CT may show hyperdense areas after intra-arterial thrombolysis due to contrast accumulation and/or hemorrhage and these are considered negative prognostic signs. The authors studied 48 patients at 24 and 72 hours after procedures. Of 15 patients with an occluded ICA or MCA, recanalization was achieved in 12 and hyperdense regions were seen in all (subarachnoid = 6, brain = 4, and both = 5). All hyperdense regions were considered to be clinically asymptomatic and thus did not carry a worse prognosis.
Abstract
BACKGROUND AND PURPOSE:
The aim of this study was to report the CT evolution and clinical significance of HCA after intra-arterial mechanical thrombectomy (revascularization by using retrievers and/or other mechanical devices without concomitant delivery of intra-arterial thrombolytics) in our patients. These lesions are common after intra-arterial thrombolysis, being considered a negative prognostic sign. Their significance after pure mechanical thrombectomy remains unknown.
MATERIALS AND METHODS:
Forty-eight patients were treated with mechanical thrombectomy by using retrievable stents between April 2010 and February 2011. All patients underwent initial (first 24 hours) and follow-up (48–72 hours) nonenhanced CT. We retrospectively analyzed the clinical and radiologic data of the patients with HCA and compared them with controls.
RESULTS:
Fifteen of 48 patients presented with HCA. The site of occlusion was the MCA in 7 patients, both the extra- and intracranial segments of the ICA in 6, and the intracranial ICA in 2. In 7 patients, previous intravenous thrombolysis was administered. Complete recanalization (TICI 3) was achieved in 12 patients, and incomplete recanalization (TICI 2b), in 3. The location of HCA was the subarachnoid space in 6 patients, the brain parenchyma in 4 patients, and both in 5 patients. The HCA were asymptomatic in all patients. There was no statistical difference in final NIHSS score reduction (NIHSS pretreatment–NIHSS at discharge) between patients and controls.
CONCLUSIONS:
In our series, HCA are common after mechanical thrombectomy but do not carry an increased risk of symptomatic hemorrhage or negative prognosis. These data might be related to the high rate of recanalization and the absence of intra-arterial thrombolytics.
Intraparenchymal HCA are commonly seen on posttherapeutic CT scans after intra-arterial reperfusion therapy and may be secondary to contrast extravasation or hemorrhage transformation.1–5 The incidence and prognosis of HCA after intra-arterial delivery of thrombolytics have been reported in a few studies, and their presence has been established as a risk factor for parenchymal hemorrhagic transformation and symptomatic hemorrhage.3–5 However, the imaging evolution and clinical significance of these lesions after pure mechanical thrombectomy have not been reported. In this series, we report 48 consecutive mechanical thrombectomies in which we observed 15 cases of HCA, describe their imaging and clinical findings, and compare them with those in controls.
Materials and Methods
This series includes all patients with acute stroke who were treated with mechanical thrombectomy at our hospital between April 2010 and February 2011. Patients were selected for this therapy after an imaging and clinical protocol that is performed at our hospital in all patients with acute stroke who are considered candidates for intravenous thrombolysis and/or intra-arterial thrombectomy.
Initial imaging protocol includes urgent noncontrast CT, CT angiography, and CT perfusion. Candidates for intravenous therapy are selected according to the National Institute of Neurological Disorders and Stroke6 and the Third European Cooperative Acute Stroke Study criteria.7,8 These patients receive full-dose intravenous rtPA (0.9 mg/kg with a maximum total dose of 90 mg), with an initial bolus of 10% of the total dose and the remaining 90% administered within 1 hour.
Indications for intra-arterial thrombectomy included: 1) rescue therapy, performed in those patients in whom intravenous therapy was not accompanied by a clinical response (improvement in NIHSS score ≥ 4) in the first 60 minutes; and 2) direct intra-arterial thrombectomy, which is performed in those patients with large-vessel strokes who arrive at our hospital between 4.5 and 8 hours after symptom onset.9–13 A second noncontrast CT to rule out hemorrhage is obtained before angiography in patients who undergo rescue therapy. Before angiography and mechanical thrombectomy, all patients or relatives give informed consent.
