Abstract
BACKGROUND AND PURPOSE:
Juvenile angiofibromas are hypervascular tumors that may benefit from preoperative devascularization to reduce intraoperative blood loss. Our purpose was to evaluate the extent of angiographic devascularization and intraoperative blood loss by using only Onyx for percutaneous juvenile angiofibroma tumor embolization.
MATERIALS AND METHODS:
We reviewed the clinical records and preoperative and postoperative imaging studies of a consecutive series of 9 patients with juvenile angiofibromas who were treated with preoperative embolization with direct percutaneous injection of Onyx followed by resection from a standard open surgical or endoscopic approach.
RESULTS:
Two Fisch type I, 1 Fisch type II, 5 Fisch type IIIa, and 1 Fisch type IVa tumor were treated. Complete devascularization was achieved in all cases percutaneously with only Onyx. There were no complications. The average intraoperative blood loss was 567.7 mL (range, 10–1700 mL). An average of 2.2 needles (range, 1–5 needles) was placed into the tumor. An average of 14.6 mL of Onyx (range, 2–25 mL) was injected into each tumor. Four Fisch type IIIa tumors were removed completely from only an ENE approach.
CONCLUSIONS:
Presurgical direct percutaneous embolization of a juvenile angiofibroma with only EVOH before surgical resection is safe and feasible. Our preliminary experience suggests that Onyx may offer a higher degree of devascularization compared with other embolic agents. This may facilitate an easier surgical resection with lower blood loss.
Juvenile angiofibroma is a rare benign fibrovascular tumor affecting young adolescent boys, originating from the posterolateral wall of the nasal cavity.1 Epistaxis and nasal obstruction are the most common presenting symptoms.2 Despite the benign nature of a juvenile angiofibroma, its growth pattern is locally destructive and it tends to spread into the anterior nasal cavity, maxillary sinus, pterygoid region, infratemporal fossa, orbit, and middle cranial fossa.2,3 Surgical resection is the treatment of choice for a juvenile angiofibroma.4 Tumor resection can be either through an endoscopic or an open surgical approach, depending on the Fisch classification or surgical staging system of Radkowski et al.5 Juvenile angiofibroma resection is often associated with significant intraoperative bleeding due to the hypervascular nature of the lesion. To reduce intraoperative blood loss and shorten the surgical resection time and morbidity, preoperative embolization has become an important adjunctive tool for the operating surgeon.6
Traditionally preoperative embolization is performed from a transarterial approach with particulate material. However, transarterial particulate embolization may be limited in cases in which it is difficult to place a microcatheter in the arterial feeder supplying the tumor. This is most common in large tumors, involving the skull base with intracranial extension and blood supply from the ICA. On the basis of the current limitations of transarterial particulate embolization and the properties of EVOH (Onyx; ev3, Irvine, California), we hypothesized that direct percutaneous embolization with only EVOH maybe a better option for the preoperative devascularization of a juvenile angiofibroma. Previously, we reported a single case describing this technique.7 Herman et al8 reported the use of the direct percutaneous embolization technique with EVOH in 4 patients using a direct endoscopic puncture of the tumor. We report our preliminary experience in a consecutive series of 9 juvenile angiofibromas that were treated with direct percutaneous embolization by using EVOH as the sole agent. Our purpose was to evaluate the extent of angiographic devascularization and intraoperative blood loss by using only EVOH for percutaneous juvenile angiofibroma embolization. Secondary measures were room time and blood transfusion requirements during surgical resection.
Materials and Methods
The institutional review board at the University of Michigan, Ann Arbor, approved this study. We retrospectively reviewed the medical records for the past 15 months to obtain a consecutive series of 9 juvenile angiofibromas in which EVOH was used as the sole agent for direct percutaneous embolization. Cross-sectional imaging studies, angiographic notes, clinic summaries, and operative reports were reviewed. All procedures were performed with the patient under general anesthesia with monitoring of the patient's electroencephalogram, visual-evoked potentials, and somatosensory-evoked potentials. The size of the tumor was determined on angiography by the hypervascular blush. The maximum anteroposterior, transverse, and cranial caudal dimensions of each tumor are shown in the Table. The staging systems of Fisch9 and Radkowski et al10 were assessed by preoperative cross-sectional MR imaging. The approach and number of needles placed into the tumor, embolization time, operative time, surgical approach, blood transfusion requirement, and estimated blood loss from surgical excision were recorded from retrospective review of medical records.
