angle, 15°; NSA (number of signal averages), 2; velocity-encoding, 60–100 cm/s; sensitivity encoding factor, 3; <36 cardiac phases; and retrospective cardiac gating. Scan duration including anatomic sequences was approximately 35 minutes. All measurements were obtained at the cavernous ICA.
Through-plane velocity was measured in a section perpendicular to the artery of interest. The acquired fast-field echo images, showing anatomic structures, and phase images, containing velocity information, were combined and analyzed by using custom-built software in Matlab (MathWorks, Natick, Massachusetts).14,15
Intra-Arterial BFV Measurements
Intra-arterial BFV was measured with the use of a sensor wire (ComboWire) mounted with a 100-Hz Doppler sensor at the tip and connected to a personal computer (ComboMap system).12 The maximal velocity was measured and recorded in a cone-shaped envelope approximately 5 mm from sensor to base at 100 Hz and was stored at 200 Hz after interpolation. The ComboWire sampled in 1 place, for approximately 30 seconds or ∼30 cardiac cycles.
Anesthesia and Intervention
The neurointerventional procedures were performed with the patient under general anesthesia. Choice of anesthetic was independent of the research protocol and was determined by the attending anesthesiologist. The anesthetics applied were either continuous inhalation of sevoflurane (group S) or intravenously administered propofol target-controlled infusion (group P). During the intervention, the end-tidal CO2 pressure was monitored.
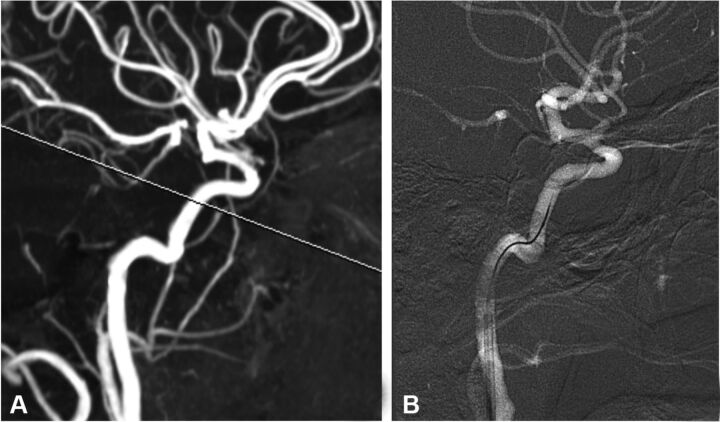
Navigation to the cranial arteries was performed following normal interventional procedures, by using fluoroscopy in combination with a standard guidewire and guiding catheter. A 7F sheath was placed in the right femoral artery, followed by placement of a 6F guiding catheter in the cervical ICA. A microguidewire with a microcatheter was placed in the vertical portion of the cavernous segment of the internal carotid artery through the guiding catheter. The standard microguidewire was then replaced by the ComboWire, which has a slightly more rigid design compared with standard microguidewires and therefore is never used as a navigational device. Minute maneuvering was sometimes needed for the ComboWire to derive the optimal velocity signal, matched with the location of the PC-MR imaging measurements (Fig 1).
Fig 1.
A, PC-MR imaging section projected on a single carotid maximum intensity projection. B, Angiographic snapshot of the ComboWire in place.
The measurement was considered successful when the signal showed a clear systolic-diastolic pattern for at least 30 seconds. The cross-sectional area of a ComboWire is 0.1 mm2, approximately 0.5% of the cross-sectional area of a 5-mm-diameter vessel. No adverse effects, such as vasospasm or thromboembolic events, were encountered while using the ComboWire in intracranial arteries.
Data Analysis
The PC-MR imaging measurements contain BFV data in every voxel in a section perpendicular to the vessel in 20–36 time-steps of a cardiac cycle (On-line Fig 1). The PC-MR imaging data had to be down-sampled to 20 time-steps in 2 patients. Lumen segmentation was performed in the fast-field echo images by using a level-set evolution algorithm.15 Only the voxel with the maximal velocity per time-step was used because the Doppler echo acquisition of BFV with the ComboWire only records the maximal BFV. The data obtained with the ComboWire were averaged in an ensemble average by using the R-peak of the electrocardiogram to match the acquired cardiac cycles and to calculate a per-point average.
The ComboWire data consist of approximately 200 recorded time-steps in 1 cardiac cycle, which were averaged to 20 time-steps for each cardiac cycle, to match PC-MR imaging data (On-line Fig 1).