All procedures were performed with the patient under general anesthesia. Angiography was performed via a femoral approach by using a 9F sheath and a diagnostic catheter, generally a JB 4F (Cordis, Miami Lakes, Florida). Once the location of the clot had been identified by using angiography, the diagnostic catheter was removed and an 8F balloon guide catheter (Concentric Medical, Mountain View, California) was advanced and placed in the ICA. A Rebar-18 microcatheter (ev3 Neurovascular, Irvine, California) with a 0.014-inch Traxcess guidewire (MicroVention, Tustin, California) was then advanced distally to the clot. The microwire was then removed and a stent-retriever, Solitaire AB 4–20 (ev3 Neurovascular), was used to engage and snare the clot. Once the clot was captured, the balloon guide catheter was inflated to temporally arrest forward flow while the clot was being withdrawn. The clot was first pulled into the catheter guide and then completely out of the body while aspirating with a 50-mL syringe. The balloon was then deflated, and the flow was restored. Occasionally, additional retrievers, Trevo Retriever 4–20 (Concentric Medical) and the Solitaire AB 6–30 (ev3 Neurovascular), microwires (0.014-inch Transend; Boston Scientific, Natick, Massachusetts), or angioplasty balloons were used to disrupt the clot (“Results ” section).
Recanalization was assessed on the control angiogram after mechanical thrombectomy and was classified as complete (TICI 3), incomplete (TICI 2), or failed (TICI 0 or 1).14–16
Once the procedure was finished, the patients were admitted to the intensive care unit for 24 hours and then to the stroke unit. Antiplatelet or anticoagulation therapy was initiated after the initial postprocedural scan had ruled out symptomatic hemorrhage, whether patients had received previous intravenous thrombolysis or not. The choice of therapy depended on the suspected etiology of the stroke. Initial anticoagulation with intravenous heparin and a posterior switch to acenocoumarol was used for inferred cardioembolism in the setting of atrial fibrillation, while antiplatelet therapy (aspirin, 200 mg a day, or clopidogrel, 75 mg a day) was preferred in the setting of atherothrombotic etiologies. Control of blood pressure, fever, hyperglycemia, aspiration risk, deep venous thrombosis prophylaxis, and all other essential aspects in acute stroke management were performed following current stroke guidelines.17
All patients underwent nonenhanced CT scans within the first 24 hours after treatment (initial scan) and again, for those still alive, in the next 48–72 hours (follow-up scan). HCA were defined as newly appearing hyperdensity exhibited on the initial CT scan after reperfusion therapy. The location of the HCA was divided into parenchymal, subarachnoid, or mixed (both). The imaging evolution of HCA between initial and follow-up scans was evaluated by 3 experienced noninterventional neuroradiologists blinded for the study, according to changes in size, morphology, and density of the HCA, and was classified as the following: increasing, decreasing, no changes, or disappearance. Clinical evolution was evaluated by the preprocedural NIHSS score, 24-hour postprocedural NIHSS score, and NIHSS score at discharge. Additional clinical and image data were also obtained from both patients with HCA and controls (patients with stroke treated with mechanical thrombectomy who did not show HCA after treatment), including sex, age, vascular risk factors, previous treatment, site of occlusion, previous intravenous thrombolytic therapy, type of retriever, procedural duration, and time from symptom onset to recanalization of the vessel.
To determine the baseline differences between the hyperattenuated area group and the control group, we performed statistical analyses by using a statistical software package (Statistical Package for the Social Sciences, Version 17.0; SPSS, Chicago, Illinois). We performed univariate analyses, including the Student t and the Mann-Whitney U tests for the continuous variables and the χ2 for the categoric variables. A value of P < .05 was considered significant.
Results
Incidence
Between April 2010 and February 2011, forty-eight of the patients who presented with an acute ischemic stroke at our hospital were treated with a mechanical intra-arterial thrombectomy. Fifteen of these patients (31.2%) showed HCA in the initial scan postthrombectomy. The results in this group of patients follow (On-line Table).
Sex and Age
Nine patients were men (60%) and 6 were women (40%). The mean age was 66 years (range, 37–81 years), 67 years for men (range, 39–81 years) and 65 years for women (range, 37–81 years).
Occlusion Site
The MCA was affected in 7 patients (46.7%), 4 on the left-hand side. Both extracranial and intracranial segments of the ICA were affected in 6 patients (40%), 3 on each side. The intracranial ICA was occluded in 2 patients (13.3%), both on the right side.
Previous Antiaggregants or Anticoagulation
Only 1 of the 15 patients (6.7%) was on anticoagulant therapy (acenocumarol) before symptom onset. Five patients (33.3%) were on antiaggregants (3 aspirin, 2 clopidogrel) before the stroke.