Patient characteristics
| Patient | Tumor Size (AP × Trans × CC) (Maximum cm) | Amount of Onyx (mL) | No. of Needles in Tumor | Fisch/Radowski Grade | Room Time for Preop Embo (min) | Surgical Approach | Room Time for Surgery (min) | EBL (mL) | Blood Transfusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.0 × 6.1 × 4.8 | 23 | 5 | IIIa/IIIA | 195 | Lefort I ENE | 386 | 1700 | 2 U PRBC |
| 2 | 6 × 5.4 × 5.3 | 16 | 2 | IIIa/IIIA | 279 | ENE | 476 | 1600 | 2 U PRBC |
| 3 | 3.4 × 4.3 × 3.9 | 25 | 5 | IVa/IIIB | 240 | ENE | 503 | 400 | None |
| 4 | 3.8 × 3.3 × 4.0 | 16 | 1 | IIIa/IIIA | 140 | ENE | 381 | 400 | None |
| 5 | 6.4 × 3.2 × 3.3 | 22 | 1 | IIIa/IIIA | 279 | ENE | 488 | 400 | None |
| 6 | 2.3 × 2.1 × 1.4 | 2 | 1 | I/IA | 76 | NE | 39 | 10 | None |
| 7 | 5.9 × 5.4 × 5.0 | 10 | 1 | IIIa/IIIA | 125 | Midface deglove/medial maxillectomy | 223 | 200 | None |
| 8 | 4.3 × 2.1 × 3.3 | 14 | 2 | II/IIA | 157 | ENE | 192 | 200 | None |
| 9 | 4.5 × 4.3 × 4.9 | 4 | 2 | I/IB | 123 | NE | 267 | 200 | None |
Note:—AP indicates anteroposterior; CC, craniocaudal; Preop Embo, preoperative embolization; NE, nasal endoscopic; Trans, transverse.
Percutaneous transfemoral access was obtained using the standard Seldinger technique. Nonionic contrast, Isovue 300 (Iopamidol; Bracco Diagnostics, Milan, Italy), was used to perform diagnostic cerebral angiography. The common carotid, external carotid, and internal carotid arteries were injected, and angiograms were obtained to identify the arterial supply and hypervascularity of the lesion. The results from the diagnostic angiograms were then used to assess the feasibility of direct percutaneous embolization, delineate the targeted neovasculature of the lesion, evaluate dangerous vascular collaterals to the intracranial circulation, and determine the appropriate projection required to monitor the embolization.
The lesion was punctured with a 19-ga 10-cm needle using CT, fluoroscopy, biplane transarterial roadmaps, or bone landmarks. Needles were placed into the lesion from transzygomatic, transnasal, transoral, and transbuccal approaches. Needle position within the lesion was considered correct when blood reflux from the needle was slow but continuous. A small amount of contrast was injected through the needle, and a parenchymogram was obtained to confirm needle position and assess the neovascular compartment of the lesion filled by the needle position. Biplane angiography was also performed to confirm needle position in the lesion and to make sure the needle did not transgress the ICA or a branch of the ECA. The dead space of the needle was primed with dimethyl-sulfoxide. EVOH was injected through the needle to embolize the tumor. We have previously described the technique of direct percutaneous embolization by using EVOH in 2 prior articles.11,12 The technique was repeated as many times as necessary to achieve optimal devascularization of the tumor.
The technical success of the embolization procedures was determined by the degree of residual parenchymal staining of the tumors on biplane angiography. The extent of tumor devascularization was recorded as poor (0%–30%), moderate (30%–70%), subtotal (70%–99%), and total (100%) and was reviewed by 3 authors (J.J.G., N.C., and A.S.P.). Comments from surgeons (L.J.M., S.E.S., and E.L.M.) about the benefit of preoperative devascularization of the tumor using the direct percutaneous embolization of EVOH were recorded.
Results
A total of 9 patients with juvenile angiofibromas were treated with direct percutaneous embolization with only EVOH. There were 9 males, with a mean age of 15 years (range, 11–22 years): Two Fisch type I, 1 Fisch type II, 5 Fisch type IIIa, and 1 Fisch Type IVa tumor were treated.9 According to the classification of Radkowski et al,10 1 type IA, 1 type IB, 1 type IIA, 5 type IIIA, and 1 type IIIB tumor were treated. Complete devascularization of all 9 juvenile angiofibromas was achieved by using the direct percutaneous embolization technique with EVOH as the sole agent. An average of 2.2 needles (range, 1–5 needles) was placed. Only a single needle was needed in a majority of cases to achieve complete devascularization of the tumor. Successful complete devascularization of the tumor was demonstrated by lack of parenchymal staining on the completion angiogram in all cases. An average of 14.6 mL of EVOH (range, 2–25 mL) was injected into each tumor. The average preoperative embolization procedure time was 176 minutes (range, 76–279 minutes). Two patients experienced the trigeminocardiac reflex during the initial priming of the needle with dimethyl-sulfoxide. No patient experienced nontarget embolization of EVOH into the extracranial or intracranial circulation. Minimal nontarget embolization of EVOH was seen in the small arteries surrounding the tumor and adjacent soft-tissue planes.