All the PC-MR imaging BFV and ComboWire BFV data were measured in centimeters per second. A Mann-Whitney test was used to calculate differences between groups. Modalities were compared by using a paired t test. A P value <.05 was considered statistically significant. The level of agreement between ComboWire measurements with the different anesthesia schemes with the PC-MR imaging measurements was visualized by using Bland-Altman plots.
Results
Patient and Aneurysm Characteristics
Ten patients (6 women) with an unruptured aneurysm were included, with an average age of 54 years (range, 31–70 years). The aneurysms were located at the ophthalmic artery (n = 6), posterior communicating artery (n = 2), middle cerebral artery (n = 1), and the carotid terminus (n = 1). The aneurysm size ranged from 3 to 22 mm. Four patients received sevoflurane, and 6 received propofol. Group P had a mean end-tidal CO2 pressure of 32 mm Hg compared with 38 mm Hg in group S.
Velocity Measurements
Both the PC-MR imaging and the Doppler sonography recordings demonstrated a pulsatile blood flow pattern in all 10 patients (On-line Fig 2). The evident interpatient variation in BFV between patients was measured with both modalities and was independent from the type of anesthetic (On-line Fig 2). The maximal measured PSV with PC-MR imaging was 93 cm/s, and the minimal measured PSV was 44 cm/s. The minimal and maximal PSVs measured with the ComboWire for group S were, respectively, 46 cm/s and 87 cm/s; and for group P, respectively, 21 cm/s and 96 cm/s.
For the total group of patients, when comparing PC-MR imaging and ComboWire, the mean BFV and PSV differed by 5.7 cm/s (P = .26) and by 6.5 cm/s (P = .36), respectively (Table).
Mean and peak systolic velocitiesa
| PC-MRI |
ComboWire |
|||
|---|---|---|---|---|
| MBFV | PSV | MBFV | PSV | |
| All patients (cm/s) | 41.3 ± 11 | 61.3 ± 16 | 35.6 ± 19 | 54.8 ± 25 |
| Group S (n = 4) (cm/s) | 39.2 ± 13 | 57.9 ± 13 | 44.9 ± 18 | 66.1 ± 17 |
| Group P (n = 6) (cm/s) | 42.8 ± 11 | 63.6 ± 19 | 29.3 ± 18 | 47.3 ± 28 |
Note:—MBFV indicates mean BFV.
Values are mean ± SD.
PC-MR imaging measurements in conscious nonanesthetized patients showed no significant difference (P = .22) between groups S and P (On-line Fig 3A). In contrast, under anesthesia, group S showed mean BFV values that were 15.6 cm/s (P < .0001) higher than those in group P, when measured with the ComboWire (On-line Fig 3B).
If the patients are separated for the type of anesthetic used during the ComboWire measurements, the patients who received sevoflurane (group S) showed higher velocities during ComboWire measurements than patients who received propofol (group P). In group S, the PC-MR imaging mean BFV measurements were 5.8 cm/s (P < .0001) lower than the ComboWire measurements (On-line Fig 4A). In group P, the mean BFV PC-MR imaging measurements were 13.5 cm/s (P < .0001) higher than the ComboWire measurements (On-line Fig 4B and the Table). The PSV values for patients in group S were 8.2 cm/s higher with the ComboWire, whereas these values were 16.3 cm/s below the PC-MR imaging values measured for group P.
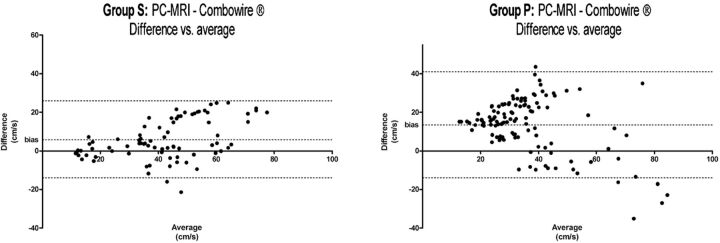
The Bland-Altman plots showed that in group P, the differences in measured BFV values between modalities were more evident than those measured in group S (Fig 2).
Fig 2.
Bland-Altman plots of groups S and P.
