We all know that chronic injury of the CST results in Wallerian degeneration and atrophy and that DWI may show this process early on, but little is known about the ultimate fate of the injured CST. Here, the authors used DTI to assess the CST in 45 patients after hemorrhage. They studied their subjects acutely within 30 days and again after 3 months and the CSTs were categorized as preserved around the hematoma (type A), interrupted around the hematoma (type B), or did not reach the hematoma (type C). Type A had the best motor function acutely and type C the worst. However, 14% changed from type A to B, 92% changed from B to A, and 16% changed from C to A. The CST may change from the acute to chronic stages during motor function recovery and DTI may predict regeneration or degeneration.
Abstract
BACKGROUND AND PURPOSE:
Little is known about the fate of the injured CST for a large number of patients with ICH. Using DTT, we investigated the longitudinal changes of injured CSTs in patients with an ICH.
MATERIALS AND METHODS:
We recruited 45 patients with CST injury by an ICH in the supratentorial subcortical area. Two longitudinal DTTs were acquired: 1 within 30 days and the other after 3 months from onset. DTTs for the CST were classified into 3 types: type A, the CST was preserved around the hematoma; type B, the CST was interrupted around the hematoma; and type C, the CST did not reach the hematoma.
RESULTS:
At the first DTT, the motor functions of type C were worse than those of types A and B (P < .01), and motor functions of type A were better than those of type C at the second DTT (P < .01). Of 14 type A, 2 changed to type B (14.3%) and 12 did not change (85.7%); of 12 type B, 11 changed to type A (91.7%) and 1 changed to type C (8.3%); of 19 type C, 3 changed to type A (15.8%) and 16 did not change (84.2%).
CONCLUSIONS:
We found that the injured CST could change from the early stage to the chronic stage during the motor recovery phase in patients with an ICH. These results would be helpful in prediction of longitudinal DTT changes from the early stage to the chronic stage following ICH.
Motor weakness is one of the most serious disabling sequelae of ICH, with over 70% of ICH patients experiencing a residual motor deficit.1 The CST is the most important neural tract for motor control in the human brain. Recovery of an injured CST is mandatory for good motor recovery after ICH, and degeneration of an injured CST accompanies poor motor function in patients with ICH.2–6 Therefore, elucidation of the CST state is an important topic for rehabilitation in patients with ICH.
Recent advances in development of DTI techniques allow 3D visualization and estimation of the CST.7–9 Several studies using DTI have reported that injury to the CST resulting from ICH could be recovered or degenerated during the critical period for motor recovery.2,3,10,11 However, little is known about the fate of the injured CST for a large number of patients with ICH. We hypothesized that the injured CST, when confirmed during the early stages of ICH, could be changed with time by the process of recovery or degeneration.
In the current study, we attempted to elucidate the longitudinal changes of the injured CST in patients with an ICH using follow-up DTI studies that were taken at early and chronic stages of ICH.
Materials and Methods
Participants
Among patients with ICH who were admitted to the rehabilitation department of a university hospital, we recruited 45 consecutive patients (26 males, 19 females; mean age, 55.4 ± 11.0 years; range, 4–82 years) according to the following selection criteria: 1) first-ever stroke; 2) age 20–85 years; 3) an ICH lesion at the level of the corona radiata or posterior limb of the internal capsule explaining the CST injury,12–17 with confirmation by a neuroradiologist; 4) severe weakness of the affected extremities, to the extent of an inability to move without gravity at ICH onset; 5) the first DTI scanned within 30 days after onset and the second after 3 months from onset; 6) patient rehabilitation from the time of first DTI to the time of second DTI; and 7) absence of serious medical complications, such as pneumonia or cardiac problems, from onset to final evaluation. Patients who showed apraxia, somatosensory problems, severe spasticity (modified Ashworth scale >2), or severe cognitive problems (Mini-Mental State Examination <25) were excluded from this study. The local ethics committee of a university hospital approved the study protocol.