Surgical resection was completed within approximately 24 hours of the embolization procedure with pathologic confirmation of a juvenile angiofibroma made from submitted tissue samples. Average operative blood loss was estimated to be 567.7 mL (range, 10–1700 mL) with an average total operative room time of 328.3 minutes (range, 39–503 minutes). Six patients had supply to the tumor from the ICA. All 3 ear, nose, and throat surgeons reported the effectiveness of preoperative devascularization as excellent in all cases on the basis of personal communication and review of the patient medical records. It was reported that the tumor could be removed easily in a piecemeal fashion with bipolar cautery. The tumor margins were also readily demarcated from normal tissue due to direct visualization of the tantalum within the EVOH staining the lesion. Furthermore, given the complete devascularization of the tumor, surgical planning in 4 cases was changed from an open surgical procedure to an ENE approach.
Illustrative Cases
Case 1.
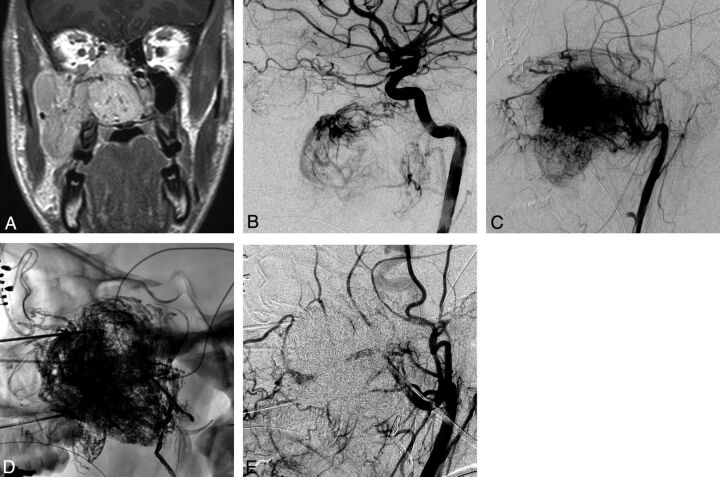
A 16-year-old boy presented with epistaxis and nasal stuffiness. Diagnostic work-up revealed a nasopharyngeal mass measuring 6.0 × 6.1 × 4.8 cm (Fig 1 A). The initial angiogram revealed a hypervascular mass showing arterial supply from feeders originating from the ECAs and ICAs (Fig 1B, -C). The tumor was punctured 5 times with a 19-ga needle from transnasal, infrazygomatic, and transbuccal approaches under CT and with fluoroscopic guidance. A total of 23 mL of Onyx was injected into the tumor (Fig 1D). Postembolization external carotid angiograms demonstrated no significant contrast filling of the tumor (Fig 1E).
Fig 1.
A 16-year-old boy who presented with epistaxis and nasal stuffiness with a Fisch grade IIIa juvenile angiofibroma. A, Coronal contrast-enhanced T1-weighted MR image shows a large contrast-enhancing mass within the right nasal cavity, extending into the right ethmoid sinus, right maxillary sinus, and right buccal space. B, Lateral right internal carotid angiogram (RICA) shows supply to the upper portion of the hypervascular mass from the inferior lateral trunk. C, Lateral right external carotid angiogram (RECA) shows the hypervascular mass with supply off the distal right internal maxillary artery, accessory meningeal artery, facial artery, and ascending pharyngeal artery. D, Lateral radiography shows the EVOH (Onyx) cast within the tumor. E, Lateral RECA postembolization shows no filling of the hypervascular mass.
Case 2.