Previous Intravenous Thrombolytic Therapy
Seven patients (46.7%) were treated with intravenous thrombolytic therapy before mechanical intra-arterial therapy, and 8 patients (57.3%) were not.
Recanalization
Complete recanalization after therapy (TICI 3) was obtained in 12 of the 15 patients (80%), whereas incomplete recanalization (TICI 2) was achieved in the remaining 3 patients (20%). These 3 patients corresponded to subtype TICI 2b. There was no case of failed recanalization (TICI 0 or 1).
Retrievers
In cases 1–9, 11, 12, and 13, a Solitaire AB 4-20 was used to engage and snare the clot. In cases 10 and 15, the Trevo Retriever 4-20 was used. In case 14, the Solitaire AB 4-20, Solitaire AB 6-30, and Trevo Retriever 4-20 had to be used to open the occluded artery.
Duration of the Revascularization Procedure
Procedure duration time ranged from 50 to 291 minutes. The mean was 139.4 ± 65.4 minutes.
Time from Symptom Onset to Recanalization of the Vessel
The time from symptom onset to vessel recanalization ranged from 222 to 573 minutes. The mean was 360.2 ± 110.5 minutes.
Location
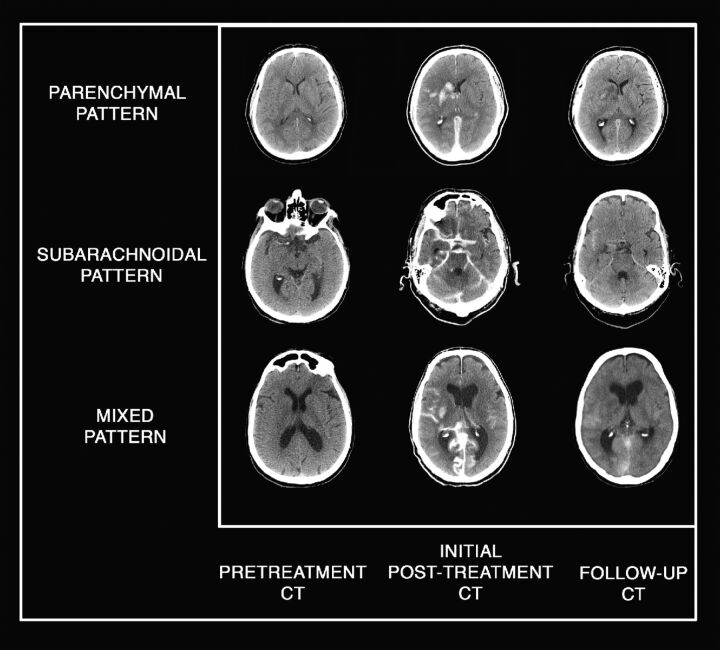
In 6 patients, location of HCA was limited to the subarachnoid space (40%); in 4 patients, it was limited to brain parenchyma (26.7%) (Fig 1). The remaining 5 patients (33.3%) had a mixed location of HCA, affecting both the parenchyma and subarachnoid space.
Fig 1.
Various patterns of localization of HCA. Plain CT axial images show pretreatment CT with the absence of early ischemic signs; initial posttreatment CT shows parenchymal, subarachnoid, and mixed patterns of HCA; and follow-up CT shows progressive resolution of HCA.
Pre- and Postprocedural NIHSS Scores
Thirteen of the 15 patients (86.7%) experienced clinical improvement after mechanical thrombectomy, ranging from 2 to 17 points in the NIHSS score (mean, 9.8). Two patients (13.3%) did not improve after treatment.
Imaging Evolution
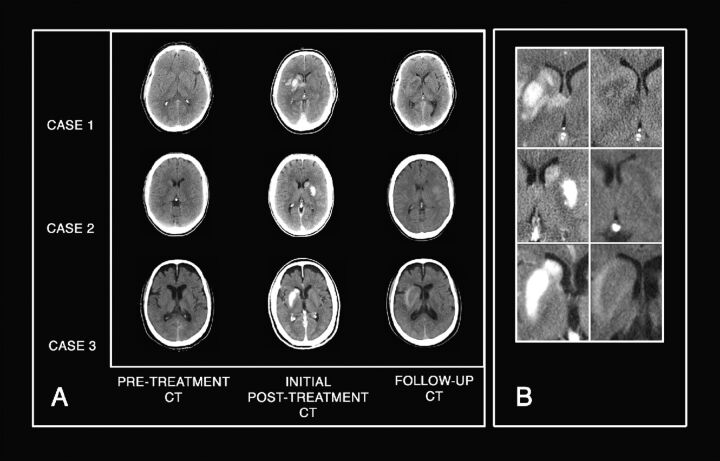
In 9 patients (60%), HCA detected in the initial CT disappeared in the follow-up scans, and in 6 patients (40%), these decreased (Figs 1 and 2). No cases of massive hemorrhagic transformation were found.