Clinical Evaluation
Motor function of patients was evaluated twice: at onset (within 24 hours of symptom onset) and at the time of the second DTI scanning (> 3 months after onset). The MI was used for measurement of motor function, with a maximum score of 100.18 Function of the affected hand was categorized according to the MBC19,20: 0 (unable to move fingers voluntarily); 1 (able to move fingers voluntarily); 2 (able to close hand voluntarily, unable to open hand); 3 (able to grasp a card between the thumb and the medial side of the index finger, able to extend fingers slightly); 4 (able to pick up and hold a glass, able to extend fingers); and 5 (able to catch and throw a ball in a near-normal fashion, able to button and unbutton a shirt). Walking ability was determined according to the FAC.21 Six categories are included in the FAC: 0 (nonambulatory); 1 (needs continuous support from 1 person); 2 (needs intermittent support from 1 person); 3 (needs only verbal supervision); 4 (help is required on stairs and uneven surfaces); and 5 (can walk independently anywhere). The reliability and validity of the MI, MBC, and FAC are well established.18–21
Diffusion Tensor Imaging
Two longitudinal diffusion tensor MR images were acquired from each patient (first DTI, mean duration 20.3 ± 7.9 days; second DTI, mean duration 430.7 ± 592.3 days), using a 1.5T Gyroscan Intera system (Philips Healthcare, Best, the Netherlands) equipped with a Synergy-L SENSE head coil, with a single-shot spin-echo-planar imaging sequence. For each of the 32 noncollinear and noncoplanar diffusion-sensitizing gradients, we acquired 60 contiguous sections parallel to the anterior/posterior commissure line. Imaging parameters were as follows: matrix = 128 × 128, field of view = 221 × 221 mm2, TE = 76 ms, TR = 10,726 ms, SENSE factor = 2, echo-planar imaging factor = 67, b = 600 mm2s−1, NEX = 1, and section thickness of 2.3 mm. Preprocessing of DTI datasets was performed using the Oxford Centre FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl). Eddy current-induced image distortions and motion artifacts were removed using affine multiscale 2D registration. DTT for CST was depicted using DTI Studio software (Johns Hopkins Medical Institute, Baltimore, Maryland). Fiber-tracking was based on the FACT algorithm, a brute-force reconstruction approach, and a multiple ROIs approach. The CST was ascertained by selection of the fibers passing through both of the ROIs on the color map.7,16,17,22 Fiber-tracking was initiated at the center of the seed voxel with fractional anisotropy >0.2 and ended at the voxel having a fiber assignment <0.2 and a tract turning-angle <60.22
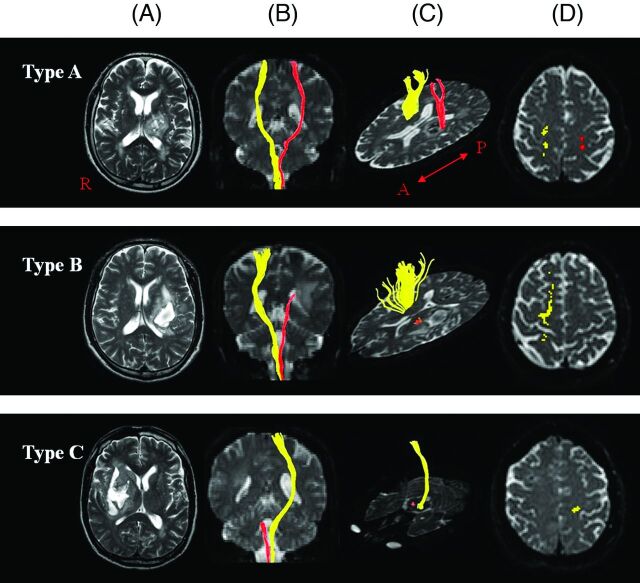
DTT was classified into 3 types: type A, the CST originating from the cerebral cortex was preserved around the hematoma; type B, the CST was interrupted (discontinuation of the integrity of the CST) at or around the hematoma; and type C, the CST did not reach the hematoma (Fig 1).
Fig 1.
Classification of DTT for an injured CST (red). A, T2-weighted brain MR images, coronal images of DTT (B), axial images of DTT at or around or below the lesion level and at the cortex level (showing the integrity or disintegrity of DTT) (C and D): type A, the CST originating from the cerebral cortex is preserved around the hematoma; type B, the CST is interrupted at or around the hematoma; and type C, the CST does not reach the hematoma due to wallerian degeneration.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, Chicago, Illinois) 17.0 software. We used the parametric 1-way ANOVA test for comparison of sex ratio, age, the ratio of affected side to unaffected side, duration from onset to rehabilitation, and motor function—MI, MBC, and FAC—scores at the time of second DTT between 3 types of CST on each DTI. The Fisher least significant difference test was used as the post hoc comparison of motor function scores between 3 types of CST on each DTI. The level of statistical significance was set at P < .01.