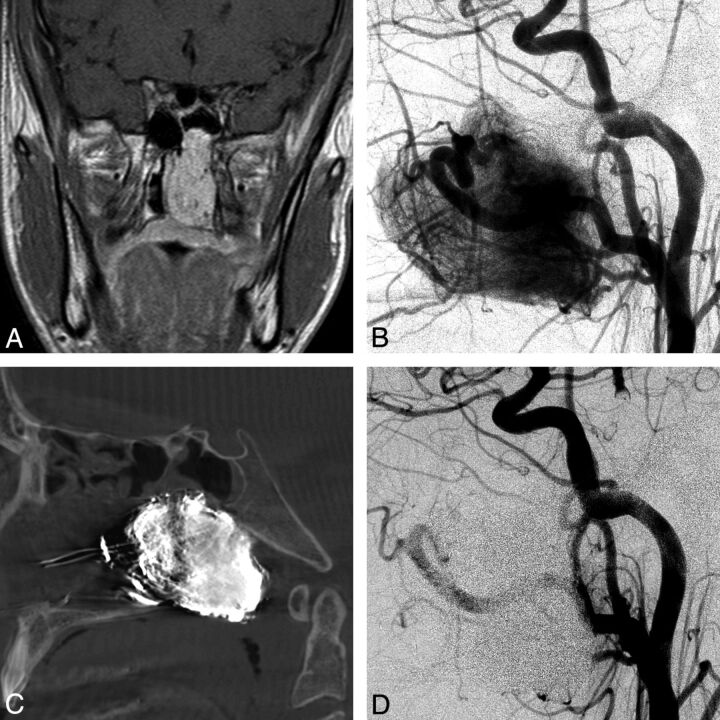
A 13-year-old boy presented with nasal stuffiness. Cross-sectional imaging revealed a nasopharyngeal hypervascular mass measuring 4.3 × 2.1 × 3.3 cm (Fig 2 A). The initial angiogram revealed a hypervascular mass with arterial supply from feeders originating from external and internal carotid arteries (Fig 2B). The tumor was punctured 2 times from a transnasal approach with a 19-ga needle under fluoroscopic guidance. A total of 14 mL of Onyx was injected into the tumor (Fig 2C). Postembolization external carotid angiograms demonstrated no significant contrast filling of the tumor (Fig 2D).
Fig 2.
A 13-year-old boy with nasal stuffiness and a Fisch Ib juvenile angiofibroma. A, Coronal contrast-enhanced T1-weighted MR image shows a contrast-enhancing mass within the left nasal cavity and posterior nasopharynx, extending into the left sphenoid sinus. B, Lateral left common carotid angiogram (LCCA) shows the hypervascular mass with supply off the ECA and ICA branches. C, Lateral DynaCT (Siemens, Erlangen, Germany) image shows the EVOH (Onyx) cast within the tumor. D, Lateral LCCA postembolization shows no filling of the hypervascular mass.
Discussion
Juvenile angiofibroma is a highly vascular tumor that can cause significant morbidity during surgical resection due to substantial intraoperative blood loss. This, in turn, may lead to a subtotal resection of the tumor or a prolonged operative time, each of which carries its own inherent risks. Given the potential for significant blood loss during surgical resection of a juvenile angiofibroma, preoperative embolization has become an important adjunctive tool. Preoperative embolization has been shown to reduce intraoperative blood loss and operative times.6 This has been traditionally performed with transarterial particulate embolization; however, small arterial feeders, vessel tortuosity, and/or vasospasm may limit this technique. Given these limitations of particulate embolization, direct percutaneous embolization of the tumor with EVOH may offer several advantages.
Gemmete et al,13 with a group of 15 hypervascular tumors of the head and neck, showed that percutaneous injection of EVOH in conjunction with particulate embolization enhanced the ability to devascularize these tumors before operative removal. In addition, there are 2 other series in the literature, with a limited number of patients, describing the direct percutaneous embolization technique using only EVOH in the preoperative devascularization of a carotid body tumor.12,14 Blood loss appears to be less with operative removal of a carotid body tumor after direct percutaneous embolization with EVOH than with particulate material. Two recent case reports describe the use of the direct percutaneous embolization technique with EVOH in the preoperative embolization of a juvenile angiofibroma before surgical resection.7,15 Complete angiographic devascularization was achieved in both cases, with an estimated blood loss of 200 and 400 mL, respectively. The largest series to date of 4 patients describes the use of needle insertion into a juvenile angiofibroma under direct endoscopic visualization, with EVOH injected into the tumor under fluoroscopic and endoscopic guidance.8 Average EBL during surgery was 412.5 mL (range, 150–800 mL), with an average operating room time of 228 minutes (range, 95–485 minutes).8 The patients experienced no bleeding from the tumor or its attachment with this approach. Two patients experienced mild numbness in the V2 distribution, which began to resolve 1 week postoperatively.