Fig 2.
Example of temporal evolution of abnormalities. A, Plain CT axial images show the appearance and evolution of HCA in the basal ganglia in 3 different patients in our series. Pretreatment CT shows the absence of early ischemic signs. Initial posttreatment CT clearly demonstrates newly appearing HCA located in basal ganglia in all. Follow up CT shows complete disappearance of HCA in cases 1 and 2 and a decrease of HCA with persistence of minimal scattered hyperattenuation in case 3. B, Magnified images of the 3 cases offer better visualization.
NIHSS Score at Discharge
Nine of the 15 patients (60%) experienced a decrease between postprocedural NIHSS score and NIHSS score at discharge (range, 1–8). In 5 patients, the NIHSS score did not change (33.3%), and 1 patient died (6.6%).
HCA versus Controls
Results in the group of patients with HCA (n = 15) were compared with those in the group of controls (n = 34), which was formed by patients with stroke treated with mechanical thrombectomy who did not show HCA after treatment (Table). Age, sex, vascular risk factors, previous medication, associated intravenous therapy, recanalization rates, duration of the recanalization procedure, time from symptom onset to revascularization, pretreatment NIHSS score, and NIHSS score reduction at discharge in the control group can be seen in the Table.
Comparison between patients with HCA and controls
| Patients with HCA | Controls | P Value | ||
|---|---|---|---|---|
| No. | 15 | 33 | ||
| Age (yr) | 66, 30 | 65, 27 | NS | |
| Sex | M/F | 9:6 | 18:15 | NS |
| Artery | EICA | 6 | 3 | NS |
| IICA | 2 | 4 | ||
| MCA | 7 | 19 | ||
| BA | 0 | 6 | ||
| PCA | 0 | 1 | ||
| Atrial fibrillation | 6 | 14 | NS | |
| High blood pressure | 8 | 23 | NS | |
| Diabetes mellitus | 2 | 10 | NS | |
| Dyslipidemia | 5 | 9 | NS | |
| Smoking | 6 | 11 | NS | |
| Previous antiplatelet therapy | 5 | 13 | NS | |
| Previous anticoagulant therapy | 1 | 5 | NS | |
| Intravenous therapy | Yes | 7 | 13 | NS |
| No | 8 | 20 | ||
| Recanalization | Complete | 12 | 26 | NS |
| Incomplete | 3 | 7 | ||
| Duration of recanalization procedure (min, mean) | 139, 40 | 113, 18 | NS | |
| Time from symptom onset to revascularization (min, mean) | 360, 20 | 431, 70 | NS | |
| NIHSS score pretreatment | 15, 13 | 18, 47 | NS | |
| NIHSS score reduction at discharge | 10, 79 | 8, 69 | NS |
Note:—NS indicates not significant; EICA, extra- and intracranial segments of the ICA; IICA, intracranial segment of the ICA; BA, basilar artery; PCA, posterior cerebral artery.
There was no statistical significance between the 2 groups with regard to sex, atrial fibrillation, high blood pressure, diabetes mellitus, dyslipidemia, smoking, previous antiplatelet or anticoagulant therapy, site of occlusion, previous thrombolytic intravenous therapy, time of procedure, percentages of recanalization, time between symptom onset and reperfusion, and preprocedural NIHSS scores.
There was no statistical difference in the final NIHSS score reduction (NIHSS pretreatment–NIHSS at discharge) between the 2 groups. We observed a trend toward a difference in the occlusion site (P = .06), probably related to the high percentage of extra- and intracranial ICA occlusions in the HCA group compared with controls (40% and 8.8% respectively) and the presence of 6 cases of basilar occlusion and 1 posterior cerebral artery occlusion in controls (no cases in patients). However, this trend did not reach statistical significance.