Results
Demographic data for patients are shown in Table 1. There were 2 significant differences between 3 types of CST at the time of first DTT, in terms of age (P = .0066), and at the time of second DTT, in terms of duration from ICH onset to beginning of rehabilitation (P = .0098). The other variables showed no significant difference (P > .01). Clinical data for patients are shown in Table 2. At the time of first DTT, the motor functions of type C—in terms of MI, MBC, and FAC—were significantly worse than those of type A and B (P < .01), and there were no significant differences between type A and B in all motor functions (P > .01). At the time of the second DTT, we could observe that the motor functions—MI, MBC, and FAC—of type A were significantly better than those of type C (P < .01), and type B showed no significant differences compared with type A and C in all motor functions (P > .01).
Table 1:
Demographic data according to corticospinal tract type on diffusion tensor tractography
| CST Type on 1st DTT |
CST Type on 2nd DTT |
Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | ||
| No. (%) | 14 (31.1) | 12 (26.7) | 19 (42.2) | 26 (57.8) | 2 (4.4) | 17 (37.8) | 45 (100.0) |
| Sex (M:F) | 8:6 | 6:6 | 12:7 | 15:11 | 1:1 | 10:7 | 26:19 |
| Age (years) | 61.6 ± 11.3 | 56.7 ± 9.9 | 49.9 ± 9.1 | 57.5 ± 11.9 | 65.0 ± 2.8 | 50.9 ± 8.6 | 55.4 ± 11.0 |
| ICH side (R:L) | 4:10 | 5:7 | 9:10 | 10:16 | 0:2 | 8:9 | 18:27 |
| Duration (days) | 17.0 ± 9.0 | 18.8 ± 7.8 | 24.8 ± 9.7 | 17.3 ± 7.3 | 28.5 ± 13.4 | 25.3 ± 10.2 | 20.8 ± 9.5 |
Note:—For Age and Duration, values are means ± standard deviation. Duration is the duration from stroke onset to rehabilitation.
Table 2:
Motor function according to corticospinal tract type on diffusion tensor tractography
| CST Type on 1st DTT |
CST Type on 2nd DTT |
Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | ||
| MI | 81.4 ± 18.2 | 74.2 ± 20.4 | 40.4 ± 24.3ab | 81.1 ± 16.7 | 57.8 ± 29.0 | 33.7 ± 16.7a | 62.2 ± 28.4 |
| MBC | 4.43 ± 1.09 | 3.83 ± 1.34 | 1.53 ± 1.50ab | 4.31 ± 0.93 | 3.00 ± 2.83 | 1.12 ± 1.11a | 3.04 ± 1.87 |
| FAC | 4.43 ± 1.02 | 3.92 ± 1.00 | 2.32 ± 1.53ab | 4.35 ± 0.85 | 3.50 ± 2.12 | 1.94 ± 1.25a | 3.40 ± 1.56 |
Note:—Values are means ± standard deviation.
P < 0.01 when compared with type A.
P < 0.01 when compared with type B.
On the first DTT, 14 patients (31.1%) belonged to type A, 12 patients (26.7%) to type B, and 19 patients (42.2%) to type C. By contrast, on the second DTT, 26 patients (57.8%) were classified as type A, 2 patients (4.4%) as type B, and 17 patients (37.8%) as type C. We found that the CST changed according to the CST type: On the first DTT, of 14 patients of type A, 2 patients (14.3%) showed a change to type B, and no change was observed in 12 patients (85.7%); on the first DTT, of 12 patients of type B, 11 patients (91.7%) showed a change to type A and 1 patient (8.3%) showed a change to type C; on the first DTT, of 19 patients of type C, 3 patients (15.8%) showed a change to type A, and no change was observed in 16 patients (84.2%) (Table 3).