Nicolai et al16 in a group of 15 juvenile angiofibromas treated with polyvinyl alcohol preoperative embolization and simple endoscopic removal of the tumor, had blood loss ranging from 80 to 600 mL (mean, 372 mL), with no patient requiring a transfusion. However, in this series, a majority of the tumors were small. There were 2 patients with type I, 9 with type II, 3 with a type IIIA, and 1 with at type IIIB juvenile angiofibroma.16
Our results compare favorably with the only other report from the Miami group in the literature.8 The average operative time in our series was 328.3 minutes with an average EBL of 567.7 mL, compared with an average procedure time of 228 minutes and an average EBL of 412.5 mL, respectively.8 The average amount of EVOH injected into the tumor in our series was 14.6 mL of EVOH, which is considerably more than the 5.5 mL reported in the only other series.8 Nearly all of our tumors were Radkowski stage IIIA, whereas a majority of the tumors from the Miami report were Radkowski stage IIB. This difference in Radkowski tumor stage can account for the difference in average procedure time, EBL, and the amount of EVOH injected into the tumor. In addition, all of the tumors in the Miami series were removed from a simple endoscopic approach. Endoscopic removal of a juvenile angiofibroma has been shown to significantly decrease blood loss during resection compared with a standard surgical approach.17
It is difficult to compare our results with those in the series from Nicolai et al.16 Eleven of the tumors in their series were small (grade II or less), according to the classification of Andrews et al,18 and all of the tumors were removed by a simple endoscopic approach. In contrast, our series had only 3 tumors that were small (grade II or less), and only 2 were removed by a simple endoscopic approach. If one compares the data of the 3 tumors of grade II or less in our series (Table), the average blood loss was 70 mL. This is below the average blood loss in their series of 372 mL and also below that in the patient with the least amount of blood loss of 80 mL in their series.
Significant blood loss encountered in 2 patients in our series was due to difficulty in controlling venous bleeding related to bony erosion of the skull base from the tumor and not due to the embolization technique, which also may have contributed to our slightly increased average blood loss in comparison with that in the other study. Overall, there is an improvement in intraoperative blood loss during surgical resection after direct percutaneous embolization with Onyx compared with using transarterial particulate material.6 Time in the operating theater also appears less, presumably due to more complete preoperative devascularization of the tumor with EVOH, allowing easier tumor removal during surgical resection. Together with decreased blood loss and easier operative experience, these improvements theoretically reduce the time that the patient is under general anesthesia and reduce its associated morbidity during surgical resection. Furthermore, given the more complete devascularization of the tumor with EVOH, the risk of blood transfusion requirements is decreased with the associated risk of acquiring blood-borne disease.
We recently compared the results of preoperative devascularization of juvenile angiofibromas in 39 patients treated with particulate material versus the 9 patients treated with direct percutaneous embolization by using only EVOH (Onyx) before surgical resection. The mean EBL for the Onyx group was 567.7 mL, and the mean EBL for the particulate group was 1258.6 mL (1-tailed Student t test, P = .043). The mean unit of PRBCs used in the Onyx group was 0.29 ± 0.76 U (P = .003), and the mean PBRC used in the particulate cohort was 1.56 ± 2.01 U (unpublished data, J.J. Gemmete, personal communication, August 11, 2011).
The direct percutaneous embolization technique provides a more complete and targeted embolization of the tumor. In addition to decreased blood loss, the ease of puncturing the juvenile angiofibroma with a needle under image guidance and the ability to readily fill the tumor with EVOH under fluoroscopy appear to decrease the radiation exposure to the patient during the preoperative embolization procedure. In the current series, we have shown an average room time for preoperative embolization of 176 minutes. This is an improvement from traditional fluoroscopic times for preoperative embolization and intraoperative blood loss from surgical resection with transarterial particulate material.6
As previously described, EVOH has been shown to have superior properties in comparison with modified acrylic glue.19 Its ability to form a nonpolymerized liquid inner core while undergoing cohesive polymerization of the outer layer, which is in direct contact with blood, produces a “lavalike” flow. Antegrade injection is, therefore, more controlled, with better penetration of the tumor parenchyma. Just as important, EVOH injections may be halted and restarted with the ability to perform intermittent angiography. This feature is critical in monitoring the embolization progress and avoiding potential nontarget embolization of EVOH.