To evaluate this possible confusing factor, we performed a new statistical analysis of final NIHSS score reduction (NIHSS pretreatment–NIHSS at discharge), excluding the 7 cases of posterior circulation involvement in controls. With this method, the mean final NIHSS reduction in patients was 10.7 ± 6.8, and in controls, 7.1 ± 5.4, with a P = .09 (nonsignificant).
Discussion
In the context of thrombolytic therapy in acute stroke, the term “hyperdense areas” has been used to define “newly appeared hyperdensities seen on a CT scan after reperfusion.”3 The clinical significance and physiopathology of these lesions were first suggested by Wildenhain et al in 1994,1 who described these lesions in 6 patients. They noticed that the density of the lesions might be secondary to hemorrhage and/or contrast extravasation and also suggested a possible relation between the image findings and clinical prognosis. Since these initial findings, a few articles have confirmed the frequent existence of hyperdense areas (also called HCA) after thrombolytic intra-arterial therapy and have helped to clarify their meaning.3–5 In one of the most relevant studies, Nakano et al3 concluded that HCA are a significant risk factor for hemorrhagic transformation and also increase the risk of symptomatic hemorrhage after intra-arterial thrombolysis.
The main aim of our study was to report our experience in the CT evolution and the clinical significance of HCA areas after intra-arterial mechanical thrombectomy, a kind of revascularization technique that is gradually taking the place of intra-arterial thrombolytics, due, in great part, to the rapid improvement of retrievers and other mechanical devices. Previous studies of postrevascularization HCA have focused on intra-arterial thrombolytics, but studies of these lesions after mechanical thrombectomy are lacking, to our knowledge.
The frequency of HCA in our series was 31.2%, which is very similar to the 33.9% reported by Yoon et al,4 and the 32.9% reported by Jang et al,5 but inferior to that reported by other authors like Wildenhain et al1 or Nakano et al3 (60% and 48%, respectively). However, these different series of patients are difficult to compare because they all differ in the number of patients (from 10 to 94), revascularization techniques (intra-arterial thrombolytics versus thrombectomy), and percentages of revascularization (from 50% to 100%), factors that probably determine the different percentages of HCA.
In our series of patients, no statistical difference in the final NIHSS score reduction (NIHSS pretreatment–NIHSS at discharge) was found between patients with HCA and controls, which suggests that these lesions may not imply a negative clinical prognosis after mechanical thrombectomy. The presence of HCA is still frequent and similar to that reported in previous articles,4,5 but in our series, none of the patients showed associated clinical decline (symptomatic hemorrhagic transformation). This benign evolution of HCA after mechanical therapy is probably related to 2 main factors: the absence of intra-arterial thrombolytics during the procedure and the high rate of successful recanalization.
Plasminogen activation carries a risk of hemorrhage by altering the platelet plug framework, vascular permeability, and vascular basal integrity at sites of injury. Intra-arterial thrombolytics produce an exogenous plasminogen activation, which might accelerate the dissolution of the blood-brain barrier, microvascular basal lamina, and the extracellular matrix and platelet-fibrin plugs, thereby increasing the risk of hemorrhage.18–20 These mechanisms are also shared by thrombolytics that are administered intravenously, but the direct administration of thrombolytics into the core of the infarct is probably linked to a higher risk of hemorrhage. Cases of hemorrhage in intra-arterial studies are higher than in intravenous ones,6,21–23 and in some registers (Safe Implementation of Thrombolysis in Stroke-Monitoring Study, 6483 patients), the percentages of symptomatic hemorrhage after intravenous therapy are as low as 1.7%,24 which might justify the absence of symptomatic hemorrhages in the 7 patients who had been treated with previous intravenous therapy in our series. On the other hand, several studies with intra-arterial thrombolytics were terminated because of safety concerns. In these trials, the risk of symptomatic hemorrhage associated with plasminogen activation was significantly greater than that with a placebo (odds ratio for symptomatic hemorrhage agent/placebo: 6.8 in MAST-E, 10.5 in MAST-I, 4.9 in ASK).21–23 Therefore, the avoidance of direct deliverance of intra-arterial thrombolytics probably plays an important role in the absence of symptomatic hemorrhages in our series.