Table 3:
The change of corticospinal tract type
| CST Type on 1st DTT | CST Type on 2nd DTT |
||
|---|---|---|---|
| A | B | C | |
| A (n = 14) | 12 (85.7%) | 2 (14.3%) | 0 (0%) |
| B (n = 12) | 11 (91.7%) | 0 (0%) | 1 (8.3%) |
| C (n = 19) | 3 (15.8%) | 0 (0%) | 16 (84.2%) |
Discussion
In the current study, we found that DTT findings obtained at an early stage of ICH could change 3 months after onset. Seventeen (37.8%) of 45 patients showed change of CST type and the others did not change their types; 14.3% of patients of type A changed to type B, 91.7% of patients of type B changed to type A, 8.3% of patients of type B changed to type C, and 15.8% of patients of type C changed to type A. In addition, we found that the motor functions were different according to the types of CST at each time of DTT scanning. The CST can recover by adaptive plasticity or show wallerian degeneration after injury. No studies of the repeatability of an injured CST on DTT in patients with ICH have been conducted; therefore, although we cannot be certain that the DTT changes of the current study are directly related to recovery or degeneration of the CST, we think that there seems to be a possibility that these changes of an injured CST on DTT could be associated with recovery or degeneration of an injured CST. On the other hand, there is another possibility that the DTT for a CST could show false-positive or false-negative results, especially in the patients who showed changes of DTT from type C at the first DTT to type A at the second DTT. However, we believe that the results of this study would be available as a reference for prediction of longitudinal DTT changes from early stage to chronic stage following ICH.
Several DTI follow-up studies of the CST in patients with ICH have been conducted.2,3,10,11 In 2006, Jang et al demonstrated the recovery of a partially damaged CST in a patient with an ICH using changes in DTI parameters at the site of a CST that was injured due to hematoma.2 During the same year, Jang et al also reported on a patient who showed decompression of the CST compressed by subcortical ICH.10 Subsequently, in 2007, Jang et al reported on a patient with an ICH in the left corona radiata and basal ganglia who experienced nearly full recovery from complete paralysis of the affected extremities at onset through the normalization process of the damaged lateral CST of the affected hemisphere.11 DTT showed that the origin of the CST had changed from the posterior parietal cortex, primary sensory cortex, and primary motor cortex. In 2008, using follow-up DTT and transcranial magnetic stimulation, Yang et al demonstrated recovery of a CST that was disrupted due to hematoma.3 On the other hand, although there were no serial DTI studies on degeneration of the CST in ICH, a few studies have reported cerebral infarct.23–25 In 2007, Kim et al measured the degeneration speed of the CST with 5 DTTs within an interval of 7 days, starting at 2 days after onset in 2 patients with cerebral infarct and found that CST degeneration could be detected at day 9 DTT, and the most rapid CST degeneration was noted for 7 days at 16 days from onset.23 In 2009, Yu et al performed 5 consecutive DTIs (within 1 week, and at 2 weeks, 1 month, 3 months, and 1 year after onset) in 9 patients with a degenerated CST and calculated the ratios of DTI parameters for the CST between the affected and unaffected hemisphere.24 They found that the ratios changed significantly until 3 months after stroke and then remained unchanged. Recently, in 2010, Puig et al measured relative fractional anisotropy value between affected and unaffected pons at onset, 3 days, and 30 days after onset in 60 patients with middle cerebral infarct and found that the relative fractional anisotropy value was correlated with the motor weakness at 30 days after onset.25 Consequently, to the best of our knowledge, this is the first study to investigate DTT changes from early stage to chronic stage using longitudinal DTT studies in a large number of patients with ICH.
Conclusions
We found that the injured CST could change from early stage to chronic stage during the motor recovery phase in patients with an ICH: 14.3% of patients of type A showed a change to type B, 91.7% of patients of type B showed a change to type A, 8.3% of patients of type B showed a change to type C, and 15.8% of patients of type C showed a change to type A. These results would be helpful in prediction of longitudinal DTT changes from early stage to chronic stage after stroke. However, the relationship of the injured CST with regeneration or degeneration should be confirmed by further studies on the reliability and validity of the injured CST. This study is limited by a small number of patients. Further complementary studies involving larger case numbers are warranted.
ABBREVIATIONS:
- CST
corticospinal tract
- DTT
diffusion tensor tractography
- FAC
functional ambulation category
- FACT
fiber assignment continuous tracking
- ICH
intracerebral hemorrhage
- MI
Motricity Index
- MBC
modified Brunnstrom classification
- SENSE
sensitivity encoding
Footnotes
This work was supported by the Daegu Innopolis R&BD project by the Korea Institute for Advancement of Technology.