In our limited experience, we have seen retrograde opacification of arterial feeders from the ICA and ECA supplying the tumor during the EVOH injection. If this occurs, we stop the injection of EVOH for 2 minutes to let it solidify. On restarting the injection, EVOH will find a pathway of lesser resistance and fill a different portion of the tumor. We have seen retrograde filling of the mandibulovidian artery and arteries from the inferior lateral trunk and meningohypophyseal trunk during direct percutaneous embolization with EVOH. The identification of these vessels on the lateral roadmaps during embolization is important to prevent intracranial embolization of EVOH into the ophthalmic artery or intracranial vasculature. Inflation of a balloon across the origin of these arteries in the ICA may be theoretically helpful in preventing nontarget embolization; however, we do not routinely inflate a balloon in the ICA to prevent nontarget embolization. We think that this increases the complexity of the case and may possibly subject the patient to the additional risk of dissection or a thromboembolic event.
Casasco et al20 described 2 cases of nontarget embolization of glue, Histoacryl (Braun, Melsungen, Germany), into the left ophthalmic and right middle cerebral arteries during percutaneous embolization of skull base tumors. We have an extensive experience with the percutaneous injection of both glue and Onyx into these tumors. We think that the injection of Onyx compared with glue is easier to perform and control. This may limit nontarget embolization of the material.
The ICA branches, which can supply a large juvenile angiofibroma, are very difficult to select with a microcatheter due to their small size and the tortuous course off the ICA. Furthermore, if selection of an ICA branch supplying the tumor with a microcatheter is possible, this is nearly always occlusive to antegrade flow, thus making injection of particles very dangerous due to possible reflux into the intracranial circulation. With the direct percutaneous embolization technique, we have commonly seen devascularization of the portion of the tumor supplied by ICA branches. We theorize that EVOH has a better more uniform penetration into the tumor bed and has the ability to penetrate the entire tumor. This should allow easier surgical resection because the most difficult portion of the tumor to be removed is the deep portion usually supplied by the ICA. In turn, it is possible that a majority of these lesions can be surgically removed from an endoscopic approach rather than by using an open surgical technique. A number of authors have compared endoscopic resection of a juvenile angiofibroma with the open surgical approaches and have found endoscopic resection to be safe and effective, with similar recurrence rates.17,21 Furthermore, patients undergoing endoscopic resection demonstrated fewer complications, less operative blood loss, and shorter hospital stays.17 Tumor resection in our series was from an endoscopic approach in 7 cases and from a standard open surgical approach in 2 cases. Tumor resection in 4 patients was changed from a standard open surgical technique to an ENE approach due to the complete preoperative devascularization of the tumor from the EVOH injection.
In addition to the benefits of direct percutaneous embolization with EVOH in devascularizing tumor parenchyma supplied by the ICA and its branches, this technique has shown benefits in devascularizing tumor supplied by bilateral ECA branches. Most juvenile angiofibromas have ipsilateral blood supply from the distal internal maxillary, accessory meningeal, ascending pharyngeal, and facial arteries as well as ICA branches. There are reports in the literature stating that up to 36% of juvenile angiofibromas could potentially have bilateral ECA supply on angiography.22 Given the possibility of dual blood supply, which is not always evaluated by angiography, and the theoretic concern of microcatheter-induced vasospasm within the catheterized feeding artery, there is the potential for persistent vascularization of the tumor, even following transarterial particulate embolization. This could result in uncontrolled bleeding at the time of surgical resection. Because the tumor parenchyma is directly targeted using the direct percutaneous embolization technique, this scenario is less likely due to the more complete devascularization of the tumor.
A rare possible complication with the use of EVOH is the trigeminocardiac reflex.23 This is a rare phenomenon seen generally during invasive head and neck procedures in which there is manipulation or stimulation of the trigeminal nerve or gassarian ganglion.24 Through activation of parasympathetic efferent pathways, the trigeminocardiac reflex leads to acute cardiac dysrhythmias, bradycardia, profound hypotension, apnea, and gastric hypermobility. A previous report by our group showed that the trigeminocardiac reflex could be activated during percutaneous injection of EVOH in a juvenile angiofibroma, specifically with priming of the syringe with dimethyl-sulfoxide, an organic solvent used to prevent premature polymerization of EVOH.25 The exact mechanism is unclear, but this may be related to the direct neurotoxic properties of dimethyl-sulfoxide. Once the reflex is encountered, the procedure should be halted temporarily while vasolytic drugs (eg, atropine and/or glycopyrrolate) or sympathomimetics are administered.26 This was seen in 2 patients in this series.