In addition, the high rate of successful recanalization associated with mechanical thrombectomy in our series (80% TICI 3 and 20% TICI 2b, compared with 50%–62% of good recanalization reported in previous studies with intra-arterial thrombolytics1–5) is probably a further determining factor in good prognosis, as is suggested by the changes in cerebral microvasculature that follow ischemic injury. Relevant experimental studies have shown that massive hemorrhagic transformation, with the distinguishing features of a rapid extravasation of blood leading to the compression of contiguous tissues with a loss of neurologic function (symptomatic hemorrhage), occurs when the integrity of the blood-brain barrier and the basal lamina has been lost,25 a situation that appears after severe and prolonged ischemic injury.26,27 A high percentage of good revascularization may preclude the loss of integrity of the blood-brain barrier/basal lamina, thus preventing symptomatic hemorrhagic transformations, and several clinical studies have proved that severe hemorrhagic transformation is more common when recanalization has failed.28–30
It also seems possible that the current evolution of mechanical thrombectomy tools, which makes them more effective and less damaging to the endothelium of cerebral arteries (retrievable stents representing a positive evolutionary leap relative to the Merci retriever [Concentric Medical]), may also play a role in the successful outcome of these patients. In this sense, a recent article that examined the safety of a retrievable stent by serial angiography and histology of treated vessels in a swine model found no evidence of vessel damage (dissection, perforation, clot formation) related to device deployment.31
Drawbacks in our study are mainly related to the low number of patients, and it seems likely that with a higher number of patients, some symptomatic hemorrhagic transformation of HCA would occur. However, all the series with intra-arterial thrombolytics have clearly reported higher percentages of symptomatic hemorrhage, even in the smallest ones (30% in 10 patients1), which seems to support the idea that HCA after mechanical thrombectomy may be associated with a less ominous prognosis than thrombolysis.
Another aspect that may also be open to discussion is the different proportion of occluded vessels in patients and controls, which shows a trend toward statistical difference (P = .06) in probable relation to the presence of 6 cases affecting the posterior circulation in controls and no such cases in patients. However, this trend did not reach statistical significance, and a subanalysis of final NIHSS score reduction, excluding the 7 cases of posterior circulation involvement in controls, again showed no statistical difference, with an even superior P value (P = .09, see “Results”), which reinforces the concept that HCA postthrombectomy is not associated with a clinical decline with respect to controls.
Conclusions
In our series, HCA were common after mechanical thrombectomy but did not carry an increased risk of symptomatic hemorrhage or negative prognosis. These data might be related to the high rate of recanalization and the absence of intra-arterial thrombolytics and may indicate that the type of intra-arterial revascularization technique (thrombolytics or thrombectomy) could be a prognostic factor in postreperfusion HCA. Studies comparing HCA evolution with both techniques are needed to reach appropriate conclusions.
Supplementary Material
ABBREVIATIONS:
- ASK
Australian Streptokinase Trial
- HCA
hemorrhage/contrast staining areas
- Mast-E
Multicentre Acute Stroke Trial-Europe
- MAST-I
Multicentre Acute Stroke Trial-Italy
- TICI
thrombolysis in cerebral infarction
References
- 1. Wildenhain SL, Jungreis CA, Barr J, et al. CT after intracranial intraarterial thrombolysis for acute stroke. AJNR Am J Neuroradiol 1994; 15: 487– 92 [PMC free article] [PubMed] [Google Scholar]
- 2. Komiyama M, Nishijima Y, Nishio A, et al. Extravasation of contrast medium from the lenticulostriate artery following local intracarotid fibrinolysis. Surg Neurol 1993; 39: 315– 19 [DOI] [PubMed] [Google Scholar]
- 3. Nakano S, Iseda T, Kawano H, et al. Parenchymal hyperdensity on computed tomography after intraarterial reperfusion therapy for acute middle cerebral artery occlusion. Stroke 2001; 32: 2042– 48 [DOI] [PubMed] [Google Scholar]
- 4. Yoon W, Jin Seo J, Kyu Kim J, et al. Contrast enhancement and contrast extravasation on computed tomography after intraarterial thrombolysis in patients with acute ischemic stroke. Stroke 2004; 35: 876– 81 [DOI] [PubMed] [Google Scholar]