Discussion
In this study, we measured profound interpatient differences in PSV and mean BFV in the cavernous segment of the ICA. The large differences between patients were measured with both PC-MR imaging and the ComboWire and thus independent of the technique used. These large differences show that accurate patient-specific CFD simulations require accurate measurements of patient-specific inflow boundaries.6 Accurate data of local hemodynamics in unruptured aneurysms are valuable because they can provide more insight into aneurysm rupture risk.4,16,17 PC-MR imaging can supply data on local hemodynamics in patients with unruptured aneurysms in a noninvasive manner. Our data show that PC-MR imaging and ComboWire measurements in the same patient are within acceptable ranges for providing patient-specific inflow boundaries for CFD simulations. Consequently, ComboWire measurements can be used during endovascular treatment of the acutely ill patient with a ruptured aneurysm in whom the use of PC-MR imaging is usually not feasible.
Both PC-MR imaging and the ComboWire were able to acquire a pulsatile flow-velocity pattern throughout the cardiac cycle. The slightly higher BFV values with PC-MR imaging versus ComboWire BFV measurements may be attributed to anesthesia. A reduction in ComboWire measured cerebral BFV in patients who received propofol contrasted with the maintained cerebral BFV in patients receiving sevoflurane. Nevertheless, both techniques were applicable for measuring intracranial flow. For patients with unruptured aneurysms, PC-MR imaging can supply data on local hemodynamics in a noninvasive manner. Deriving PC-MR imaging on local hemodynamics is more difficult in patients with ruptured aneurysms. Under those circumstances, the ComboWire may provide BFV as well as locally measured intracranial arterial pressure in and around cerebral aneurysms.12
Patient-specific velocity values are essential for the correct calculation of flow-dependent parameters in CFD. Local wall shear stress is calculated with the BFV near the vessel wall; a change in magnitude of the BFV will affect the magnitude of the wall shear stress. Previous research showed that the flow rate can vary up to 25% without a substantial difference in flow patterns and impingement zones.18 In this study, the maximal interpatient difference in PC-MR imaging–determined BFV was more than 2-fold, consistent with conclusions from Venugopal et al,6 supporting the belief that patient-specific boundary conditions should be used in CFD research.
A limitation of the use of an intra-arterial Doppler sensor is that adequate flow signals are sometimes difficult to acquire by using a flexible wire with a velocity sensor in the tip. In our study, the neuroradiologist maneuvered the wire until a clear diastolic-systolic signal was shown. The need for maneuvering the wire shows that it is theoretically possible that the wire could be in a suboptimal position for picking up the main flow signal. It is possible that the ComboWire measures flow in the inner curve of a curved artery, while the main flow is situated in the outer curve. However, measurements were only scored when a clear and optimal signal was obtained during the intervention, by using fluoroscopy as a visual control. Hence, we think the chance of measuring flow other than the main flow is negligible.
A second limitation is that due to the large number of data points, the ComboWire data can be slightly under- or overestimated as a consequence of data processing. The ComboWire and ComboMap system sample the measured data at a rate of 200 Hz, recording consecutive systolic-diastolic velocity curves. This series of curves is processed by using an ensemble average. The use of an ensemble average smooths the curve, in contrast to the velocity curve obtained with PC-MR imaging, which has not been smoothed. As a consequence, intrapatient differences can be slightly altered; the intrapatient differences are increased if the ComboWire measurements are, on average, lower than the PC-MR imaging measurements and are decreased if the ComboWire measurements are, on average, higher than the PC-MR imaging measurements. Subjects have physiologic variations in cerebral BFV during the day; depending on activity, this could have had an effect on our measurements and subsequently on the reported inter- and intrapatient variability. However, because all measurements were performed under similar circumstances, we believe that the profound interpatient differences in BFV are due to interpatient variability rather than to physiologic variance.
Previous studies have shown that PC-MR imaging underestimates the peak systolic velocity by 4%–35% compared with TCD.19–21 We measured a 12.4% underestimation of PSV with PC-MR imaging compared with the ComboWire in group S, which is in line with these TCD data. On the contrary, PC-MR imaging measurements in group P were higher than those with the ComboWire, which should be attributed to the use of propofol as an anesthetic. The overall lower velocities measured with PC-MR imaging can be the result of the lower spatial and temporal resolution of PC-MR imaging compared with Doppler echo-based techniques.19–22
Anesthesia during the endovascular intracranial procedures affects CBF and cerebral BFV.23 The finding of a lower cerebral BFV with propofol versus sevoflurane is related to the reduction of cerebral metabolism and CBF/cerebral BFV by propofol.23,24 Moreover, cerebral BFV measurements of sevoflurane-anesthetized patients showed a much better agreement with the PC-MR imaging measurements, compared with propofol-anesthetized patients (Fig 2).