References
- 1. Arboix A, Garcia-Eroles L, Massons J, et al. Haemorrhagic pure motor stroke. Eur J Neurol 2007; 14: 219– 23 [DOI] [PubMed] [Google Scholar]
- 2. Jang SH, Byun WM, Han BS, et al. Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: a diffusion tensor image study. Restor Neurol Neurosci 2006; 24: 25– 29 [PubMed] [Google Scholar]
- 3. Yang DS, Kim DS, Kim YH, et al. Demonstration of recovery of a severely damaged corticospinal tract: a diffusion tensor tractography and transcranial magnetic stimulation follow-up study. J Comput Assist Tomogr 2008; 32: 418– 20 [DOI] [PubMed] [Google Scholar]
- 4. Yoshioka H, Horikoshi T, Aoki S, et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery 2008; 62: 97– 103 [DOI] [PubMed] [Google Scholar]
- 5. Cho SH, Kim SH, Choi BY, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett 2007; 421: 142– 46 [DOI] [PubMed] [Google Scholar]
- 6. Xu SW, Chen ZQ, Chen JH, et al. Correlation between muscular strength and basal nuclei ischemic/hemorrhagic stroke-induced corticospinal tract injury, as detected by diffusion tensor imaging and tractography. Neural Regen Res 2010; 5: 1010– 14 [Google Scholar]
- 7. Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45: 265– 69 [DOI] [PubMed] [Google Scholar]
- 8. Hsieh CT, Chen CY, Chiang YH, et al. Role of diffusion tensor imaging in a patient with spontaneous intracerebral hematoma treated by stereotactic evacuation. Surg Neurol 2008; 70: 75– 78 [DOI] [PubMed] [Google Scholar]
- 9. Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005; 25: 53– 68 [DOI] [PubMed] [Google Scholar]
- 10. Jang SH, Kim YH, Kwon YH, et al. Restoration of the corticospinal tract compressed by hematoma. Arch Neurol 2006; 63: 140– 41 [DOI] [PubMed] [Google Scholar]
- 11. Jang SH, Kim SH, Cho SH, et al. Demonstration of motor recovery process in a patient with intracerebral hemorrhage. NeuroRehabilitation 2007; 22: 141– 45 [PubMed] [Google Scholar]
- 12. Han BS, Hong JH, Hong C, et al. Location of the corticospinal tract at the corona radiata in human brain. Brain Res 2010; 1326: 75– 80 [DOI] [PubMed] [Google Scholar]
- 13. Kim YH, Kim DS, Hong JH, et al. Corticospinal tract location in internal capsule of human brain: diffusion tensor tractography and functional MRI study. Neuroreport 2008; 19: 817– 20 [DOI] [PubMed] [Google Scholar]
- 14. Holodny AI, Gor DM, Watts R, et al. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology 2005; 234: 649– 53 [DOI] [PubMed] [Google Scholar]
- 15. Ino T, Nakai R, Azuma T, et al. Somatotopy of corticospinal tract in the internal capsule shown by functional MRI and diffusion tensor images. Neuroreport 2007; 18: 665– 68 [DOI] [PubMed] [Google Scholar]
- 16. Hong JH, Son SM, Jang SH. Somatotopic location of corticospinal tract at pons in human brain: a diffusion tensor tractography study. NeuroImage 2010; 51: 952– 55 [DOI] [PubMed] [Google Scholar]
- 17. Jang SH. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation 2009; 24: 279– 83 [DOI] [PubMed] [Google Scholar]
- 18. Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980; 19: 382– 89 [DOI] [PubMed] [Google Scholar]
- 19. Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 1966; 46: 357– 75 [DOI] [PubMed] [Google Scholar]
- 20. Fujii Y, Nakada T. Cortical reorganization in patients with subcortical hemiparesis: neural mechanisms of functional recovery and prognostic implication. J Neurosurg 2003; 98: 64– 73 [DOI] [PubMed] [Google Scholar]
- 21. Cunha IT, Lim PA, Henson H, et al. Performance-based gait tests for acute stroke patients. Am J Phys Med Rehabil 2002; 81: 848– 56 [DOI] [PubMed] [Google Scholar]
- 22. Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 2004; 3: 11– 17 [DOI] [PubMed] [Google Scholar]
- 23. Kim DG, Ahn YH, Byun WM, et al. Degeneration speed of corticospinal tract in patients with cerebral infarct. NeuroRehabilitation 2007; 22: 273– 77 [PubMed] [Google Scholar]
- 24. Yu C, Zhu C, Zhang Y, et al. A longitudinal diffusion tensor imaging study on wallerian degeneration of corticospinal tract after motor pathway stroke. NeuroImage 2009; 47: 451– 58 [DOI] [PubMed] [Google Scholar]
- 25. Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010; 31: 1324– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]