- 5. Jang Y, Lee D, Kim H, et al. The fate of high density lesions on the non-contrast CT obtained immediately after intraarterial thrombolysis in ischemic stroke patients. Korean J Radiol 2006; 7: 221– 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tissue plasminogen activator for acute ischemic stroke: the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581– 87 [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Bluhmki E, et al. for the ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317– 29 [DOI] [PubMed] [Google Scholar]
- 8. Wahlgren N, Ahmed N, Dávalos A, et al. for the SITS investigators . Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study Lancet 2008; 372: 1303– 09 [DOI] [PubMed] [Google Scholar]
- 9. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005; 36: 1432– 38 [DOI] [PubMed] [Google Scholar]
- 10. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008; 39: 1205– 12 [DOI] [PubMed] [Google Scholar]
- 11. Castaño C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010; 41: 1836– 40 [DOI] [PubMed] [Google Scholar]
- 12. Wehrschuetz M, Wehrschuetz E, Augustin M, et al. Early single center experience with the Solitaire thrombectomy device for the treatment of acute ischemic stroke. Interv Neuroradiol 2011; 17: 235– 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kan PT, Orion D, Yashar P, et al. Intra-arterial thrombolysis and thrombectomy for acute ischemic stroke: technique and results. J Neurosurg Sci 2011; 55: 151– 60 [PubMed] [Google Scholar]
- 14. Thrombolysis in Myocardial Infarction (TIMI) Trial: phase I findings—TIMI study group. N Engl J Med 1985; 312: 932– 36 [DOI] [PubMed] [Google Scholar]
- 15. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: 109– 37 [DOI] [PubMed] [Google Scholar]
- 16. Tomsick T. TIMI, TIBI, TICI: I Came, I Saw, I Got Confused. AJNR Am J Neuroradiol 2007; 28: 382– 84 [PMC free article] [PubMed] [Google Scholar]
- 17. Adams HP, del Zoppo G, Alberts HJ, et al. Guidelines for the early management of adults with ischemic stroke. Circulation 2007; 115: 478– 534 [DOI] [PubMed] [Google Scholar]
- 18. Rudd MA, Johnstone MT, Rabbani LE, et al. Thrombolytic therapy causes an increase in vascular permeability that is reversed by 1-deamino-8-D-vasopressin. Circulation 1991; 84: 2568– 73 [DOI] [PubMed] [Google Scholar]
- 19. Okajima K, Abe H, Binder BR. Endothelial cell injury induced by plasmin in vitro. J Lab Clin Med 1995; 126: 377– 84 [PubMed] [Google Scholar]
- 20. Rabbani LE, Johnstone MT, Rudd MA, et al. PPACK attenuates plasmin induced changes in endothelial integrity. Thromb Res 1993; 70: 425– 36 [DOI] [PubMed] [Google Scholar]
- 21. Hommel M, Boissel JP, Cornu C, et al. Termination of trial of streptokinase in severe acute ischemic stroke (letter). Lancet 1995; 345: 578– 79 [DOI] [PubMed] [Google Scholar]
- 22. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischemic stroke: Multicentre Acute Stroke Trial-Italy (MAST-I) Group. Lancet 1995; 346: 1509– 14 [PubMed] [Google Scholar]
- 23. Thrombolytic therapy with streptokinase in acute ischemic stroke: the Multicentre Acute Stroke Trial-Europe Study Group. N Engl J Med 1996; 335: 145– 50 [DOI] [PubMed] [Google Scholar]
- 24. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275– 82 [DOI] [PubMed] [Google Scholar]
- 25. Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab 1996; 16: 1373– 78 [DOI] [PubMed] [Google Scholar]
- 26. Meyer JS. Importance of ischemic damage to small vessels in experimental cerebral infarction. J Neuropathol Exp Neurol 1958; 17: 577– 85 [DOI] [PubMed] [Google Scholar]
- 27. Fisher CM, Adams RD. Observations on brain embolism with special reference to the mechanism of hemorrhagic infarction. In: Furlan AJ. ed. The Heart and Stroke: Exploring Mutual Cerebrovascular and Cardiovascular Issues. New York: Springer-Verlag; 1987: 17– 36 [Google Scholar]
- 28. Yokogami K, Nakano S, Ohta H, et al. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 1996; 39: 1102– 07 [DOI] [PubMed] [Google Scholar]
- 29. Kidwell CS, Saver JL, Carneado J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 2002; 33: 717– 24 [DOI] [PubMed] [Google Scholar]
- 30. Bourekas EC, Slivka A, Shah R, et al. Intra-arterial thrombolysis within three hours of stroke onset in middle cerebral artery strokes. Neurocrit Care 2009; 11: 217– 22 [DOI] [PubMed] [Google Scholar]
- 31. Jahan R. Solitaire flow-restoration device for treatment of acute ischemic stroke: safety and recanalization efficacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol 2010; 31: 1938– 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.