Cerebrovascular responses are modulated by alterations in PaCO2, with an increase in CBF when PaCO2 increases and vice versa.24,25 In the supine position during anesthesia, measured end-tidal CO2 pressure values are an adequate reflection of PaCO2.26 To correct for the difference between groups S and P, we applied the values given by Eng et al (1992),24 in which the slope of the velocity increases by 1.5 cm/s per 1 mm Hg end-tidal CO2 pressure. The mean velocity of group P increased by 9 cm/s to an average value of 38.3 cm/s, or 6.6 cm/s (15%) below the mean velocity in group S. Compared with the ComboWire measurements in group P, the mean BFV was 11% higher in the PC-MR imaging measurements.
Instead of patient-specific measurements, several researchers scaled the flow rate with the radius of the inflow artery to obtain a fixed mean wall shear stress and, consequently, impose a more patient-specific inflow boundary for CFD.27 This method is based on the assumption that every patient has a similar mean wall shear stress in the parent artery of the aneurysm. Although this provides some correction for interpatient variation, direct patient-specific measurements remain preferable.
Evaluation of the flow rate and distribution in the efferent arteries will further increase the accuracy of the CFD simulations. In the future, the ComboWire could potentially be used to perform measurements in the A1 segment of the ACA or M1 segment of the MCA. However, performing measurements in even smaller efferent arteries requires additional manipulation, which may increase the risk of complications because the ComboWire is slightly more rigid than conventional guidewires.
Recent developments in the treatment of cerebral aneurysms include flow-diverting stents for modulation of intra-aneurysmal hemodynamics.28,29 The hemodynamic effects of these stents have been evaluated by using CFD simulations with non-patient-specific boundary conditions.30 Patient-specific input data in CFD modeling have the potential to provide an improved prediction of the hemodynamic changes and subsequent aneurysm occlusion following flow-diversion techniques.
Conclusions
Results of PC-MR imaging and direct intra-arterial BFV measurements in the same patient are within an acceptable range for use as inflow-boundary conditions for CFD simulations. The marked interpatient variation warrants the use of patient-specific BFV values as boundary conditions for accurate CFD simulations in intracranial aneurysms.
Supplementary Material
ABBREVIATIONS:
- BFV
blood flow velocity
- CFD
computational fluid dynamics
- CI
confidence interval
- PC-MRI
phase contrast MR imaging
- PaCO2
arterial CO2 pressure
- PSV
peak systolic velocity
- TCD
transcranial Doppler
Footnotes
Disclosures: Johannes Van Lieshout—UNRELATED: Consultancy: BMEYE inventive hemodynamics,* Grants/Grants Pending: Dutch Heart Foundation.* *Money paid to the institution.
References
- 1. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006; 355: 928– 39 [DOI] [PubMed] [Google Scholar]
- 2. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention—International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 1998; 339: 1725 [DOI] [PubMed] [Google Scholar]
- 3. Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809– 17 [DOI] [PubMed] [Google Scholar]
- 4. Cebral JR, Castro MA, Burgess JE, et al. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol 2005; 26: 2550– 59 [PMC free article] [PubMed] [Google Scholar]
- 5. Chatziprodromou I, Tricoli A, Poulikakos D, et al. Haemodynamics and wall remodelling of a growing cerebral aneurysm: a computational model. J Biomech 2007; 40: 412– 26 [DOI] [PubMed] [Google Scholar]
- 6. Venugopal P, Valentino D, Schmitt H, et al. Sensitivity of patient-specific numerical simulation of cerebral aneurysm hemodynamics to inflow boundary conditions. J Neurosurg 2007; 106: 1051– 60 [DOI] [PubMed] [Google Scholar]
- 7. Karmonik C, Yen C, Grossman RG, et al. Intra-aneurysmal flow patterns and wall shear stresses calculated with computational flow dynamics in an anterior communicating artery aneurysm depend on knowledge of patient-specific inflow rates. Acta Neurochir 2009; 151: 479– 85 [DOI] [PubMed] [Google Scholar]
- 8. Markl M, Chan FP, Alley MT, et al. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging 2003; 17: 499– 506 [DOI] [PubMed] [Google Scholar]