The cost of using EVOH to perform preoperative direct percutaneous embolization of a juvenile angiofibroma is very high. On average, a total of 15 vials of EVOH were needed to perform complete preoperative devascularization of a tumor. Each vial of EVOH in the United States is $1800; therefore, the total cost is $27,000. This is significantly more than the average cost of a vial of particulate material, $300, or a single pushable coil, which costs approximately $300. However, for a direct cost comparison, the room time in the angiography suite for preoperative embolization, the operating theater time, blood transfusion requirements during tumor resection, and operative approach (endoscopic versus an open surgical approach) must be taken into consideration for a more accurate measure of the total costs of the direct percutaneous embolization technique with EVOH compared with particulate embolization.
There are several limitations to our study. The small number of patients makes generalization to a larger cohort of patients very difficult. All tumors were embolized with only EVOH; therefore, a direct comparison of the effectiveness among particulate material, modified acrylic glue, and direct percutaneous embolization with EVOH cannot be made.
There is a limited amount of data in the literature regarding the use of EVOH as a sole agent for direct percutaneous embolization of a juvenile angiofibroma before surgical resection. Our series, while consisting of a small cohort, offers additional data on the effectiveness and safety of such a procedure. Future studies should directly compare direct percutaneous embolization with only EVOH with traditional intra-arterial particulate embolization in the preoperative devascularization of a juvenile angiofibroma. In addition, given the complete devascularization of the tumor from the direct percutaneous embolization technique, it would interesting to see if all juvenile angiofibromas without intracranial extension can removed solely from an endoscopic approach.
Conclusions
Presurgical direct percutaneous embolization of a juvenile angiofibroma with only EVOH before surgical resection is safe and feasible. Our preliminary experience suggests that direct percutaneous embolization with EVOH may offer a higher degree of devascularization of the tumor compared with transarterial particulate embolization. This may facilitate a less challenging surgical resection with less intraoperative blood loss. This approach may allow a majority of juvenile angiofibromas to be removed from an endoscopic approach.
ABBREVIATIONS:
- EBL
estimated blood loss
- ECA
external carotid artery
- ENE
expanded nasal endoscopic approach
- EVOH
ethylene-vinyl alcohol copolymer
- PRBC
packed red blood cells
Footnotes
Disclosures: Joseph Gemmete—RELATED: Consulting Fee or Honorarium: Covidien, Comments: paid consultant for company. Aditya Pandey—UNRELATED: Grants/Grants Pending: ev3.
References
- 1. Valavanis A, Christoforidis G. Applications of interventional neuroradiology in the head and neck. Semin Roentgenol 2000; 35: 72– 83 [DOI] [PubMed] [Google Scholar]
- 2. Sivanandan R, Willard EF. Benign and malignant tumors of the nasopharynx. In: Cummings CW, Haughey BH, Thomas JR, et al., eds. Cummings Otolaryngology: Head and Neck Surgery 4th ed. Philadelphia: Mosby; 2005: 1669– 84 [Google Scholar]
- 3. Roche PH, Paris J, Regis J, et al. Management of invasive juvenile nasopharyngeal angiofibromas: the role of a multimodality approach. Neurosurgery 2007; 61: 768– 77, discussion 77 [DOI] [PubMed] [Google Scholar]
- 4. Lin Y, Qiu JH, Qiao L, et al. Le Fort I osteotomy for extensive juvenile nasopharyngeal angiofibroma: a retrospective study. Adv Ther 2008; 25: 1057– 64 [DOI] [PubMed] [Google Scholar]
- 5. Renkonen S, Hagstrom J, Vuola J, et al. The changing surgical management of juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol 2011; 268: 599– 607 [DOI] [PubMed] [Google Scholar]
- 6. Moulin G, Chagnaud C, Gras R, et al. Juvenile nasopharyngeal angiofibroma: comparison of blood loss during removal in embolized group versus nonembolized group. Cardiovasc Intervent Radiol 1995; 18: 158– 61 [DOI] [PubMed] [Google Scholar]
- 7. Gemmete JJ, Chaudhary N, Pandey AS, et al. Complete devascularization of a juvenile angiofibroma by direct percutaneous embolization with only ethylene vinyl alcohol copolymer (Onyx) through a single needle placement. J Neurointerv Surg 2011; 3: 191– 93 [DOI] [PubMed] [Google Scholar]
- 8. Herman B, Bublik M, Ruiz J, et al. Endoscopic embolization with Onyx prior to resection of JNA: a new approach. Int J Pediatr Otorhinolaryngol 2011; 75: 53– 56 [DOI] [PubMed] [Google Scholar]
- 9. Fisch U. The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope 1983; 93: 36– 44 [DOI] [PubMed] [Google Scholar]
- 10. Radkowski D, McGill T, Healy GB, et al. Angiofibroma: changes in staging and treatment. Arch Otolaryngol Head Neck Surg 1996; 122: 122– 29 [DOI] [PubMed] [Google Scholar]
- 11. Gemmete JJ, Ansari SA, McHugh J, et al. Embolization of vascular tumors of the head and neck. Neuroimaging Clin N Am 2009; 19: 181– 98 [DOI] [PubMed] [Google Scholar]
- 12. Shah HM, Gemmete JJ, Chaudhary N, et al. Preliminary experience with the percutaneous embolization of paragangliomas at the carotid bifurcation using only ethylene vinyl alcohol copolymer (EVOH) Onyx. J Neurointerv Surg 2012; 4: 125– 29 [DOI] [PubMed] [Google Scholar]
- 13. Gemmete JJ, Chaudhary N, Pandey A, et al. Usefulness of percutaneously injected ethylene-vinyl alcohol copolymer in conjunction with standard endovascular embolization techniques for preoperative devascularization of hypervascular head and neck tumors: technique, initial experience, and correlation with surgical observations. AJNR Am J Neuroradiol 2010; 31: 961– 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wanke I, Jackel MC, Goericke S, et al. Percutaneous embolization of carotid paragangliomas using solely Onyx. AJNR Am J Neuroradiol 2009; 30: 1594– 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehmann M, Ulrich S, Reineke U, et al. Intratumoral Onyx embolisation in the management of juvenile nasopharyngeal angiofibroma [in German]. HNO 2010; 58: 853– 57 [DOI] [PubMed] [Google Scholar]
- 16. Nicolai P, Berlucchi M, Tomenzoli D, et al. Endoscopic surgery for juvenile angiofibroma: when and how. Laryngoscope 2003; 113: 775– 82 [DOI] [PubMed] [Google Scholar]
- 17. Pryor SG, Moore EJ, Kasperbauer JL. Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma. Laryngoscope 2005; 115: 1201– 07 [DOI] [PubMed] [Google Scholar]
- 18. Andrews JC, Fisch U, Valavanis A, et al. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope 1989; 99: 429– 37 [DOI] [PubMed] [Google Scholar]
- 19. Quadros RS, Gallas S, Delcourt C, et al. Preoperative embolization of a cervicodorsal paraganglioma by direct percutaneous injection of Onyx and endovascular delivery of particles. AJNR Am J Neuroradiol 2006; 27: 1907– 09 [PMC free article] [PubMed] [Google Scholar]
- 20. Casasco A, Houdart E, Biondi A, et al. Major complications of percutaneous embolization of skull-base tumors. AJNR Am J Neuroradiol 1999; 20: 179– 81 [PubMed] [Google Scholar]
- 21. Andrade NA, Pinto JA, Nobrega Mde O, et al. Exclusively endoscopic surgery for juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg 2007; 137: 492– 96 [DOI] [PubMed] [Google Scholar]
- 22. Wu AW, Mowry SE, Vinuela F, et al. Bilateral vascular supply in juvenile nasopharyngeal angiofibromas. Laryngoscope 2011; 121: 639– 43 [DOI] [PubMed] [Google Scholar]
- 23. Lv X, Li Y, Jiang C, et al. The incidence of trigeminocardiac reflex in endovascular treatment of dural arteriovenous fistula with Onyx. Interv Neuroradiol 2010; 16: 59– 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schaller B. Trigeminocardiac reflex: a clinical phenomenon or a new physiological entity? J Neurol 2004; 251: 658– 65 [DOI] [PubMed] [Google Scholar]
- 25. Potti TA, Gemmete JJ, Pandey AS, et al. Trigeminocardiac reflex during the percutaneous injection of ethylene vinyl alcohol copolymer (Onyx) into a juvenile nasopharyngeal angiofibroma: a report of two cases. J Neurointerv Surg 2011; 3: 263– 65 [DOI] [PubMed] [Google Scholar]
- 26. Arasho B, Sandu N, Spiriev T, et al. Management of the trigeminocardiac reflex: facts and own experience. Neurol India 2009; 57: 375– 80 [DOI] [PubMed] [Google Scholar]