- 9. Markl M, Harloff A, Bley T, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007; 25: 824– 31 [DOI] [PubMed] [Google Scholar]
- 10. Isoda H, Hirano M, Takeda H, et al. Visualization of hemodynamics in a silicon aneurysm model using time-resolved, 3D, phase-contrast MRI. AJNR Am J Neuroradiol 2006; 27: 1119– 22 [PMC free article] [PubMed] [Google Scholar]
- 11. Benndorf G, Wellnhofer E, Lanksch W, et al. Intraaneurysmal flow: evaluation with Doppler guidewires. AJNR Am J Neuroradiol 1996; 17: 1333– 37 [PMC free article] [PubMed] [Google Scholar]
- 12. Ferns SP, Schneiders JJ, Siebes M, et al. Intracranial blood-flow velocity and pressure measurements using an intra-arterial dual-sensor guidewire. AJNR Am J Neuroradiol 2009; 31: 324– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siebes M, Verhoeff B-J, Meuwissen M, et al. Single-wire pressure and flow velocity measurement to quantify coronary stenosis hemodynamics and effects of percutaneous interventions. Circulation 2004; 109: 756– 62 [DOI] [PubMed] [Google Scholar]
- 14. van Ooij P, Guédon A, Poelma C, et al. Complex flow patterns in a real-size intracranial aneurysm phantom: phase contrast MRI compared with particle image velocimetry and computational fluid dynamics. NMR Biomed 2012; 25: 14– 26 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Xu C, Gui C, et al. Level set evolution without re-initialization: a new variational formulation. In: Proceedings of the 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Diego, California. June 20–25, 2005: 430– 36 [Google Scholar]
- 16. Cebral JR, Mut F, Weir J, et al. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol 2011; 32: 264– 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiang J, Natarajan SK, Tremmel M, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 2011; 42: 144– 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cebral JR, Castro MA, Appanaboyina S, et al. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans Med Imaging 2005; 24: 457– 67 [DOI] [PubMed] [Google Scholar]
- 19. Seitz J, Strotzer M, Wild T, et al. Quantification of blood flow in the carotid arteries: comparison of Doppler ultrasound and three different phase-contrast magnetic resonance imaging sequences. Invest Radiol 2001; 36: 642– 47 [DOI] [PubMed] [Google Scholar]
- 20. Balédent O, Fin L, Khuoy L, et al. Brain hydrodynamics study by phase-contrast magnetic resonance imaging and transcranial color Doppler. J Magn Reson Imaging 2006; 24: 995– 1004 [DOI] [PubMed] [Google Scholar]
- 21. Chang W, Landgraf B, Johnson KM, et al. Velocity measurements in the middle cerebral arteries of healthy volunteers using 3D radial phase-contrast HYPRFlow: comparison with transcranial Doppler sonography and 2D phase-contrast MR imaging. AJNR Am J Neuroradiol 2011; 32: 54– 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lotz J, Meier C, Leppert A, et al. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics 2002; 22: 651– 71 [DOI] [PubMed] [Google Scholar]
- 23. Fodale V, Schifilliti D, Conti A, et al. Transcranial Doppler and anesthetics. Acta Anaesthesiol Scand 2007; 51: 839– 47 [DOI] [PubMed] [Google Scholar]
- 24. Eng C, Lam AM, Mayberg TS, et al. The influence of propofol with and without nitrous oxide on cerebral blood flow velocity and CO2 reactivity in humans. Anesthesiology 1992; 77: 872– 79 [DOI] [PubMed] [Google Scholar]
- 25. Lennox WG, Gibbs EL. The blood flow in the brain and the leg of man, and the changes induced by alteration of blood gases. J Clin Invest 1932; 11: 1155– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Immink RV, Secher NH, Roos CM, et al. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 2006; 96: 609– 14 [DOI] [PubMed] [Google Scholar]
- 27. Castro MA, Putman CM, Sheridan MJ, et al. Hemodynamic patterns of anterior communicating artery aneurysms: a possible association with rupture. AJNR Am J Neuroradiol 2009; 30: 297– 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009; 64: 632– 42 [DOI] [PubMed] [Google Scholar]
- 29. Byrne JV, Beltechi R, Yarnold J, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PloS One 2010; 5: 1– 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radaelli AG, Augsburger L, Cebral JR, et al. Reproducibility of haemodynamical simulations in a subject-specific stented aneurysm model: a report on the Virtual Intracranial Stenting Challenge 2007. J Biomech 2008; 41: 2069– 81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.